Diarrhea is a common side effect of bortezomib. The pathogenesis of this unpredictable complication is unclear. Up until now, it was believed that this agent does not induce direct mucosal damage, with few reports of colon mucositis in the literature in recent years.1,2
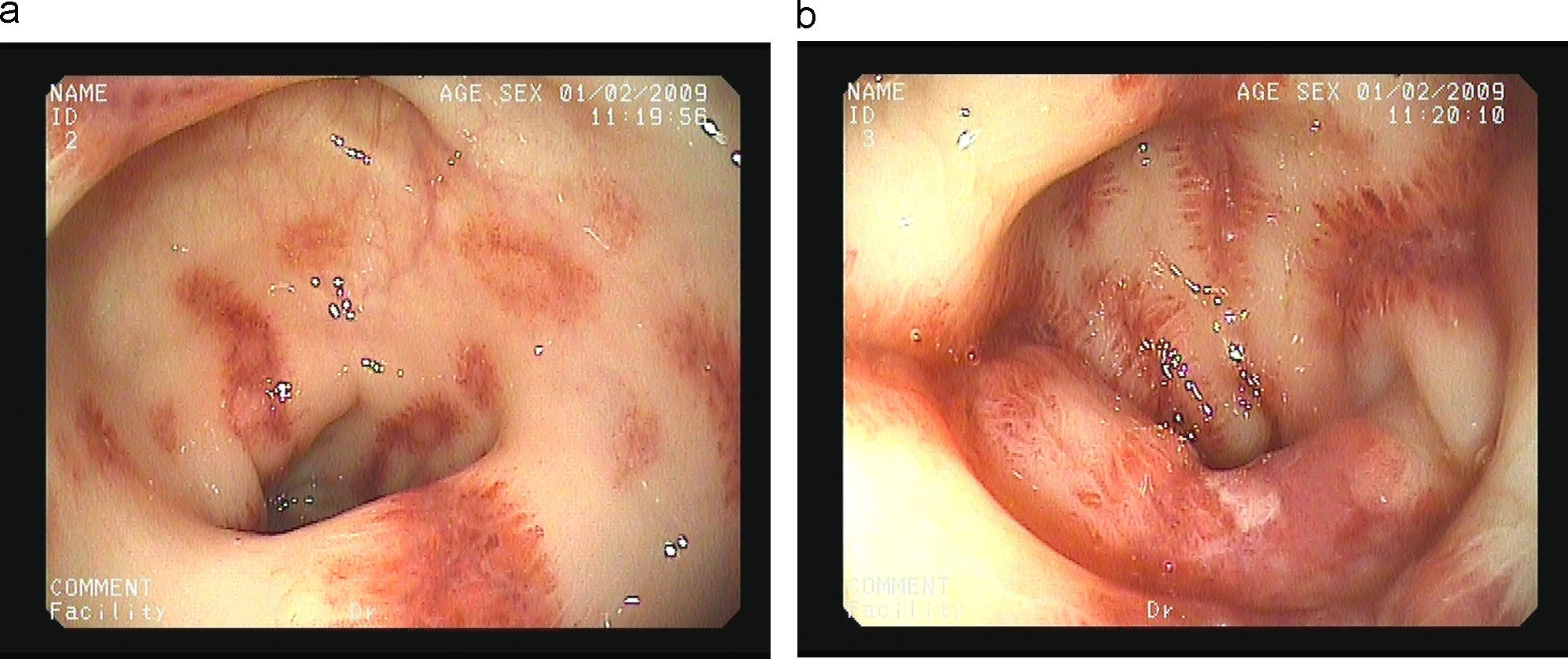
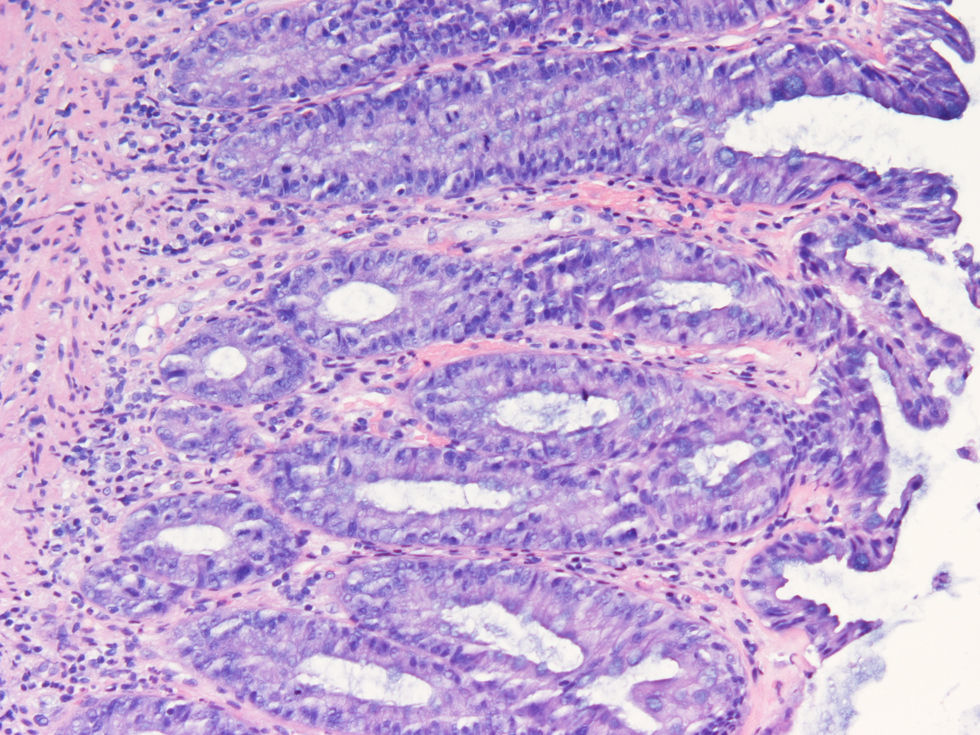
We report the case of a 65-year-old man with history of multiple myeloma (IgD) who presented with acute bloody diarrhea. The patient was under treatment with melphalan and prednisone, and due to progression of his disease, recently started bortezomib (a proteosome inhibitor) 1.3mg/m2 by intravenous bolus injection on days 1, 4, 8 and 11, to be repeated every 3 weeks. Bloody diarrhea started 24h after the fourth dose of bortezomib and was not accompanied by mucus or fever; the patient referred an autolimited non-bloody diarrhea after the third dose of bortezomib. Laboratory tests showed a previously unknown plaquetopenia (58.000plaq/μL (140.000–400.000plaq/μL). The emergency sigmoidoscopy revealed multiple ecchymosis and small colonic ulcers covered by fibrin and perilesional inflammation with a trend to stenose the lumen (Fig. 1a, b). Biopsy specimens showed a normal epithelium with a chronic and unspecific inflammatory infiltrate in the crypts (Fig. 2) with lack of amiloyd or clonicity of Kappa/Lambda chains; the bacterial and viral cultures were negative. Bloody diarrhea and plaquetopenia disappeared after the suspension of bortezomib, without new episodes after 6 months under treatment with melphalan, prednisone and talidomide.
Frequent watery diarrhea following bortezomib is oftenly related to other mechanisms rather than mucositis. However, a more intense surveillance of patients under this treatment could be justified to exclude more severe pathology.