The response to SARS-CoV-2 vaccination decreases in inflammatory bowel disease (IBD) patients, specially under anti-TNF treatment. However, data on medium-term effectiveness are limited, specially using new recommended seroconversion rate (>260BAU/mL). Our aim was to evaluate the 6-month>260 BAU-seroconversion rate after full vaccination and after booster-dose.
MethodsVACOVEII is a Spanish multicenter, prospective study promoted by GETECCU. IBD patients full vaccinated against SARS-CoV-2 and without previous COVID-19 infection, treated or not with immunosuppressants, were included. The booster dose was administered 6 months after the full vaccination. Seroconversion was set at 260BAU/mL, according to most recent recommendations and was assessed 6 months after the full vaccination and 6 months after booster-dose.
ResultsBetween October 2021 and March 2022, 313 patients were included (124 no treatment or mesalazine; 55 immunomodulators; 87 anti-TNF; 19 anti-integrin; and 28 ustekinumab). Most patients received mRNA-vaccines (86%). Six months after full vaccination, overall seroconversion rate was 44.1%, being significantly lower among patients on anti-TNF (19.5%, p<0.001) and ustekinumab (35.7%, p=0.031). The seroconversion rate after booster was 92%. Again, anti-TNF patients had a significantly lower seroconversion rate (67%, p<0.001). mRNA-vaccine improved seroconversion rate (OR 11.720 [95% CI 2.26–60.512]).
ConclusionThe full vaccination regimen achieves suboptimal response in IBD patients, specially among those anti-TNF or ustekinumab. The booster dose improves seroconversion rate in all patients, although it remains limited in those treated with anti-TNF. These results reinforce the need to prioritize future booster doses in patients on immunosuppressants therapy, specially under anti-TNF, and using mRNA-vaccines.
La respuesta a la vacunación contra el SARS-CoV-2 disminuye en pacientes con enfermedad inflamatoria intestinal (EII), especialmente bajo tratamiento con anti-TNF. Sin embargo, los datos a medio plazo son limitados, especialmente siguiendo las nuevas recomendaciones de tasa de seroconversión (>260 BAU/mL). Nuestro objetivo es evaluar la tasa de seroconversión a seis meses tras la pauta completa de vacunación y tras la dosis de refuerzo.
Material y métodosVACOVEII es un estudio español multicéntrico, prospectivo, promovido por GETECCU. Se incluyeron pacientes con EII vacunados con la dosis completa contra el SARS-CoV-2 sin infección previa, tratados o no con inmunosupresores. La dosis de refuerzo se administró seis meses después de la pauta completa. El dintel de seroconversión se estableció en 260 BAU/mL y se evaluó a los seis meses de la dosis de refuerzo.
ResultadosEntre octubre de 2021 y marzo de 2022 se incluyeron 313 pacientes (124 sin tratamiento o con mesalazina exclusivamente; 55 con inmunomoduladores; 87 con anti-TNF; 19 antiintegrina y 28 ustekinumab). La mayoría de los pacientes recibieron vacunas mRNA (86%). Seis meses tras la pauta completa, la tasa de seroconversión global fue de 44,1%, siendo significativamente inferior entre los pacientes con anti-TNF (19,5%, p < 0,001) y ustekinumab (35,7%, p = 0,031). La tasa de seroconversión tras el refuerzo fue de 92%. De nuevo, los pacientes con anti-TNF presentaban una tasa significativamente inferior (67%, p < 0,001). El uso de vacunas mRNA aumentó la probabilidad de seroconversión (OR 11,720 [IC 95%; 2,26-60,512]).
ConclusionesEl régimen de vacunación completo logra resultados subóptimos en pacientes con EII, especialmente entre aquellos bajo tratamiento con anti-TNF o ustekinumab. La dosis de refuerzo mejora las tasas de seroconversión en todos los grupos de pacientes, aunque de manera más limitada en el grupo de anti-TNF. Estos resultados refuerzan la necesidad de priorizar futuras dosis de refuerzo en pacientes bajo tratamiento inmunosupresor, especialmente bajo tratamiento anti-TNF así como la priorización de vacunas mRNA.
The COVID-19 pandemic, due to SARS-CoV-2, has resulted in more than 7 million deaths worldwide since its onset in December 2019. The development of vaccines against COVID-19 has been the best weapon to control the disease. It has shown to be extremely effective in reducing hospitalization and death rates worldwide.1
Inflammatory bowel disease (IBD) is a chronic, immune-mediated condition that causes lesions in the digestive tract, encompassing Crohn's disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC). IBD is often managed with immunosuppressants (thiopurines, methotrexate, steroids, JAK-inhibitors and biologic agents, such as anti-TNF, vedolizumab, and ustekinumab), therefore vaccination is essential to prevent infectious complications. IBD itself and its treatment may have a negative impact on the effectiveness of vaccines, and this is the reason why specific recommendations are often developed for this population.2–5 However, patients with IBD have been excluded from most clinical trials of SARS-CoV-2 vaccines, as were patients with other immune-mediated diseases, so data on effectiveness in this population are scarce.
Regarding vaccination against SARS-CoV-2–19 in patients with IBD, all the vaccines that have been licensed by the European Medicines Agency so far, can be used, regardless of their immunosuppression status and treatments.6 Early studies already suggested that the response to these vaccines may be appropriate in the short term in patients with IBD,3 although with particularities yet to be defined. Thus, their effectiveness, as measured by antibody titer response, appears to be lower in patients on anti-TNF therapy than in those on vedolizumab7–9 or other immunosuppressants.6 Studies published to date indicate that, at least in the very short term, antibody titers increase after the administration of a booster dose.10–14 Unfortunately, although the duration of response to vaccination seems to be a key aspect in order to define the vaccination strategy in immunosuppressed populations (particularly regarding the need for additional booster doses and its timing), medium-term data are scarce,15 with only one study assessing response at 6 months.16
Furthermore, it is currently accepted that an antibody level of 260BAU/mL (according to WHO standard units17) is the minimum threshold for achieving an effective immunization, based on studies evaluating protection after vaccination in the COV0021417 trial, COVE trial18 and other cohorts.19–23 In these studies, levels below 260BAU/mL showed to provide a very low protection, and official organizations responsible for pandemic management, such as the Spanish24 and French25 Health Ministries, established this threshold as the cut-off point for indicating the administration of SARS-CoV-2 monoclonal antibody treatments. The use of this cut-off point may profoundly change the interpretation of the results obtained to date. In fact, no study in patients with IBD used this threshold as a primary endpoint.
