Water deficit constitutes a severe limitation to agricultural productivity. In the context of sustainable crop production, the potential of microbial biotechnology to increase plant drought tolerance and improve crop yields under adverse conditions is gaining relevance. This work aimed to compare the performance of Azospirillumargentinense strain Az19 to that of strain Az39, the most widely used for commercial inoculants, when inoculated in maize plants exposed to water deficit. For this purpose, greenhouse and field assays were conducted. In the greenhouse experiment, strain Az19 prevented the adverse effect of water deficit at V2 stage on maize growth. Moreover, the percentage of fertile plants and the ear weight decreased significantly under water deficits imposed at V2 and flowering in Az39-inoculated plants but not in Az19-inoculated plants. In the first field trial with the commercial maize hybrid DOW DS 515 PW, Az19-inoculated plants were those which better tolerated the water deficit imposed. In the second field trial, two maize genotypes with differential drought sensitivity (LP 29×LP 2542, sensitive; LP 882 (923)×LP 4703, tolerant) were tested. Higher tolerance to water deficit was detected in plants inoculated with A. argentinense Az19, with a noticeable effect on grain yield components in the sensitive genotype. Based on these results, we propose the use of A. argentinense Az19 for the formulation of more targeted Azospirillum-based inoculants, suitable for agroecological areas subjected to seasonal water deficits.
El déficit hídrico constituye una severa limitación a la productividad agrícola. En el marco de la producción sostenible de cultivos, la biotecnología microbiana está cobrando relevancia para aumentar la tolerancia a la sequía y mejorar el rendimiento de los cultivos en condiciones adversas. El propósito de este trabajo fue comparar la acción de la cepa de Azospirillum argentinense Az19, con tolerancia in vitro a estresores abióticos, con la cepa Az39, utilizada ampliamente para la formulación de inoculantes comerciales, al inocularlas en plantas sometidas a déficit hídrico. Se realizaron ensayos de invernadero y de campo. En invernadero, la cepa Az19 evitó el impacto adverso del déficit hídrico en el estadio V2 sobre el crecimiento del maíz. Además, el porcentaje de plantas con espigas y el peso de la espiga disminuyó significativamente con la restricción hídrica aplicada en V2 y en floración en plantas inoculadas con la cepa Az39, pero no en las inoculadas con Az19. En el primer ensayo de campo con el maíz híbrido comercial DOW DS 515 PW las plantas inoculadas con Az19 fueron las que mejor toleraron la deficiencia hídrica. En el segundo ensayo de campo se utilizaron dos genotipos de maíz con sensibilidad diferencial a la sequía. La inoculación con Az19 condujo a una mayor tolerancia al déficit hídrico, con un efecto detectable en algunos componentes del rendimiento en el genotipo sensible. Sobre la base de estos resultados, proponemos el empleo de A. argentinense Az19 para la formulación de inoculantes basados en Azospirillum especialmente indicados para áreas agroecológicas que experimenten períodos de déficit hídrico.
In Argentina, maize (Zea mays L.) was traditionally cultivated in the Undulating Pampa region (Pampa Ondulada) (32° to 35.8° S and 58° to 62° W), a temperate-humid region without climatic constraints and adequate for extensive agriculture. However, due to the massive cultivation of soybean in the last decades, maize production has been expanded outside that optimal zone to semiarid environments (<700mm precipitation per year), where high temperatures and water restrictions are relatively frequent events20.
Flowering is a key phenological stage for cereal production, and water deficit occurring during that stage adversely impacts yield due to its negative effect on grain development. Water limitation at flowering leads to impaired reproductive performance, resulting in a decreased number of grains per hectare and decreased final grain weight19.
In addition to plant breeding techniques, microbial biotechnology is increasingly positioned as a valuable strategy to improve crop performance under adverse conditions. In this context, the introduction of plant growth-promoting rhizobacteria (PGPR) is an environmental-friendly alternative to chemical treatments for sustainable production systems. PGPR comprise those microorganisms that colonize the rhizosphere of plants and promote plant growth. Within this group, the genus Azospirillum has been one of the most studied and is usually regarded as a model bacterium to study plant-bacteria interactions27. Azospirillum can interact with a wide range of plant species due to its recognized metabolic versatility27. For example, the strain Az39 of Azospirillum argentinense, used in most commercial formulations in Argentina, was tested in various cereals and was found to increase yield significantly7,9,30.
