At present, different reports have shown that children reach similar SARS-CoV-2 viral load (VL) levels compared to adults; however, the impact of VL on children remains ambiguous when asymptomatic versus symptomatic cases are compared. Thus, the aim of this study was to assess VL at the time of diagnosis in asymptomatic and symptomatic SARS-CoV-2 infected children. VL analysis was retrospectively carried out from nasopharyngeal swabs on 82 SARS-CoV-2 infected children, from March to October 2020. Of the 82 children, 31 were asymptomatic. Symptomatic patients had significantly higher VL values compared to asymptomatic ones (median=7.41 vs 4.35log10 copies/ml, respectively). Notwithstanding, 8 out of 31 asymptomatic children had high VL levels, overlapping levels observed above the first quartile in the symptomatic group. Analysis of different age groups revealed that median VL values were higher in the symptomatic groups, although there was only a significant difference in children younger than 5 years of age. On the other hand, there was no significant difference between the VL values from the 82 SARS-CoV-2 infected children according to age, sex, underlying disease, symptoms or severity of COVID-19 related disease. This study emphasizes the importance of VL analysis in SARS-CoV-2 infected children, who could contribute to viral spread in the community. This concern could be extended to healthcare workers, who are in contact with children.
Diferentes informes han demostrado que los niños alcanzan niveles de carga viral (CV) de SARS-CoV-2 similares a los de los adultos, pero el impacto de la CV en los niños continua siendo incierto cuando se compara entre aquellos que son asintomáticos y sintomáticos. El objetivo de este estudio fue evaluar la CV al momento del diagnóstico en niños asintomáticos y sintomáticos infectados por SARS-CoV-2. El análisis de CV se realizó retrospectivamente a partir de muestras de hisopados nasofaríngeos de 82 niños infectados por SARS-CoV-2 entre marzo y octubre de 2020. De ellos, 31 eran asintomáticos. Encontramos que el grupo sintomático tenía valores de CV significativamente más altos en comparación con el grupo asintomático (mediana=7,41 vs. 4,35log10 copias/ml, respectivamente). No obstante, 8 de los 31 niños asintomáticos presentaron valores de CV elevados, equivalentes a los observados por encima del primer cuartil del grupo sintomático. El análisis por grupos de edad reveló que la mediana de CV fue más alta en los niños sintomáticos, aunque esta diferencia fue significativa solamente en los menores de 5 años. A su vez, los valores de CV obtenidos a partir de los 82 niños infectados por SARS-CoV-2 no mostraron diferencias significativas según el grupo etario, el sexo, la enfermedad de base, los síntomas y la gravedad de la COVID-19. Este estudio enfatiza la necesidad del análisis de la CV en niños infectados por SARS-CoV-2, quienes podrían contribuir a la propagación del virus en la comunidad. Esta preocupación podría extenderse a los trabajadores de la salud que están en contacto con los niños.
At the end of December 2019, a novel coronavirus, now called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China and was responsible for the COVID-19 pandemic21,37. Until the end of January 2022, the COVID-19 pandemic has caused more than 400 million infections worldwide36. In the same period, 8441341 cases have been reported in Argentina, 992605 of which were children under 20 years of age25, while at the national referral public Hospital de Pediatría “Prof. Dr. Juan P. Garrahan” 3247 patients were diagnosed with SARS-CoV-2 infections16.
Although people of all age groups are susceptible to SARS-CoV-2 infection, most children are asymptomatic or develop a milder form of the disease compared to adults. Fever, respiratory as well as gastrointestinal symptoms are the most frequent clinical manifestations in children4,12,26,30. Nevertheless, some children may develop severe COVID-19 disease, as reported in adult patients. These cases mainly occur in children with underlying diseases such as immune deficiency, diabetes, obesity, asthma and hematological or oncological malignancy. The number of deaths registered in children by COVID-19 is low and mainly occur in those with underlying diseases3,4,33.