In VACOVEII study, our main aim was to evaluate the humoral response 6 months after full SARS-CoV-2 vaccination and after the first booster dose, using the cut-off point of 260BAU/mL to define response, as well as to assess the impact of different immunosuppressive therapies on it.
MethodsStudy designVACOVEII was a multicentre, prospective, non-interventional study aimed to evaluate the humoral effectiveness of vaccination against SARS-CoV-2 in patients with IBD and the potential impact of immunosuppressive therapy on it. This study has been carried out under the auspices of GETECCU (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa), and was approved by its research committee. Thirteen Spanish-GETECCU hospitals participated in the study.
Patients diagnosed with IBD by the usual criteria, over 18 years of age, with and without immunosuppressive treatment, full vaccinated against SARS-CoV-2 with any of the vaccines marketed in Spain, were included. A full regimen was considered according to the data sheet of the different vaccines (two doses of the ChAdOx1 nCoV-19, BNT162b2 or mRNA1273 vaccine; and one dose of the JNJ-78436735 vaccine). Patients with known previous infection with SARS-CoV 2, demonstrated by a positive PCR or serology prior to vaccination, whether symptomatic or not, were excluded. However, they were not excluded from the analyses, for practical reasons, if the infection was subsequently acquired. Patients were also excluded if they had other causes of immunodeficiency, whether primary or secondary (active neoplastic disease, human immunodeficiency virus infection, solid organ transplantation, hematological disease, liver cirrhosis, chronic kidney failure, etc.), were undergoing treatment with chemotherapy and/or immunotherapy or those whose immunosuppressive treatment had been indicated for a reason other than IBD, and/or who were on any concomitant immunosuppressive/immunomodulatory drug not used in IBD.
All patients who met the selection criteria coming from the 13 participating centers were consecutively offered to participate in the study. Those who agreed to participate and signed an informed consent were included and underwent a personal interview to collect all study variables.
The effectiveness of the vaccination was evaluated in two phases: (a) first phase effectiveness, 180 days (±28 days) after the full vaccination; and (b) second phase effectiveness, 180 days (±28 days) after the first booster dose. Those patients who did not accept the booster dose but did have a serology at the second phase date (360±28 days) after the full vaccination were included in the analysis. However, patients whose antibody determination was performed outside the protocol time frame were excluded from the analysis.
VariablesDemographic variables including sex, age, and smoking status were collected. Body mass index (BMI) was calculated using weight and height measurements obtained at the consultation. Comorbidity was assessed using the age-adjusted Charlson's index.26 IBD-related variables included the type of disease (CD, UC or IC), its extent, location, and behavior, as well as disease duration. Disease activity was assessed using the Harvey–Bradshaw index27 for CD and the partial Mayo score28 for UC.
Regarding IBD treatment, five groups were established: no treatment or mesalazine; immunomodulatory (thiopurines or methotrexate); anti-TNF agents; anti-integrins and ustekinumab. Treatment doses, regimen, and date of last pre-vaccination administration were collected. All these data were obtained by interview with the patient and/or according to the electronic records in each participating center.
Regarding vaccination, the type of vaccine (mRNA or no-mRNA), dose, and date(s) of administration were collected directly from the electronic medical record in each center.
Assessment of humoral immune responseThe humoral effectiveness of vaccination (at the two-time points analyzed) was assessed by both determining the anti-S antibody titer and the seroconversion rate. Seroconversion is defined as the presence of anti-S levels above 260BAU/mL according to WHO standard units.17
To evaluate the contribution of the booster dose, the individual effect was assessed through the gain in anti-S antibody titers (the difference between the titer 180 days after the booster dose and that previously obtained 180 days after the full dose) or in seroconversion (going from levels below 260BAU/mL before the booster dose to higher levels after the booster dose) in each subject.
The analyses of the antibody response induced by the SARS-CoV-2 vaccine were performed centrally at the Clinical Microbiology Service of University Hospital Miguel Servet (Zaragoza, Spain). The LIAISON® SARS-CoV-2 Trimerics IgG (DiaSorin, Saluggia, Italy), an indirect chemiluminescence immunoassay (CLIA) was used for the detection of IgG antibodies to SARS-CoV-2 in human serum and plasma samples. The principal components of the test are magnetic particles (solid phase) coated with recombinant trimeric SARS-CoV-2 spike protein and a conjugate reagent containing an anti-human IgG mouse monoclonal antibody linked to an isoluminol derivative. The light signal, hence, the amount of isoluminol-antibody conjugate, was measured by a photomultiplier as relative light units (RLU) and is indicative of antibodies to SARS-CoV-2 present in calibrators, samples, or controls. The conversion of RLU units to BAU units and the threshold value (33.8 BAU units) were stated according to manufacturer instructions.
Moreover, at both time points all patients were searched for detection of unnoticed previously passed SARS-CoV-2 infection with the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Mannheim, Germany). This electrochemiluminescence immunoassay (ECLIA) uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS-CoV-2. These antibodies are present only in natural infections. Results are determined automatically by the software by comparing the electrochemiluminescence signal obtained from the reaction product of the sample with the signal of the cut-off value previously obtained by calibration, with a threshold value>1 (cut-off index).
Data collectionStudy data were collected and managed using REDCap electronic data capture tools hosted at AEG (Asociación Española de Gastroenterología). REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.29
Ethical considerationsAll patients included in the study were of legal age on the date of inclusion and gave informed consent for their participation in the study. This study was evaluated and approved by GETECCU's research committee and by the Aragón Health Care Ethics Committee (CEICA) with study code EPA21/028.
Statistical analysisContinuous variables were expressed as mean and standard deviation or median and interquartile range and qualitative variables as total number and percentage. The comparison of groups was carried out using Chi-square for qualitative variables and Student's T test or Mann–Whitney's U test for qualitative variables, depending on the normality or non-normality of the sample. When more than two groups were involved, ANOVA of independent groups was used. Normality was assessed using the Kolmogorov–Smirnov test. Multivariate analysis was performed using logistic regression models. For the construction of the model, seroconversion was the independent variable. Gender, age, vaccine type, treatment group, SARS-CoV-2 infection and smoking status were included as independent variables. Age was treated as a linear variable and its coefficient was expressed per incremental year. All analyses were carried out considering all other potentially confounding variables and limiting the analysis to specific cohorts if necessary.
All tests were two-tailed with significance set at a p-value of less than 0.05. Analyses were carried out with Jamovi software, version 2.3.16 (https://www.jamovi.org, accessed 21.2.23).