It has also been documented that plants inoculated with PGPR exhibit greater tolerance to abiotic stresses such as hydric and osmotic stresses18. The beneficial effects of PGPR inoculation in plants subjected to osmotic stress include biomass increase and water status improvement17. Under field conditions, wheat inoculation with A. baldaniorum Sp245 increased cell wall elasticity in the flag leaf and reduced yield losses due to moderate drought at the anthesis stage. In addition, this strain mitigated the negative effect of drought and led to increased grain yield and mineral content (Mg, K, and Ca) compared to non-inoculated plants6.
Previously, we characterized the strain Az19 of A. argentinense from the PGPR collection of IMyZA INTA and observed that this strain had similar PGPR features but better attributes to cope with osmotic stress than A. argentinense Az39, the strain typically included in commercial inoculants11. Az19 is a collection strain isolated in 1982 from wheat field fallow. Since this strain has already demonstrated higher in vitro survival under saline and osmotic stress than Az39 and higher trehalose production11, we hypothesized that this bacterium could also display higher survival in the soil/root interface and thus contribute to sustain plant growth and yield under water deficit, which usually entails osmotic imbalances. The purpose of this study was to assess if seed inoculation with the strain Az19 ameliorates the negative effects of water restriction at specific phenological stages on the yield of a commercial maize hybrid to a greater extent than the Az39 strain (greenhouse experiment and field assay 1) and on different contrasting maize genotypes for their sensitivity to drought (field assay 2).
Materials and methodsPlant materialTwo commercial maize hybrids, DOW 510 PW (greenhouse experiment) and DOW DS 515 PW (field experiment 1), and two maize genotypes obtained at INTA Pergamino, Argentina, LP 29×LP 2542 (drought-sensitive) and LP 882 (923)×LP 4703 (drought-tolerant) (field experiment 2), were used.
Azospirillum argentinense strains used for maize inoculationAz39 and Az19 were obtained from the PGPR collection of IMyZA INTA Castelar. Az39 was used as the reference strain.
Both A. brasilense strains were cultured in OAB liquid medium25 supplemented with 1g/l NH4Cl (for an optimal bacterial growth) at 30°C in a rotary shaker at 180rpm for 48h. Viable cell number (CFU/ml) was quantified using the drop plate method13 on plates containing Congo Red medium28.
Greenhouse experimentSurface disinfected (ethanol 80% and sodium hypochlorite 4%, v/v) seeds of the maize hybrid DOW 510 PW were pregerminated in a wet chamber at 25°C, and uniform-developed seedlings were inoculated 24h later by immersion for 2h in an inoculum containing 5×107 colony-forming units (CFU) of the corresponding A. argentinense strain per seedling. A set of non-inoculated seedlings was included. Emerging seedlings were subsequently transferred to 10l plastic pots containing a mixture of sterile sand, soil, vermiculite, and perlite (3:3:3:1) as substrate.
All treatments (including controls) were irrigated with Hoagland nutrient solution. Water restriction was applied once at V2 or twice at V2 and flowering, and plants were harvested at V3 or physiological maturity, respectively. Water deficit at V2 was achieved by withholding the irrigation for 10 days while water deficit at flowering was achieved by withholding irrigation for 20 days after flowering. Male and female flowering dates were recorded when 50% of the plants were shedding pollen or showed 1-cm long stigmas, respectively.
A randomized complete block design consisting of 3 blocks with 12 plants per treatment was used. Length, fresh weight, and dry weight of the aerial part and the relative water content (RWC) of the last expanded leaf were measured at V3 (plants exposed to water deficit at V2). RWC was determined according to the method described by Barrs and Weatherley2 based on the following formula:
At physiological maturity, the percentage of fertile plants (grain-producing plants), the ear weight, and the grain weight per plant were determined (plants exposed to water deficit at V2 and flowering).
Field assaysBoth field assays were conducted in plots belonging to the agricultural experimental station of the Instituto Nacional de Tecnología Agropecuaria in San Juan province (INTA EEA San Juan), located near Pocito city (31°39′0″S, 68°33′0″W), during the maize campaign 2015/16. We selected this location because of the recognized scarcity of rainfall, which is appropriate to mimic a water-deficient condition requiring controlled irrigation.
