Retroperitoneal liposarcoma constitutes an uncommon and locally aggressive malignancy. We performed a retrospective analysis of 10 patients (6 males; mean age: 63.2±11 years) with histologically proven retroperitoneal liposarcoma seen at our institution between 1999 and 2007. Presence of a palpable abdominal mass was the main symptom at diagnosis. All patients underwent complete surgical resection. Negative microscopic margin was achieved in four cases. Histological analysis revealed the following subtypes: well-differentiated (6 cases), dedifferentiated (two cases), pleomorphic, and myxoid/round cell (one case each). Concomitant resection of adjacent organs was needed in five cases. Half of the patients developed tumor recurrence, mainly limited to the retroperitoneum or abdominal cavity. The mean recurrence-free survival was 43.3 months (95%CI: 25.7–60.8), with 3- and 5-year overall survival rates of 79% and 61%, respectively. Patients undergoing complete resection with clear margins showed a near-significant trend toward increased recurrence-free survival (62.9 vs. 29.3 months; P=0.06).
El liposarcoma retroperitoneal constituye una neoplasia infrecuente y localmente agresiva. Realizamos un análisis retrospectivo de los 10 pacientes (6 varones; edad media: 63,2±11años) con diagnóstico histológico de liposarcoma retroperitoneal atendidos en nuestra institución entre 1999–2007. La presencia de una masa abdominal palpable constituyó la forma de presentación más frecuente. Todos los pacientes fueron sometidos a resección quirúrgica, obteniéndose en cuatro casos márgenes negativos. El análisis histológico reveló lo siguientes subtipos: bien diferenciado (6 casos), desdiferenciado (dos casos), pleomórfico (un caso) y mixoide/células redondas (un caso). En cinco casos fue necesaria la resección concomitante de órganos adyacentes. La mitad de los pacientes presentaron recurrencia tumoral, fundamentalmente limitada al retroperitoneo o a la cavidad abdominal. La supervivencia media libre de recurrencia fue de 43,3 meses (IC 95%: 25,7–60,8), con una probabilidad de supervivencia global a los 3 y 5 años del 79 y 61%. Los pacientes sometidos a resección quirúrgica completa con márgenes negativos presentaron una tendencia hacia una mayor supervivencia libre de recurrencia (62,9 vs. 29,3 meses; p=0.06).
Retroperitoneal sarcomas are rare mesenchymal tumors that exhibit a vast array of histological subtypes, accounting for less than 1% of all solid malignancies.1 About one third of malignant tumors that arise in the retroperitoneum are sarcomas, and approximately 10–20% of soft tissue sarcomas are located in this region.2,3 Their peak incidence is in the fourth–sixth decades of life, although they may occur in any age group.1 Sarcomas are believed to develop from mesenchymal stem cells located in muscle, fat, and connective tissues, and specific histological types include liposarcomas, leiomyosarcomas, malignant fibrous histiocytomas, fibrosarcomas, and malignant peripheral nerve sheath tumors, among others.3 Surgical excision is the most effective therapeutic approach and constitutes the only chance for potential long-term survival.4 However, the absence of early symptoms leads to a delay in diagnosis, resulting in tumors of considerable size with frequent involvement of adjacent organs.2,4,5
Retroperitoneal liposarcoma (RPLS) is the single most common subtype of soft tissue sarcoma arising in the retroperitoneum, comprising about 30–50% of all sarcomas in this region.2–5 However, because of the rarity of RPLS, little is known about its clinical presentation, biological behaviour, and optimal therapeutic approach. Although surgical resection constitutes the mainstay of the management in patients with RPLS, the role of adjuvant therapies is still poorly established.5 Effective adjuvant regimens of chemotherapy remain to be identified, whereas the feasibility of radiation therapy in this particular location is limited by toxicity to adjacent structures. Gholami et al.6 have concluded that adjuvant chemotherapy or radiation therapy did not significantly affect recurrence-free survival in a recently published series of 41 patients with localized retroperitoneal sarcoma. On the contrary, other studies have suggested that adjuvant radiation therapy may decrease the probability of local recurrence.7 In our experience, surgery is primarily indicated in patients with no evidence of distant metastasis or major vessel involvement, good performance status (Karnofsky score >70), and acceptable anesthesia risk as determined through routine preoperative screening. In the absence of metastatic disease, surgery is equally the choice for locally recurrent tumors. A multidisciplinary team individualizes the use of adjuvant therapy, usually reserved for patients with poor prognostic factors (i.e., incomplete resection, extensive disease, or high grade tumors).