Sample sizeThe sample size was calculated considering the limited data available on the effectiveness of SARS-CoV-2 vaccine in patients with IBD after administration of the full regimen.30 No data on the effectiveness of the booster dose were available when the present study was designed. A two-sided alpha significance level of 99% was established, with a power of 80%. The ratio of exposed/not exposed to immunosuppressive treatment was set at 0.65. The difference in seroconversion rates was set at 15 percentage points. Using these parameters, a sample size of 283 patients (171 exposed and 112 unexposed) was obtained.
ResultsBaseline characteristics of the cohort325 patients were initially included, of which 12 were excluded (on steroid monotherapy or tofacitinib) due to the low sample size of these treatment subgroups, with 313 patients finally being analyzed. The complete flow chart of the study is summarized in Fig. 1.
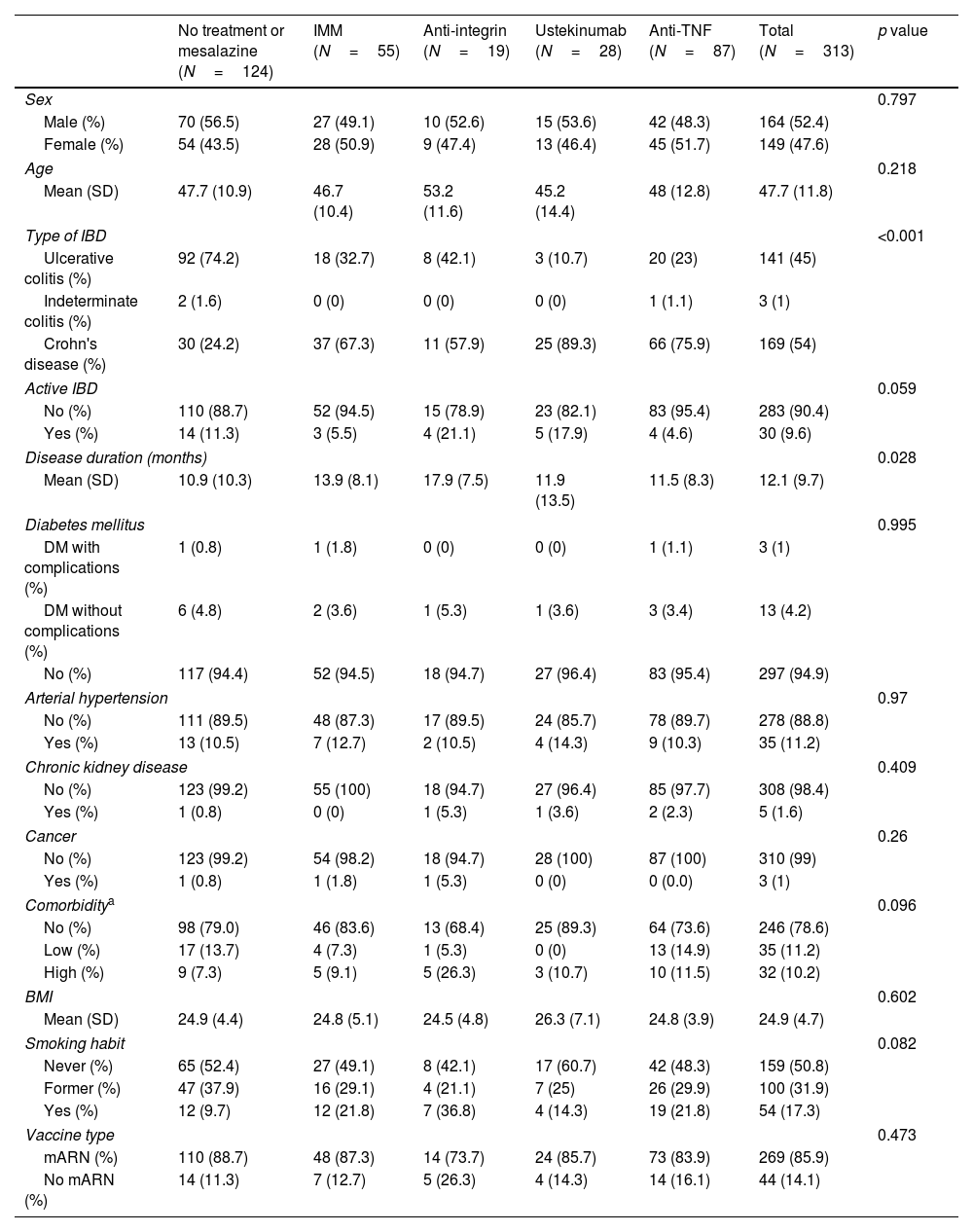
In these 313 patients, the mean age was 47.7 (±11.8) years and 47.6% were women; 169 patients (54%) had CD, 141 UC and 3 IC. Regarding baseline treatments, 124 (39.6%) patients received no treatment or mesalazine, 55 (17.6%) immunomodulators, 87 (39.1%) anti-TNF in mono or combo-therapy, 19 (6.1%) anti-integrin and 28 (8.9%) ustekinumab. Most of the patients received mRNA vaccines (269 patients, 85.9%). Baseline characteristics of the cohort, according to treatment, can be found in Table 1.
Baseline characteristics according to treatment.