Since the beginning of the pandemic, the detection of SARS-CoV-2 RNA by qualitative real-time reverse transcription polymerase chain reaction (RT-PCR) is considered the gold standard methodology for diagnosis. On the other hand, VL determined by the standardized quantitative RT-PCR (RT-qPCR) assay provides additional data on the virus behavior. However, the types of samples frequently collected (nasopharyngeal, nasal or oropharyngeal swabs) are quite heterogeneous and their quality is largely dependent on operator and sampling techniques. Therefore, it is important to take into account the tests used (qualitative or quantitative assays) for the interpretation of the data.
Different studies have observed low viral load in the initial days of infection, generally increasing in upper respiratory tract samples at the time of symptom onset or in the first week of illness, followed by a gradual decline during the next one to three weeks9,10,35. Although, different reports have shown that children reach similar VL levels compared to adults, there are scarce data on VL in asymptomatic and symptomatic children and how they actually contribute to the spread of SARS-CoV-2 is still unclear2,11,15,22,28,31,35.
Thus, the aim of this study was to assess VL at the time of diagnosis in asymptomatic and symptomatic SARS-CoV-2 infected children.
Materials and methodsDesign and study populationFrom March to the end of October 2020, 516 children were diagnosed with SARS-CoV-2 infection by RT-PCR at the national referral public pediatric hospital “Hospital de Pediatría, Prof. Dr. Juan P. Garrahan” (Buenos Aires, Argentina). The median age was 62 months (IQR: 16–129) and 48% were male. This cross-sectional retrospective study was conducted in 82 SARS-CoV-2-infected children with available samples. Approximately, 20 clinical samples were randomly selected for each age group: (i) ≤1 year; (ii)>1 to ≤5 years; (iii) >5 to ≤10 years and (iv) >10 years. Patients were classified at the moment of sampling as asymptomatic or symptomatic, depending on whether they presented with fever, respiratory and/or gastrointestinal symptoms, according to the WHO criteria38.
The study protocol was approved by the Institutional Review Board (Comité Revisor y de Ética en la Investigación, Hospital de Pediatría “Prof. Dr. Juan P. Garrahan”, Protocol No. 1359).
Viral load analysisViral RNA was extracted from 500μl of nasopharyngeal swabs within 24h since the sample was collected, using the automated MagNA Pure 96 DNA and Viral NA large volume kit (Roche, Germany), according to the manufacturer's instructions. Extracted RNA was stored at −80°C until used.
To measure VL, an in-house reverse transcriptase quantitative PCR (RT-qPCR) targeting a region of the N-gene of SARS-CoV-2 was performed on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The standard curve consisted of an in vitro transcribed viral RNA serially diluted in a cellular RNA matrix from negative SARS-CoV-2 nasopharyngeal samples. Briefly, a region of the N-gene of SARS-CoV-2, was amplified by RT-PCR and inserted in a cloning vector using TOPO TA Cloning® kit (Invitrogen). Then, plasmids were linearized with BamHI restriction enzyme, and transcribed in vitro using the HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs). Finally, RNA transcripts were purified using a commercial reagent (Trizol, Invitrogen) and treated with DNase I (Ambion™ (Thermo Fisher)). The concentration was determined by using NanoDrop (NanoDrop 2000, Thermo Scientific™). It is important to mention that the standard RNA developed was checked for DNA contamination by a PCR assay without the RT step in two separate runs and by quintuplicate, and there was no signal of amplification in any well.
The RT-qPCR assay included the measure of a housekeeping gene (RNase P) as an internal control and normalizer. The housekeeping gene cycle threshold (Ct) was used to correct the specific-SARS-CoV-2 Ct according to the number of cells in the sample [SARS-CoV-2 Ct value×sample RNase P Ct value/mean RNase P Ct value (historic RNase P mean value)] as was reported by Bouscambert Duchamp et al.5. Then, this corrected Ct value was extrapolated in a standard curve to obtain the copies of virus (log10) per reaction. Finally, this result was multiplied by the dilution factor of the process to obtain the copies of virus per ml of sample. In this way, the heterogeneity of the VL measurement was reduced across different amplification runs. An RNase P Ct value ranging from 19.84 to 31.90 was considered acceptable for each sample analyzed.