The soil of this area is classified as Entisol, and since 2009, these plots have been under maize production, with fallow between cycles. Plots received 200kg N/ha (100kg at sowing and 100kg at V7). The mean temperature at silking (±15 days) was 30.2°C, and from that stage to grain filling was 24.1°C. Accumulated rainfall in the same periods was 13.5 and 7.2mm, respectively.
Field assay 1. The commercial maize hybrid DOW DS 515 PW was used. Before planting, seeds were inoculated with the corresponding A. argentinense strain (12ml/kg seeds; 1×109CFU/ml of inoculant) and then sown at a plant density of 70000plants/ha, with a distance of 0.63m between rows and 0.19m between plants. A split-plot experimental design was used, the main plots corresponding to factor A, water restriction, and the secondary plots to factor B, inoculation, with 5 repetitions (plots) per treatment. Each plot included eight 5-m long rows.
Water restriction (factor A) included a full-irrigated control, irrigated at 100% of the calculated evapotranspiration (ETc) throughout the entire crop cycle, and two alternative water deficit treatments: one imposed at flowering, with the irrigation reduced to 25% of the ETc between −15 to +15 days respect to the flowering date (WR-Fl), and the other one imposed at grain filling, with the irrigation reduced to 25% of the ETc from grain set up to physiological maturity (WR-Gf). Inoculation treatments (factor B) were the same as in the greenhouse experiment: Az39, Az19, none (control).
Male and female flowering dates were recorded when more than 15 plants per row were shedding pollen and had 1-cm long stigmas, respectively. Irrigation was performed via a surface drip irrigation system with hoses of 1.27cm in diameter and emitters every 25cm, with a flow of 2.5l/h. The irrigation rate was established based on the calculated crop evapotranspiration. Yield (kg/ha) and empty ear rates (%) were evaluated at physiological maturity by sampling two random rows. The plant stand was uniform across treatments.
Field assay 2. Only strain Az19 was used as inoculation treatment, and two non-commercial hybrids provided by the Breeding Program of INTA EEA Pergamino, Argentina, were tested in this trial: the drought-sensitive genotype LP 29×LP 2542 and the drought-tolerant genotype LP 882 (923)×LP 4703. Water restriction was imposed as in field assay 1, and yield and empty ear rate were evaluated as described. Plant height was an additional parameter recorded at physiological maturity.