| No treatment or mesalazine (N=124) | IMM (N=55) | Anti-integrin (N=19) | Ustekinumab (N=28) | Anti-TNF (N=87) | Total (N=313) | p value | |
|---|---|---|---|---|---|---|---|
| Sex | 0.797 | ||||||
| Male (%) | 70 (56.5) | 27 (49.1) | 10 (52.6) | 15 (53.6) | 42 (48.3) | 164 (52.4) | |
| Female (%) | 54 (43.5) | 28 (50.9) | 9 (47.4) | 13 (46.4) | 45 (51.7) | 149 (47.6) | |
| Age | 0.218 | ||||||
| Mean (SD) | 47.7 (10.9) | 46.7 (10.4) | 53.2 (11.6) | 45.2 (14.4) | 48 (12.8) | 47.7 (11.8) | |
| Type of IBD | <0.001 | ||||||
| Ulcerative colitis (%) | 92 (74.2) | 18 (32.7) | 8 (42.1) | 3 (10.7) | 20 (23) | 141 (45) | |
| Indeterminate colitis (%) | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) | 1 (1.1) | 3 (1) | |
| Crohn's disease (%) | 30 (24.2) | 37 (67.3) | 11 (57.9) | 25 (89.3) | 66 (75.9) | 169 (54) | |
| Active IBD | 0.059 | ||||||
| No (%) | 110 (88.7) | 52 (94.5) | 15 (78.9) | 23 (82.1) | 83 (95.4) | 283 (90.4) | |
| Yes (%) | 14 (11.3) | 3 (5.5) | 4 (21.1) | 5 (17.9) | 4 (4.6) | 30 (9.6) | |
| Disease duration (months) | 0.028 | ||||||
| Mean (SD) | 10.9 (10.3) | 13.9 (8.1) | 17.9 (7.5) | 11.9 (13.5) | 11.5 (8.3) | 12.1 (9.7) | |
| Diabetes mellitus | 0.995 | ||||||
| DM with complications (%) | 1 (0.8) | 1 (1.8) | 0 (0) | 0 (0) | 1 (1.1) | 3 (1) | |
| DM without complications (%) | 6 (4.8) | 2 (3.6) | 1 (5.3) | 1 (3.6) | 3 (3.4) | 13 (4.2) | |
| No (%) | 117 (94.4) | 52 (94.5) | 18 (94.7) | 27 (96.4) | 83 (95.4) | 297 (94.9) | |
| Arterial hypertension | 0.97 | ||||||
| No (%) | 111 (89.5) | 48 (87.3) | 17 (89.5) | 24 (85.7) | 78 (89.7) | 278 (88.8) | |
| Yes (%) | 13 (10.5) | 7 (12.7) | 2 (10.5) | 4 (14.3) | 9 (10.3) | 35 (11.2) | |
| Chronic kidney disease | 0.409 | ||||||
| No (%) | 123 (99.2) | 55 (100) | 18 (94.7) | 27 (96.4) | 85 (97.7) | 308 (98.4) | |
| Yes (%) | 1 (0.8) | 0 (0) | 1 (5.3) | 1 (3.6) | 2 (2.3) | 5 (1.6) | |
| Cancer | 0.26 | ||||||
| No (%) | 123 (99.2) | 54 (98.2) | 18 (94.7) | 28 (100) | 87 (100) | 310 (99) | |
| Yes (%) | 1 (0.8) | 1 (1.8) | 1 (5.3) | 0 (0) | 0 (0.0) | 3 (1) | |
| Comorbiditya | 0.096 | ||||||
| No (%) | 98 (79.0) | 46 (83.6) | 13 (68.4) | 25 (89.3) | 64 (73.6) | 246 (78.6) | |
| Low (%) | 17 (13.7) | 4 (7.3) | 1 (5.3) | 0 (0) | 13 (14.9) | 35 (11.2) | |
| High (%) | 9 (7.3) | 5 (9.1) | 5 (26.3) | 3 (10.7) | 10 (11.5) | 32 (10.2) | |
| BMI | 0.602 | ||||||
| Mean (SD) | 24.9 (4.4) | 24.8 (5.1) | 24.5 (4.8) | 26.3 (7.1) | 24.8 (3.9) | 24.9 (4.7) | |
| Smoking habit | 0.082 | ||||||
| Never (%) | 65 (52.4) | 27 (49.1) | 8 (42.1) | 17 (60.7) | 42 (48.3) | 159 (50.8) | |
| Former (%) | 47 (37.9) | 16 (29.1) | 4 (21.1) | 7 (25) | 26 (29.9) | 100 (31.9) | |
| Yes (%) | 12 (9.7) | 12 (21.8) | 7 (36.8) | 4 (14.3) | 19 (21.8) | 54 (17.3) | |
| Vaccine type | 0.473 | ||||||
| mARN (%) | 110 (88.7) | 48 (87.3) | 14 (73.7) | 24 (85.7) | 73 (83.9) | 269 (85.9) | |
| No mARN (%) | 14 (11.3) | 7 (12.7) | 5 (26.3) | 4 (14.3) | 14 (16.1) | 44 (14.1) | |
IMM: immunomodulators; IBD: inflammatory bowel disease; BMI: body mass index; DM: diabetes mellitus.