The VL assay had an efficiency of 99%; the reproducibility and repeatability expressed as a coefficient of variation (CV) was 1.01–2.31% and 0.27–1.89%, respectively. The dynamic range was 10 to 1×108 copies per reaction (equivalent to a range from 400 to 4×109copies per ml). The specificity was 100%, tested in vitro against SARS-CoV-2 negative samples and a panel of respiratory viruses (Pan-coronavirus, Adenovirus, Influenza A and B, Metapneumovirus and Rhinovirus). All these parameters were determined according to the guidelines of in vitro quantitative diagnostic assays as previously reported by Burd et al. and Bustin et al.7,8.
Statistical analysisViral load data was log-transformed and summarized using medians and interquartile range (IQR), whereas categorical variables were presented by counts and percentages. Data normality was rejected using the Shapiro–Wilk test and as consequence non parametric Wilcoxon or Kruskal–Wallis tests were used to compare differences in continuous variables among two or more groups, respectively. Fisher's exact test was used to compare characteristics and demographic data. The correlation between age and log-transformed viral load was assessed using the Pearson correlation coefficient. All statistical analyses were performed with the software R (version 3.6.0).
ResultsMain characteristics of the study populationSARS-CoV-2 VL was retrospectively studied in 82 infected children (median age: 5 years; range: 8 days to 18 years). Of them, 31 (38%) were asymptomatic and 43 (52%) were male. Demographic data and clinical characteristics of the study group are shown in Table 1.
Demographic and clinical characteristics of SARS-CoV-2-infected children.
| Characteristic | Asymptomatic children | Symptomatic children | Total |
|---|---|---|---|
| Number (%) | 31 (38) | 51 (62) | 82 (100) |
| Gender | |||
| Male, n (%) | 14 (45) | 29 (57) | 43 (52) |
| Female, n (%) | 17 (51) | 22 (43) | 39 (48) |
| Age, median (range) | 4y (74d–15y) | 7y (8d–18y) | 5y (8d–18y) |
| Age range in years | |||
| ≤1; n (%) | 9 (29) | 13 (25) | 22 (27) |
| >1 to ≤5; n (%) | 9 (29) | 11 (22) | 20 (24) |
| >5 to ≤10; n (%) | 4 (13) | 14 (28) | 18 (22) |
| >10; n (%) | 9 (29) | 13 (25) | 22 (27) |
| Hospitalized, No. | 27 | 46 | 73 |
| Admission due to the severity of COVID-19 | 0 | 7 | 7 |
| Admission due to institutional guidelines on the management of patients with COVID-19 | 20 | 31 | 51 |
| Admission due to another reason | 7 | 8 | 15 |
Among the 82 patients, 73 (89%) were hospitalized for a median of 8 (IQR: 3–19) days. Seven children were admitted to intensive care units due to the severity of COVID-19, 51 due to institutional guidelines on the management of patients with COVID-19 at that time, and 15 due to another reason (Table 1). In the population studied, there were no reported deaths from COVID-19 within 60 days of diagnosis. A total of 35 children had underlying diseases: 28 infants had only one underlying disease and seven of them had two (Supplementary Table 1). In addition, among the patients without underlying disease, one of them was a pregnant girl (14 years of age).
Among the 51 symptomatic children, 41 underwent molecular diagnosis within 3 days from symptom onset, and 10 within a week. Fever was the most frequently observed symptom (76%), followed by upper respiratory tract infection (39%) and odynophagia (20%). All other symptoms, such as diarrhea 16%, vomiting 16%, abdominal pain 12%, shortness of breath 12%, cough 8% and anosmia/dysgeusia 6%, were less frequent. Among symptomatic children, 16 only had one symptom, 21 had two symptoms, 10 had three symptoms and four had four symptoms. Regarding the severity of the disease, 41 (80%) children had mild, 3 (6%) severe and 7 (14%) critical COVID-19 disease.