As indicated previously, in the greenhouse experiment, water restriction was achieved by withholding regular watering. In the field trials, water restriction was performed by reducing the amount of water supplied through irrigation. The phenological stage at which water restriction was imposed was the primary variable considered in both experimental settings.
Statistical analysisData was statistically analyzed as indicated in each figure legend using Prism 9 (GraphPad Software Inc., California, USA). Outlier values were detected by the ROUT method and excluded from the analyses. When required, data sets were transformed to meet the statistical test requirements.
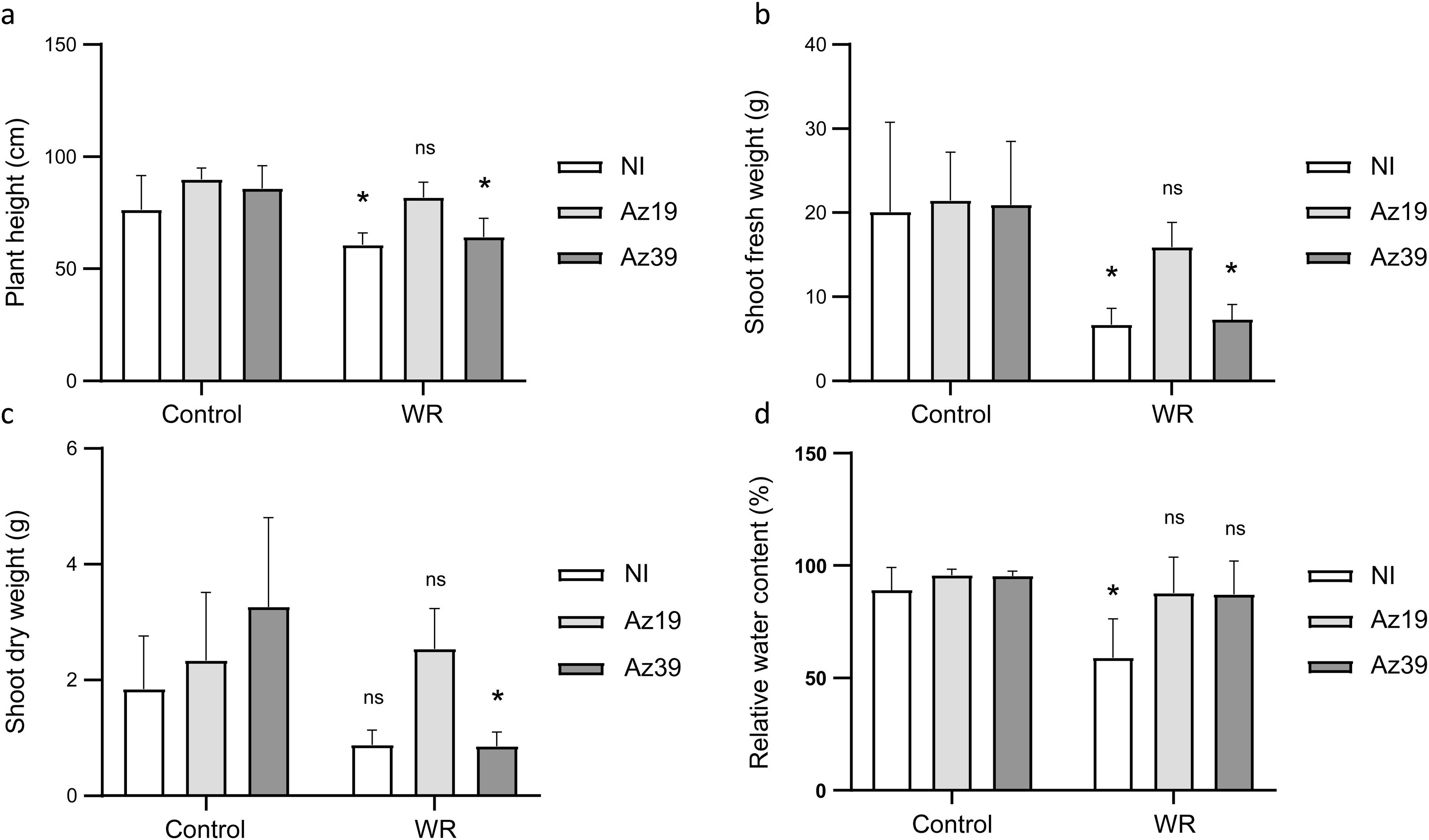
ResultsIn the greenhouse experiment using the commercial maize hybrid DOW 510 PW, water deficit imposed at V2 negatively affected height and shoot fresh weight in uninoculated plants, with reductions ranging from 20% to 67% compared to full-irrigated plants (Figs. 1a–c). Notably, inoculation with strain Az19 prevented the adverse effects of water deficit on these growth parameters, whereas Az39 did not (Figs. 1a–c). Water deficit also decreased the RWC of the last expanded leaf by 34% in the uninoculated control, but inoculated plants maintained the same RWC as irrigated plants, irrespective of the A. argentinense strain supplied (Fig. 1d).
Effect of inoculation and water deficit at V2 on maize growth and physiological parameters (greenhouse experiment). Mean values and standard deviation determined at V3 are shown (n=12). (a) Plant height; (b) shoot fresh weight; (c) shoot dry weight; (d) relative water content of the last expanded leaf. Control: no water deficit; WR: water restriction; NI: non-inoculated. Data was square root-transformed and analyzed by two-way ANOVA plus the Tukey's test. Asterisks indicate significant differences between control and WR groups within each inoculation treatment (p˂0.1).
With regard to the reproductive performance of the maize plants, none of the uninoculated plants produced ears under water restrictions (0% of fertile plants) (Table 1). Inoculation mitigated this effect irrespective of the A. argentinense strain supplied. Compared to uninoculated plants not subjected to water deficits, the grain weight per plant decreased significantly due to water restriction in Az39-inoculated plants but not in Az19-inoculated plants (Table 1).