At 6 months after the full regimen, anti-S antibody titers and seroconversion rate were assessed in 313 patients. Of these, from enrollment to the time of serology, 52 patients acquired the infection.
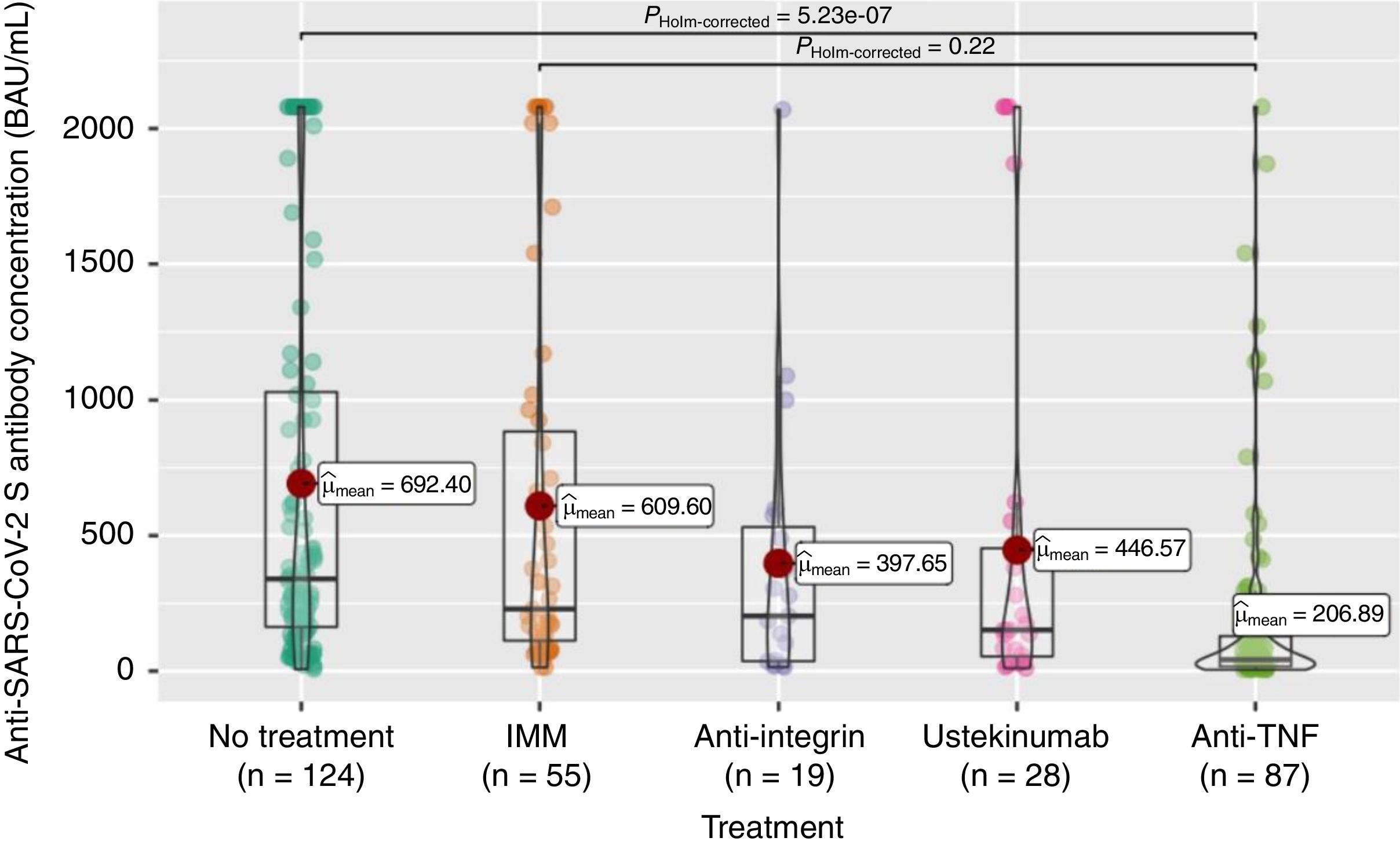
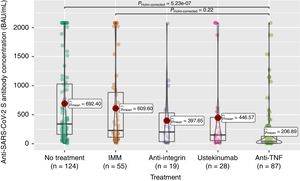
Anti-S antibody titers, within the full cohort of 313 patients, reached a mean of 503 (SD 667.4). As can be seen in Fig. 2, these titers were significantly lower in the anti-TNF treatment group compared to the non-treatment group, and even to the immunomodulatory monotherapy group.
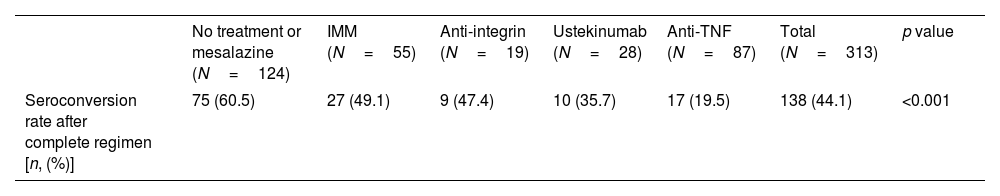
The seroconversion rate (>260BAU/mL), within the full cohort of 313 patients, was only 44% (Table 2). As shown in Table 2, there were also statistically significant differences according to treatment group, with lower rates in the anti-TNF (compared to no-treatment or mesalazine and immunomodulators groups) and ustekinumab (compared to no-treatment or mesalazine) groups.
Seroconversion rates according to treatment group after full vaccination regimen.
| No treatment or mesalazine (N=124) | IMM (N=55) | Anti-integrin (N=19) | Ustekinumab (N=28) | Anti-TNF (N=87) | Total (N=313) | p value | |
|---|---|---|---|---|---|---|---|
| Seroconversion rate after complete regimen [n, (%)] | 75 (60.5) | 27 (49.1) | 9 (47.4) | 10 (35.7) | 17 (19.5) | 138 (44.1) | <0.001 |
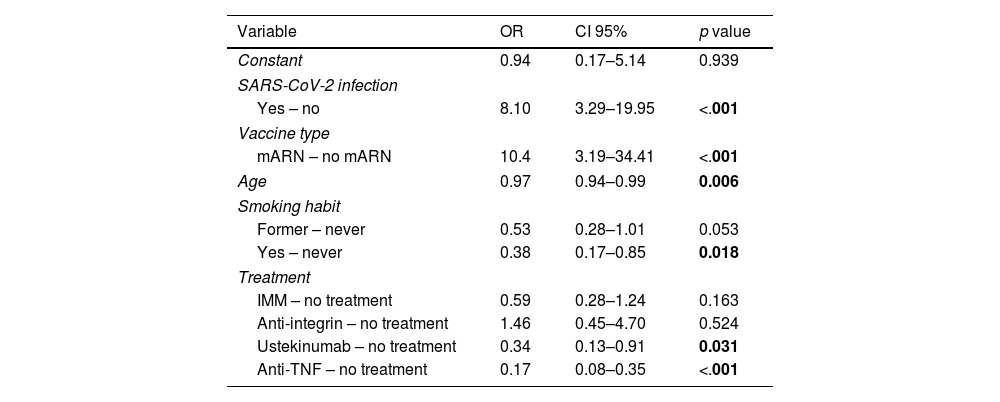
To assess the impact of relevant variables on the seroconversion rate, a multivariate analysis was performed. As shown in Table 3, the use of mRNA vaccine and having passed the infection (from full vaccination to the determination of effectiveness) were associated with a significantly higher probability of seroconversion. In contrast, treatment with anti-TNF (either in mono- or combo-therapy) or ustekinumab, older age, and smoking habit were associated with a significantly lower likelihood of seroconversion. Treatment with anti-integrin and immunomodulatory therapy did not decrease the likelihood of seroconversion compared to patients without immunosuppression (non-treatment group).
Multivariate model of factors associated with seroconversion after full vaccination schedule.
| Variable | OR | CI 95% | p value |
|---|---|---|---|
| Constant | 0.94 | 0.17–5.14 | 0.939 |
| SARS-CoV-2 infection | |||
| Yes – no | 8.10 | 3.29–19.95 | <.001 |
| Vaccine type | |||
| mARN – no mARN | 10.4 | 3.19–34.41 | <.001 |
| Age | 0.97 | 0.94–0.99 | 0.006 |
| Smoking habit | |||
| Former – never | 0.53 | 0.28–1.01 | 0.053 |
| Yes – never | 0.38 | 0.17–0.85 | 0.018 |
| Treatment | |||
| IMM – no treatment | 0.59 | 0.28–1.24 | 0.163 |
| Anti-integrin – no treatment | 1.46 | 0.45–4.70 | 0.524 |
| Ustekinumab – no treatment | 0.34 | 0.13–0.91 | 0.031 |
| Anti-TNF – no treatment | 0.17 | 0.08–0.35 | <.001 |
All 313 patients included in the study completed the 12-month clinical follow-up from the initial vaccination. However, neither all of them agreed to receive the booster dose (267, 85.3%) nor underwent the second serological effectiveness evaluation, performed in 285 patients.