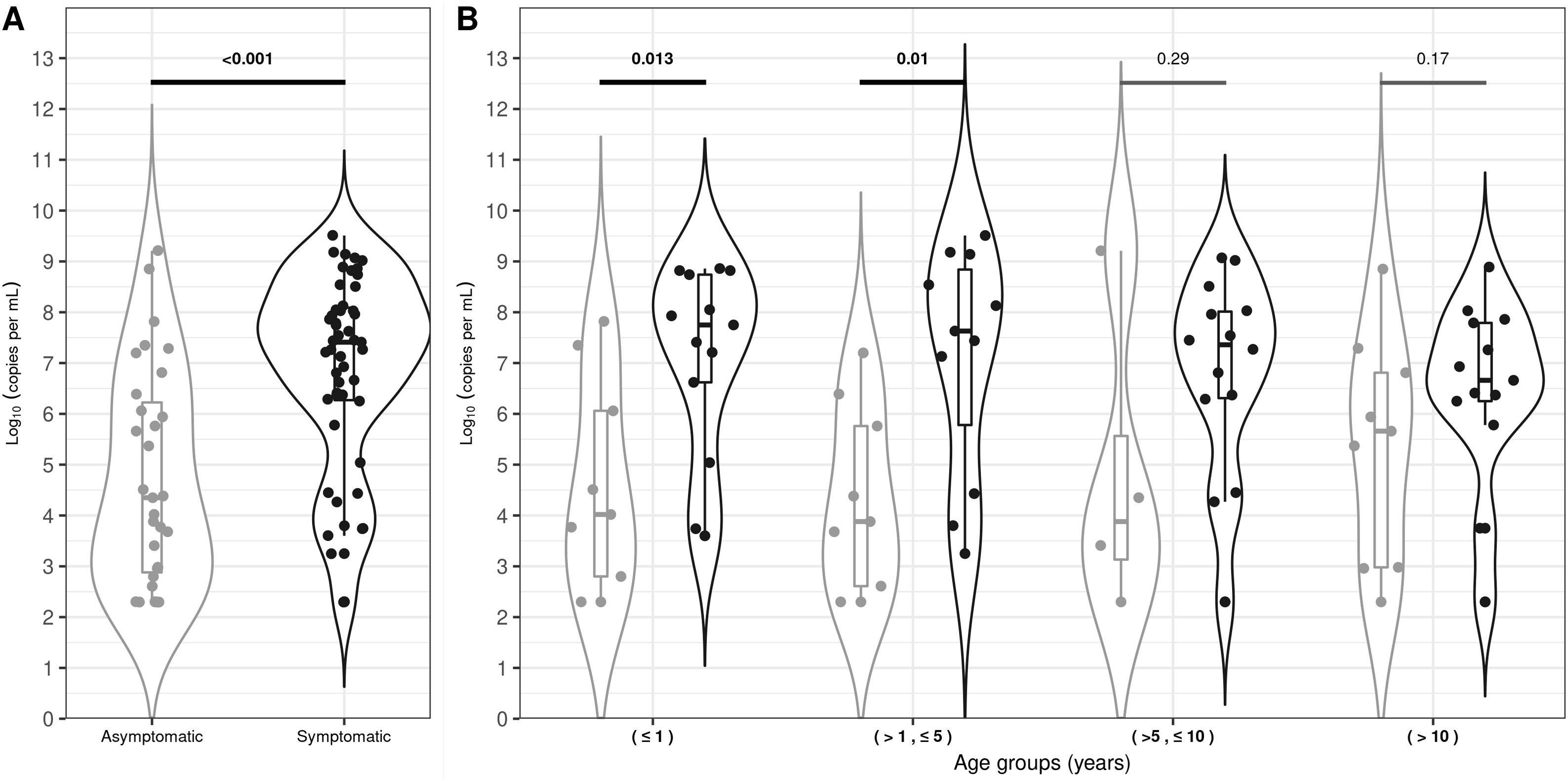
SARS-CoV-2 viral load analysis between symptomatic and asymptomatic childrenViral load quantification was performed within 2 months of sample collection in 93% of the patients. The median VL value for symptomatic children was 7.41 log10 RNA copies per ml (IQR: 6.27–8.09) and 4.35 log10 RNA copies per ml (IQR: 2.88–6.22) for asymptomatic children. This difference was statistically significant (p-value<0.001, Fig. 1A). Nevertheless, 8 out of 31 (26%) asymptomatic children had high VL levels, overlapping those levels observed above the first quartile in the symptomatic group (Fig. 1A). The median VL value was higher for symptomatic than asymptomatic children for all the age groups analyzed, but these differences were only significant in children belonging to ≤1 and >1 to ≤5 age groups (Fig. 1B). On the other hand, the VL analysis of the 82 infected children showed no significant difference between age groups (≤1; >1 to ≤5; > 5 to ≤10 and >10 years), sex, underlying disease, symptoms and severity due to COVID-19.