Effect of inoculation and water deficit imposed at V2 and flowering on maize productivity parameters (greenhouse experiment).
| Treatment | Fertile plants (%) | Ear weight (g) | Grain weight per plant (g) |
|---|---|---|---|
| C:Ni | 39.3±12.34 | 10.65±5.07 | 5.2±2.12 |
| C:Az19 | 33.0±14.75 | 8.06±4.28 | 3.66±2.26 |
| C:Az39 | 39.6±6.6 | 12.73±3.69 | 5.69±2.11 |
| WR:Ni | 0* | – | – |
| WR:Az19 | 33.2±18.75 ns | 5.62±1.66 ns | 2.35±0.02 ns |
| WR:Az39 | 13.2±8.08 ns | 2.94±1.86* | 0.44±0.43* |
Mean values obtained at physiological maturity are shown. C: irrigated on demand; WR: water restriction; Ni: not inoculated. Asterisks indicate significant differences between C and WR groups within each inoculation treatment, according to two-way ANOVA followed by Šídák's multiple range test (p≤0.1).
Despite the fact that inoculation with Az19 had no significant effect on the full-watered treatments, in water-restricted plants, inoculation with this strain mitigated to a greater extent the negative effect of water limitation on yield components, leading to a mean ear weight and a mean grain weight per plant 2-fold and 5-fold increased, respectively, compared to those recorded for plants inoculated with strain Az39 (Table 1).
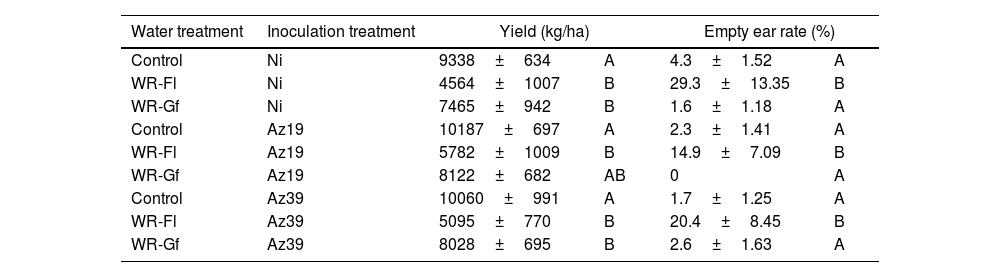
In the first field assay with a commercial hybrid, water restriction imposed at flowering significantly decreased the yield and increased the empty ear rate compared to full-watered controls, irrespective of the inoculation treatments (Table 2). In the Az19 treatment, water restriction at grain filling resulted in no significant yield reductions whereas in Az39, a significant yield decrease occurred (Table 2).
Effect of inoculation and water restriction on maize productivity parameters (field assay 1).
| Water treatment | Inoculation treatment | Yield (kg/ha) | Empty ear rate (%) | ||
|---|---|---|---|---|---|
| Control | Ni | 9338±634 | A | 4.3±1.52 | A |
| WR-Fl | Ni | 4564±1007 | B | 29.3±13.35 | B |
| WR-Gf | Ni | 7465±942 | B | 1.6±1.18 | A |
| Control | Az19 | 10187±697 | A | 2.3±1.41 | A |
| WR-Fl | Az19 | 5782±1009 | B | 14.9±7.09 | B |
| WR-Gf | Az19 | 8122±682 | AB | 0 | A |
| Control | Az39 | 10060±991 | A | 1.7±1.25 | A |
| WR-Fl | Az39 | 5095±770 | B | 20.4±8.45 | B |
| WR-Gf | Az39 | 8028±695 | B | 2.6±1.63 | A |
Mean values obtained at physiological maturity are shown. Control: full-irrigated; WR-Fl: water restriction at flowering; WR-Gf: water restriction at grain filling; NI: non-inoculated. Different letters indicate significant differences between different water treatments within each inoculation group according to two-way ANOVA followed by Tukey's multiple range test (p˂0.1).
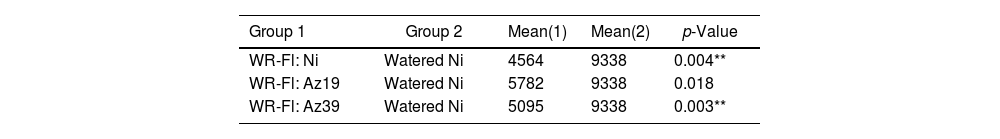
A paired sample t-test was used to analyze the incidence of inoculation treatments on grain yield under water restriction at flowering (Table 3). Only the grain yield of Az19-inoculated plants subjected to water restriction did not differ significantly from that of the watered control plants (p≤0.01).