Patients who declined the booster dose were younger (43.2 vs 48.5, p=0.005), less commonly receiving immunosuppressive treatment (58.7% vs 36.3%, p<0.001) and more often had CD (63% vs 41.9%, p=0.026). However, in the multivariate analysis (Supplementary Table 1) only age and absence of immunosuppressive treatment maintained statistical significance, while disease type did not.
Therefore, effectiveness in the second phase was assessed in 285 patients, including 247 (86.7%) who received the booster dose and 38 (13.3%) who did not. In addition, 172 (60%) of these 285 patients passed SARS-CoV-2 infection (and therefore had been able to develop natural immunity) during the study period and 113 (40%) patients did not (of whom 103 (36.1%) received the booster dose) (Fig. 1).
Out of the 172 patients infected during the second study period, 103 (30%) had asymptomatic infection. In contrast, 67 (39%) had a mild condition and 2 (<1%) had a moderate illness. No patient required admission in the intensive care unit and no deaths occurred.
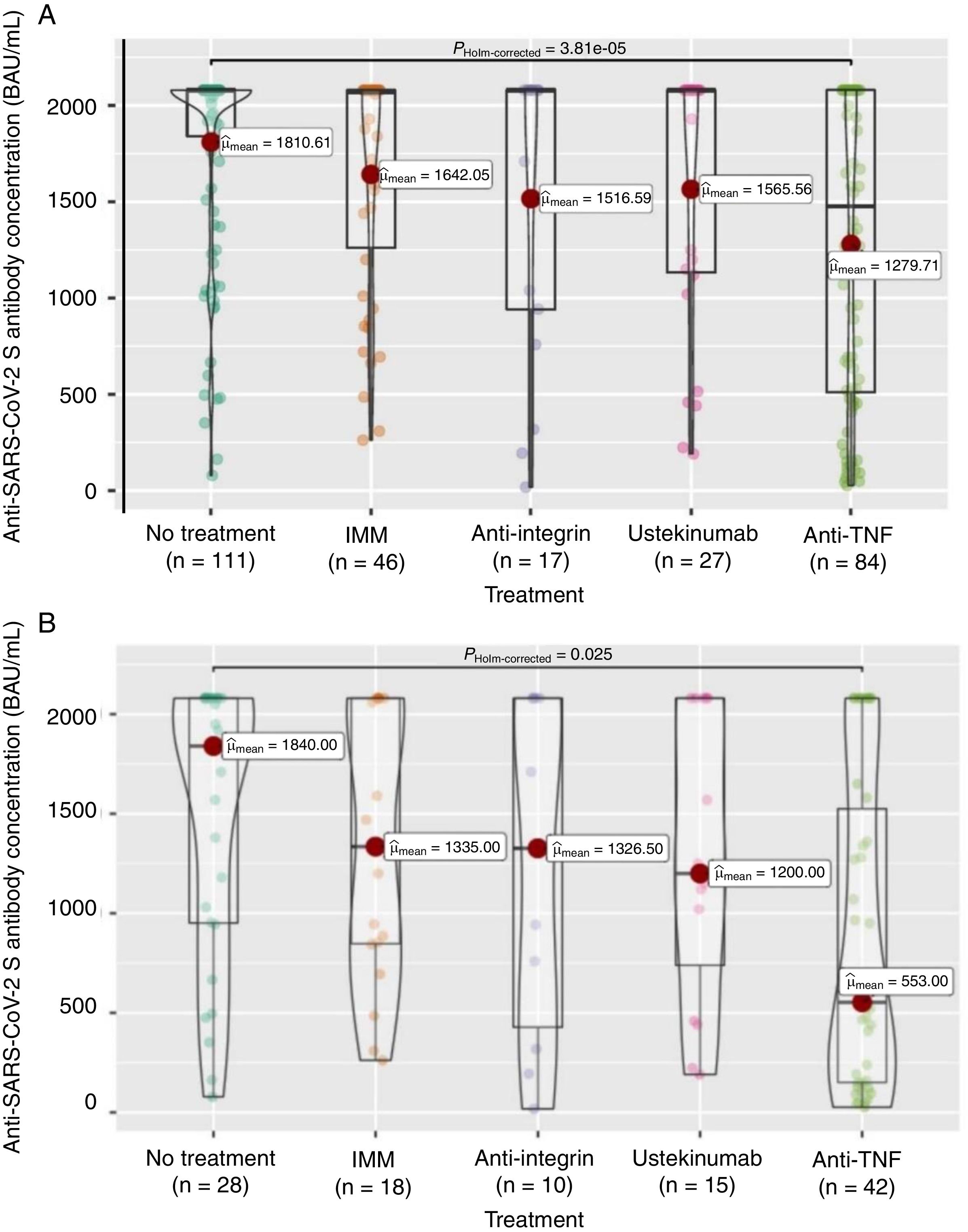
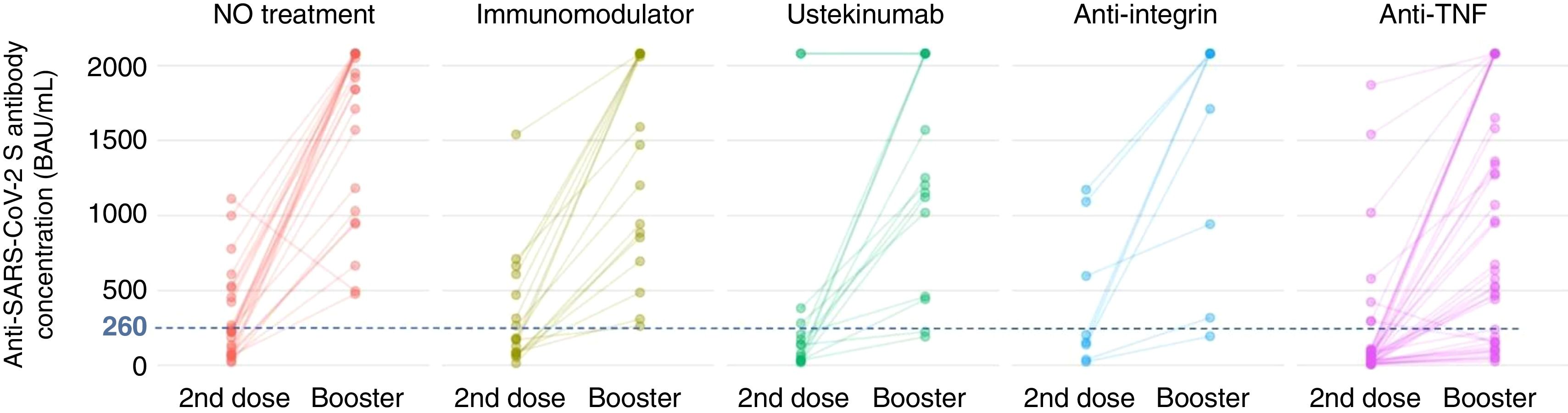
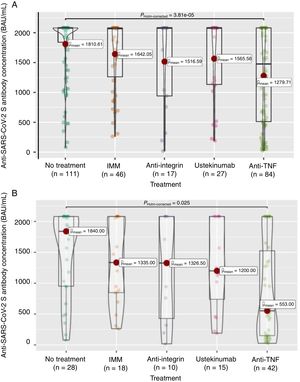
Firstly, to assess the contribution of the booster dose to vaccine effectiveness we included the 285 patients with available serology performed on schedule (180±28 days) after the booster dose date, regardless the booster was administered or not (n=38), and SARS-CoV-2 infection. In this group the mean value of anti-S antibody concentration was 1586±682BAU/mL. As shown in Fig. 3A, antibody titers were significantly lower in anti-TNF-treated patients compared to no treatment patients (1279.7 vs 1810.6BAU/mL, p<0.001). In the subgroup of patients without previous SARS-CoV-2 infection (n=113), antibody titers were 1180±774BAU/mL, and those on anti-TNF showed one more time a lower antibody concentration compared to patients without immunosuppressive treatment (553 vs 1840, p=0.008) as can be seen in Fig. 3B. In the subgroup of patients without SARS-CoV-2 infection that received the booster dose (n=103), antibody titers showed a marked increase in most of them after receiving the booster dose as shown in Fig. 4.