Violin-plot viral loads for asymptomatic and symptomatic SARS-CoV-2 infected children (A) and viral loads per age range (B). The width of the violin plot outlines represents the proportion of data points. The boxes indicate the IQR. The horizontal line bisecting the box indicates the median value. p-Value for the comparison of the respective medians is shown. Bold values denote statistical significance.
The median Ct values analysis for the internal control gene (RNasa P) of the 82 infected children showed no significant difference between age groups (≤1; >1 to ≤5; >5 to ≤10 and >10 years) nor between symptomatic vs asymptomatic groups.
DiscussionAt present, the impact of SARS-CoV-2 viral load in children continues under debate, especially when asymptomatic versus symptomatic are compared1,2,15,22. Here, we found that symptomatic children had significantly higher VL values, mainly symptomatic infants younger than 5 years old compared to asymptomatic ones, in accordance with other published studies15,31. It is important to note that the time of infection could differ considerably to the time of diagnosis, introducing bias in the interpretation of the initial levels of VL in asymptomatic group, as previously discussed by Kociolek et al.19. In fact, we observed that the majority of the symptomatic children were tested within 3 days of symptom onset, and they did not differ in VL at diagnosis with those symptomatic patients tested after 3 days. In addition, we observed a group of asymptomatic children that presented high VL, similar to that observed in symptomatic children, which raises the need for future studies to screen children with epidemiological risk factors independently of their symptoms.
In our study, 80% of the symptomatic children had mild COVID-19 disease. Compared to other respiratory viruses (for example influenza or respiratory syncytial virus), the reason why children have less severe symptoms when infected by SARS-CoV-2 is not yet well understood. A possible hypothesis is that children mount a potent innate response to SARS-CoV-2 that resolves faster than in adults, without compromising adaptive immunity as was recently reported by Vono et al., or that they are less exposed to other coronaviruses, thus lacking a cross-reactive immune response against SARS-CoV-2 infection, which may lead to milder disease4,30,34.
Considering that most children have mild illness and some reports suggest that they are less likely to transmit the infection29, it would be expected that children present a lower VL. However, we found that in the symptomatic group and some of the asymptomatic children had high level of VL. On the other hand, we observed that the age of children at the time of diagnosis was not associated with the VL, similar to what was previously reported by Aykac et al. and Madera et al.1,22, but different from that reported by Heald-Sargent et al.15, where it was observed that children younger than 5 years old had higher VL compared to adults. Nevertheless, a recent study carried out in Argentina showed that infants younger than 6 months of age had the highest viral load values (estimated by Ct values) compared to any other age group, including adults27. A similar trend was observed in our cohort; although the finding was not statistically significant, it may have been due to the low number of patients analyzed in each group. However, symptomatic patients younger than 5 years old were significantly associated with VL levels. Understanding how this population with high VL can contribute to viral spread is still unclear. Therefore, future studies are necessary to evaluate which factors have a major impact on community transmission, considering the relative low frequency of infected children in the general population.