Paired sample t-test for grain yield (field assay 1).
| Group 1 | Group 2 | Mean(1) | Mean(2) | p-Value |
|---|---|---|---|---|
| WR-Fl: Ni | Watered Ni | 4564 | 9338 | 0.004** |
| WR-Fl: Az19 | Watered Ni | 5782 | 9338 | 0.018 |
| WR-Fl: Az39 | Watered Ni | 5095 | 9338 | 0.003** |
Ni: non-inoculated; WR-Fl: plants subjected to water restriction at flowering. Asterisks indicate significant differences at p˂0.01.
In the second field assay where a drought-sensitive (GS) and a drought-tolerant (GT) genotype were used, the 3-way ANOVA indicated that the interaction between the factors (water treatment, inoculation, and genotype) was not significant. Subsequent analyses were conducted by 2-way ANOVA taking water and the inoculation treatment as independent variables.
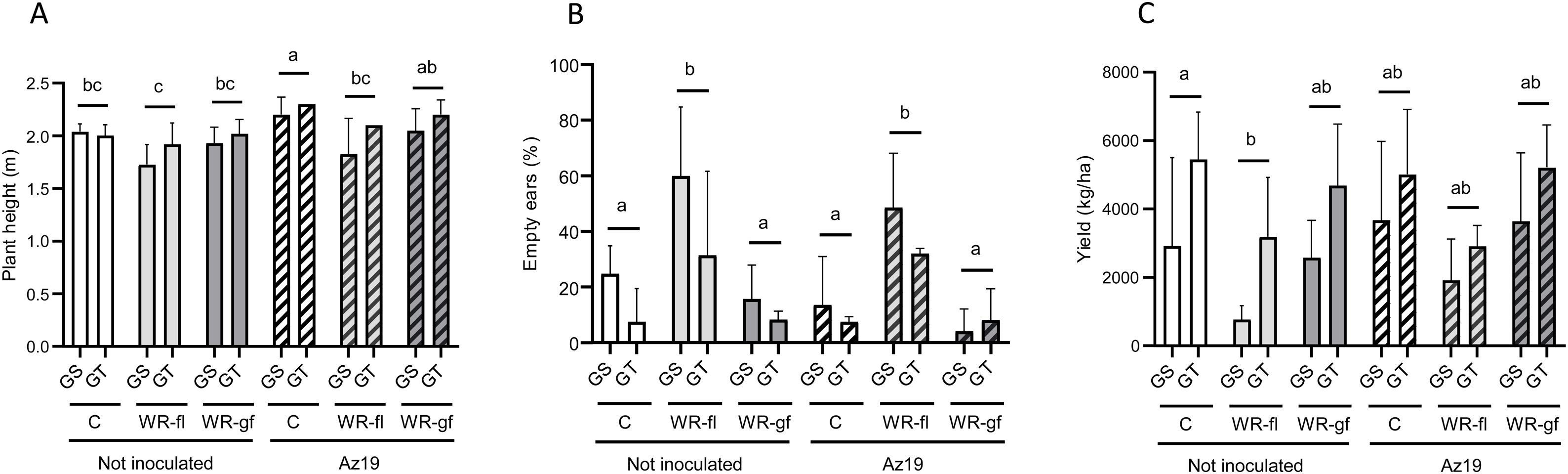
Az19 inoculation positively affected the height of plants grown under optimum irrigation conditions, but not under water deficit (Fig. 2).
Changes in maize height (A), percentage of empty ears (B), and yield (C) according to irrigation and inoculation treatments (field assay 2). Mean values and standard deviation determined at physiological maturity are shown. Sampling was performed as described in “Materials and methods” section. C: full-irrigated control; WR-Fl: water restriction at flowering; WR-Gf: water restriction at grain filling; GS: drought-sensitive genotype; GT: drought-tolerant genotype. Data was square root-transformed (B) or not transformed (A and C) and analyzed by two-way ANOVA plus the Tukey's test. Different letters indicate significant differences between treatments irrespective of the plant genotype (p˂0.1).
Our data showed that water deficit at flowering significantly increased the empty ear rate in both non-inoculated and Az19-inoculated groups when compared to their respective irrigated controls (Fig. 2). However, the significance of this increase was higher for non-inoculated plants (p=0.02 and p=0.08 for non-inoculated and Az19-inoculated plants). The yield was significantly reduced by water deficit at flowering in non-inoculated plants, but not in inoculated plants.