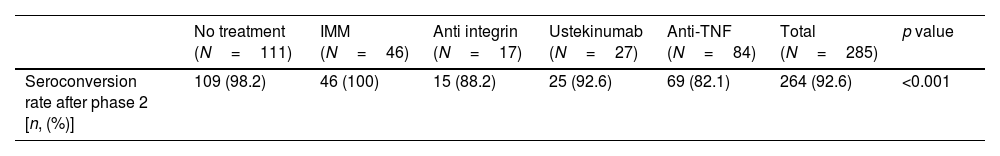
Seroconversion rate in the full cohort (N=285) was achieved in 264 patients (92.6%). As shown in Table 4, the seroconversion rate was significantly lower in patients on anti-TNF treatment. The seroconversion rate in the subgroup of patients without previous SARS-CoV-2 infection was 81.4% (92 out of 113 patients). Again, significant differences were observed in the seroconversion rate according to treatment, being lower in patients treated with anti-TNF (Table 4). In the subgroup of patients without SARS-CoV-2 infection that received the booster dose (n=103), a clear positive effect in terms of achieving seroconversion was obtained, as shown in Fig. 4. The impact of other factors in seroconversion after booster dose is shown in Supplementary Table 2.
Seroconversion rate according to treatment group after phase two.
| No treatment (N=111) | IMM (N=46) | Anti integrin (N=17) | Ustekinumab (N=27) | Anti-TNF (N=84) | Total (N=285) | p value | |
|---|---|---|---|---|---|---|---|
| Seroconversion rate after phase 2 [n, (%)] | 109 (98.2) | 46 (100) | 15 (88.2) | 25 (92.6) | 69 (82.1) | 264 (92.6) | <0.001 |
| No treatment (N=28) | IMM (N=18) | Anti integrin (N=10) | Ustekinumab (N=15) | Anti-TNF (N=42) | Total (N=113) | p value | |
|---|---|---|---|---|---|---|---|
| Seroconversion rate after phase 2, patients without SARS-CoV-2 infection [n, (%)] | 26 (92) | 18 (100) | 8 (80) | 13 (86.7) | 27 (64.3) | 92 (81.4) | 0.005 |
IMM: immunomodulators.
Focusing on the seroconversion rate, to better assess the impact of the other factors involved univariate and multivariate analysis were performed (Supplementary Table 3). Univariate analyses showed that all patients who passed SARS-CoV-2 infection during follow-up seroconverted. Therefore, given the determinant impact of previous SARS-CoV-2 infection on antibody development and seroconversion, the analysis of factors involved in vaccine effectiveness, including the contribution of the booster dose, was carried out only in those patients who were not infected by the SARS-CoV-2 during follow-up (N=113). In this subgroup, multivariate analysis (Supplementary Table 3) confirmed that anti-TNF treatment was associated with a lower probability of seroconversion (OR 0.10 [0.01–0.75]), while administration of the booster dose (OR 10.91 [1.55–76.61]), use of mRNA vaccines (OR 11.72 [2.26–60.51]), and age (1.07 [1.01–1.13]) predicted a higher probability of reaching the seroconversion threshold.
DiscussionOur study is one of the first to evaluate prospectively the effectiveness of a booster dose of SARS-CoV-2 vaccines in patients with IBD in clinical practice, using the currently recommended seroconversion values, and at medium-term. Our data show the clear benefit for IBD patients of an additional dose after the full vaccination schedule. The longitudinal follow-up of our patients allows us to assess the gain added to the full regimen. Our results show that anti-TNF treatment is the main negative factor in achieving an adequate and protective response after vaccination, even after booster dose. This is consistent with some previous studies10,12–14 but contrasts with the Canadian study conducted by Quan et al., in which anti-TNF treatment was not associated with lower antibody levels after the third dose.11 It is striking that the impact of IBD treatment observed on vaccination is much greater than that observed on the spread of the disease itself and its severity.31,32
In our study we present results after the full regimen and after the booster dose, in both cases measured 6 months later. This differs from previous studies, which analyze the response at much shorter times. Furthermore, according to the most recent data on the protective capacity of antibody levels, we have established 260BAU/mL as the seroconversion level.
This threshold is higher than that established in previous studies, which makes the assessment of seroconversion much more demanding. Similarly, we have focused our analysis on the ability to achieve seroconversion or not, rather than on antibody titer, because of its greater value in practice, hitherto unknown.