An important issue to address is that the samples for our study were obtained between March and the end of October 2020, which corresponds to the first wave of the Argentine pandemic where no vaccine was available, and considering that the 2020 original SARS-CoV-2 strain was the only one circulating at that time. As in the rest of the world, in Argentina the context has changed due to the emergence of new variants and the implementation of an extensive vaccination campaign including children older than 3 years of age and because the use of masks is no longer mandatory at school. Despite this new scenario, our study provides valuable data on the natural history of SARS-CoV-2 in children, in particular in asymptomatic ones, in whom we had the unique opportunity to hospitalize asymptomatic patients following the national guidelines on the management of patients with COVID-19 in force at that time. Another important point is that different reports have shown that VL values could vary according to the infecting viral variant24,32. However, Matic et al. have found no difference in VL values between Omicron and other variants of concern23. On the other hand, Jung et al. have shown that the initial viral load was similar between vaccinated and unvaccinated individuals, but fully vaccinated individuals had a shorter duration of viable viral shedding and a lower secondary attack rate than partially vaccinated or unvaccinated individuals17. Currently, with an epidemic phase with different SARS-CoV-2 variants and vaccination policies, the viral load analysis becomes even more complex because these factors did not exist at the beginning of the pandemic.
It is important to highlight that in our study the results obtained from the VL analysis were conducted using a single methodology based on an in-house quantitative RT-PCR. In this way, we used a standard curve to quantify the amount of virus expressed in RNA copies per ml and we did not use naive Ct values, which avoid a misunderstanding of VL kinetics for comparison across different amplification runs, as described by Han et al.14. The limitations of our study include the low number of children grouped by age and the fact that we were not able to carry out viral isolation to measure viral infectivity. The culture of SARS-CoV-2 requires the use of BSL3 facilities, which are not available in our hospital. Although the VL methodology does not allow to differentiate between the detection of infective and non-infective viruses, different reports have tried to determine a VL threshold to infer the presence of infective viruses in clinical specimens6,10,13,18,20. L’Huillier et al. observed that higher VL in children were associated with isolated replicative SARS-CoV-220 and Gniazdowski et al. in a study mainly conducted in adults, observed that prolonged viral RNA shedding was associated with positive virus growth in culture in specimens collected up to 21 days after the first positive result, but mostly in individuals who were symptomatic at the time of sample collection13. Moreover, children were classified as asymptomatic or symptomatic at the time of the SARS-CoV-2 diagnosis. Unfortunately, we have no data on symptom development after the initial sampling to perform further analyses in every patient.
In conclusion, we found that the group of symptomatic children (mainly younger than 5 years of age) had higher VL compared to the asymptomatic group. Therefore, they could be potential drivers of SARS-CoV-2 spread in the general population. However, we also observed that a small group of asymptomatic children can reach high viral loads that could contribute to the spread of SARS-CoV-2 in the community. This concern could be extended to healthcare workers, who are in contact with children, which could also be relevant for the control of infections in hospitals. Further research is needed to understand the role of pediatric infection in the global spread of SARS-CoV-2. This group is usually underrepresented in the majority of the studies and develops a milder disease compared with adults.
FundingThis work was supported by Fondo Sectorial Argentino, Ministerio de Ciencia, Tecnología e Innovación (Argentina) (Grant Name: Convocatoria Ideas Proyectos IP-COVID-19; Grant Number: IP No. 402 and No. 0012).
Authors’ contributionsAM and MDG: conceived and designed the study. MM, APA, MFF and MDG: collected data. All authors contributed to the data analysis. MDG, MM and AM: drafted the manuscript and all authors contributed to review, edit and approve the final manuscript.
Conflict of interestsThe authors declare that there is no conflict of interest regarding the publication of this article.
We thank the staff of Unidad de Virología y Epidemiología Molecular - CONICET, Hospital de Pediatría “Prof. Dr. Juan P. Garrahan” for their hard work and collaboration.