DiscussionIn nature, plants thriving in arid and semiarid regions usually have evolved adaptive features that enable them to cope with water limitations, in most cases, to the detriment of seed production3. However, cereals typically cultivated in humid/subhumid zones, such as maize, sugarcane, or wheat, do not possess such mechanisms3; thus, rhizospheric microorganisms adapted to adverse conditions may partially compensate for such environmental limitations22.
Under controlled conditions, the collection strain A. argentinense Az19 mitigated the negative effects of water deficit to a greater extent than A. argentinense Az39, the most frequent Azospirillum strain included in commercial inoculants in Argentina. This was evidenced by lower reductions in shoot fresh/dry weight, plant height, percentage of fertile plants, and ear weight11. In maize and other cereals, the percentage of fertile plants, the ear number per plant, the kernel number per ear, and the 1000-kernel weight are key yield components that determine the final grain yield. Therefore, the maximum yield potential of these crops is expected to be determined at flowering1.
It is well-known that water deficit at reproductive stages impairs grain yield29, but water restrictions at vegetative stages may also compromise yield due to the impact on photosynthetic biomass production21. In this research, only repeated water restrictions (at V2 and flowering) had adverse consequences on central yield components such as the percentage of fertile plants, in line with data reviewed by Fahad et al.8. Since both Azospirillum strains, Az39 and Az19, are good in vitro indole acetic acid producers, this differential behavior regarding plant response to water deficit seems not to be directly related to the ability of these microorganisms to stimulate root system development, and other mechanisms may be involved. In this regard, in a previous greenhouse experiment in which the same maize hybrid (DOW 510 PW) was subjected to drought, plants inoculated with Az19 showed higher RWC in the last expanded leaf and higher proline content in the roots than plants inoculated with Az3911, indicating that a more efficient osmotic adjustment was probably taking place in plants inoculated with the former strain. Other researchers also observed higher proline content in Azospirillum inoculated-maize plants subjected to drought5,14, along with changes in specific water status parameters including increased water potential and apoplastic water fraction, and decreased cell wall modulus of elasticity6. Interestingly, the high in vitro osmotic tolerance of the strain Az19 was related to high trehalose production in pure cultures subjected to osmotic stress11; this differential osmoadaptive response of strain Az19 over strain Az39 would contribute to improving plant fitness under water limitations.
In our field trial, only water deficit at flowering negatively affected plant yield, and Az19 inoculation mitigated the adverse effects caused by this water restriction. Several authors have indicated that water deficit during flowering impairs the reproductive performance of corn4,12,26. Our results agree with those of Casanovas et al.5, who found that inoculation with A. baldaniorum Sp245 resulted in double benefit in corn plants under water deficit by decreasing the number of aborted grains and maintaining the weight of individual grains. In addition, Naseri et al.24 reported that biofertilization with A. argentinense mitigated water deficit damage in field corn trials.
In the field trial using different genotypes, we observed that the inoculation effect was not dependent on the genotype, although several authors have already highlighted the importance of plant genotype in responses of cereals to inoculation with PGPR15,23. In this sense, García de Salomone and Döbereiner10 attributed differences in maize response to Azospirillum inoculation in field assays carried out in Argentina to the interactions between genotypes and strains. Likewise, in a field trial that comprised 5 wheat genotypes and two Azospirillum strains, an effect of the interaction between both factors was observed for some parameters16.
ConclusionObtaining new strains with improved plant growth-promoting abilities is a permanent challenge. Our results highlight Az19 as a promising PGPR strain, particularly useful to improve maize yield under water deficit at V2 or flowering.
Although Az19 and Az39 strains showed similar positive effects on irrigated plants, strain Az19 was more efficient than Az39 in reducing the adverse effects of water limitation on growth and yield parameters in maize.
Our results show that inoculation with selected PGPR may mitigate the adverse impact of water scarcity during critical phenological stages, such as flowering and grain filling, on maize yield. The development of biological products based on this microorganism can be available for farmers faster than resistant or tolerant cultivars obtained through plant breeding, a point that acquires particular significance in light of growing food demands and climate change.
Conflict of interestNone declared.
María Daniela Groppa and Guillermo Andrés Maroniche are researchers at the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
This work has been funded by Instituto Nacional de Tecnología Agropecuaria (PNCYO-1127033) and the University of Buenos Aires (UBACYT 20020130200052BA).