Seroconversion rates following full vaccination in our cohort are low (less than 50%), with a large variation between those patients with no treatment (60.5%) and those with ustekinumab (35.7%) and specially anti-TNF (19.5%). Using a threshold of 260BAU/mL, this rates are clearly much lower than in other series in similar populations, where rates of over 90% are reached.33 Another difference of our results in this first phase results with the literature published to date, is the negative impact of ustekinumab treatment on the likelihood of seroconverting. This effect has not been previously described in published literature and may be due to how response is assessed (seroconversion rate rather than antibody titers, and 6 months after full vaccination). In fact, in our own data, there is no difference between the ustekinumab group and the untreated group in terms of antibody levels. This is like what has been previously described in the literature.8,10,13 These findings underline the importance of using the seroconversion point of 260BAU/mL, as it clearly modifies the results obtained.
In contrast, significant differences in antibody levels were observed between the anti-TNF group and the no-treatment and immunomodulatory groups, as previously published.
We also analyzed factors related to adherence to a booster dose of vaccine. Our administration rate was 85%, like that described in the study by Wellens et al.34 Factors associated with non-adherence in our cohort were young age (although with minor differences between groups) and, specially, the absence of immunosuppressive treatment (of any type). These data suggest that patients under immunosuppressive treatment are particularly aware of the importance of vaccination, while the message needs to be reinforced in patients without treatment.
Finally, we analyzed the effectiveness and impact of the booster dose. In contrast to other studies such as VIP,10 we included a control group with IBD, as some of the patients decided not to take the booster dose. After administration of the booster dose, the seroconversion rate (>260BAU/mL) increased from 44.1% to 92.6%. Given the impact of SARS-CoV-2 infection on seroconversion, and to see more clearly the importance of the booster dose, patients without infection during the study year were analyzed separately. All treatment groups experienced a large improvement in the seroconversion rate, close to 50 percentage points. After the improvement obtained with the booster dose, the negative influence of ustekinumab on seroconversion after full vaccination disappeared, while anti-TNF-treated patients still had a significantly lower seroconversion rate. These results are not comparable to those previously described in the literature, as there are no data in IBD patients using the cut-off point of 260BAU/mL.
The evaluation using the cut-off point of 260BAU/mL is undoubtedly the first major contribution of our work. The second particularly relevant contribution of our study is the time when response is evaluated. To date, there is only one study16 that assessed medium-term response. The other studies previously mentioned evaluate response at a maximum of 2 months after administration. This is particularly relevant because numerous studies have shown that antibody loss is a constant from 3–6 months after vaccine administration.35–38 Therefore, too early assessment may provide falsely optimistic data. Our work shows that the administration of a booster dose, regardless of the effectiveness of the full regimen, allows optimal seroconversion rates to be achieved and maintained for at least 6 months after its administration. Notably, the gain is much more marked in patients without SARS-CoV-2 infection during follow-up. As we do not know the exact date of infection, it is difficult to make a precise recommendation, but it seems reasonable that, in patients with recent SARS-CoV-2 infection, deferring the administration of the booster dose should be considered.
Our study has some limitations. First, the number of patients in the vedolizumab and ustekinumab groups is small, which may have limited the ability to detect differences in these groups. Secondly, one of the largest waves of infection in Spain occurred during the study period, which explains that the infection rate during the second phase was very high and makes somewhat difficult to analyze the impact of the booster dose, as natural immunity plays a key role. Disease activity has been assessed by clinical indices, but endoscopic or biological activity has not been evaluated. SARS-CoV-2 infection has been assessed as a dichotomous variable, but the presence of different variants during different waves may have caused differential effects on immunity.
However, our study also has important strengths. Our cohort has a relevant sample size, with a one-year follow-up and an assessment of major relevance in our view for clinical practice. The use of seroconversion as the primary endpoint and measurement at 6 months allows us to obtain results that are highly relevant for daily practice. In addition, we have a control group of no treatment IBD patients, which allows us to better assess the impact of treatment compared to a cohort of healthy patients without IBD. A control group without booster vaccination is also available for the analysis of the second phase, which again allows us to draw important conclusions.
In conclusion, the administration of a booster dose provides a significant improvement in antibody titers and seroconversion rates (therefore protective titers) in patients with IBD with or without any immunosuppressive treatment, and thus it is clearly recommended. However, anti-TNF treatment not only decreases the effectiveness of the initial full vaccination regimen, but also that obtained after the booster dose. It must be considered, specially in patients at higher risk due to other associated factors such as elderly, chronic kidney disease, severe cardiovascular disease, diabetes with target organ involvement, obesity or underweight. In our opinion, IBD treatment should not be modified in these patients because of the risk of reduced seroconversion, but rather the need for correct administration of the recommended booster doses should be emphasized.
Authors’ contributionsDCD: Conceptualization, Methodology, Formal analysis, Writing-original Draft; BJG: Conceptualization, Methodology, Data curation, Writing-original Draft; SGL: Conceptualization, Methodology, Supervision, Writing-original; ECB: Formal analysis, Data curation, Project administration; EVR and EGG: Resources and Investigation; LRA, BS, VL, MJAE, LMV, RFI, APP, MC, SRI, IMY, YBN, SGM, RVL, LA, EA, PCR, MC: Data curation; BB, ED, AGC, MM, YZ, JPG and MBdA: Data curation and Writing-Review & Editing.
Data availabilityThe data presented in this study are available on request to the corresponding author with prior authorization of our Ethical Committee that can be obtained at https://www.iacs.es/investigacion/comite-de-etica-de-la-investigacion-dearagon-ceica/ceica-evaluaciones-y-otras-presentaciones [accessed 20.4.23].
FundingDCD is partially funded by a Rio Hortega Grant from Instituto de Salud Carlos III (Grant number CM21/00067). This study have been funded and supported by GETECCU.
Conflict of interestDr. Casas-Deza has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring and Faes Farma. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma. Dr. Barreiro has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. Dr. García-López has served as a speaker, advisory member for or has received research funding from AbbVie, Janssen, MSD, Pfizer, and Takeda. Dra Calafat MC has served as a speaker or has received research or education funding or advisory fees for Takeda, Janssen, Gilead, Falk and Pfizer. D. Domènech has served as a speaker or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Galapagos, Gilead, GoodGut, Imidomics, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots. Dra Ferreiro has served as a speaker or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Janssen, Kern Pharma, MSD, Takeda, Tillots, Casenrecordati, Pfizer, Palex, Shire Pharmaceuticals, Ferring. Dra Gutierrez has served as a speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Janssen, Kern Pharma,Takeda, Galapagos, Pfizer, Sandoz, Fresenius, Ferring, Faes Farma, and Adacyte. Dra Chaparro has served or has received research or education funding or advisory fees from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead and Lilly.