Most of the previously published series of primary malignant retroperitoneal tumors have included RPLS along with other retroperitoneal sarcomas with heterogeneous histology, impeding its independent characterization.3,4,6–9 Liposarcomas may represent a more indolent histologic entity among this group of malignancies, with a relatively lower incidence of metastatic disease as compared with the aggressive course of other pleomorphic sarcomas.2,10 Therefore, the aim of this study was to review our single-center experience in the management of RPLS during an 8-year period, focusing on clinical presentation, primary therapeutic modalities, and patient outcome.
Clinical observationsThe Tumor Registry of the University Hospital “12 de Octubre” compiles data on all new cancer cases in Health Area 11 of the Community of Madrid, with a reference population of 887,134 inhabitants in 2007. We performed a retrospective analysis of all the consecutive patients with histologically proven RPLS presenting to our institution between January 1999 and October 2007. No patient was excluded due to the nature of the therapeutic approach (i.e., the absence of surgical indication). The corresponding medical records were reviewed and their clinicopathological features were assessed with respect to the initial surgical procedure. The histologic features were reexamined by one of the authors (Y.R.G.). The study variables included gender, age at diagnosis, presenting symptoms and duration in months, risk classification of the American Society of Anesthesiologists (ASA), laboratory tests, preoperative imaging studies [ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI)] and histological diagnosis, tumor grade and histological subtype, tumor location and size at the time of initial resection, involvement of adjacent organs, type of surgical procedure, and use of adjuvant therapy. Tumors were divided according to its location into two groups (upper or lower abdomen) using the middle of L3 vertebra as the anatomical boundary.3 RPLS was classified into four histological subtypes based on the latest World Health Organization (WHO) criteria as well-differentiated, myxoid/round cell, dedifferentiated, and pleomorphic.10 Tumor grade was assessed according to the French Federation of Comprehensive Cancer Centers (FNCLCC) system.11 Tumor staging was performed following the sixth edition of the American Joint Committee on Cancer (AJCC) Staging System for soft tissue sarcomas.12 Results are presented as mean or median±standard deviation and range or 95% confidence interval (95%CI). The recurrence-free and overall survival times were defined as the time interval from the date of surgery to the date of first recurrence or death by any cause. Survival was estimated by the Kaplan–Meier method, and differences between groups were compared with the log-rank test. Statistical analysis was performed using the package SPSS, version 12.0 (SPSS Inc., Chicago, IL).
A total of 10 patients with histologically proven RPLS were identified. Patient characteristics are summarized in Table 1. There were 6 males and four women, with a mean age at diagnosis of 63.2±11 years (range: 47–85). Familiar history of malignant disease was present in three patients. Multiple primary malignant neoplasms were present in one case, an 85-year-old female who underwent radiotherapy following right mastectomy for breast cancer 9 years before diagnosis of RPLS (patient 6). Eight patients were symptomatic when they first visited our center. The most common complaint at presentation was self-reported presence of a palpable abdominal mass (6 cases), followed by weight loss and night sweats (one case each). One patient had been previously diagnosed with meralgia paresthetica due to lower extremity pain of 3-year duration secondary to neural involvement by the tumor mass. The other two patients were asymptomatic, and their tumors were discovered incidentally. Physical examination showed a palpable abdominal mass in 8 patients. Laboratory investigations (including preoperative determination of complete blood counts, renal and liver function tests, and serum tumor markers) did not reveal any specific abnormalities. Four of the patients had a preoperative histological diagnosis suggestive of liposarcoma, based on either fine-needle aspiration biopsy or cytology. Duration of symptoms prior to diagnosis of RPLS was available for 6 patients, and ranged from 3 to 36 months, with a median of 8.5 months. All the patients in our series underwent resection with curative intent. The median interval from diagnosis to treatment was 3.1 weeks (range: 1.9–10.6). The follow-up in the course of this period was based on abdominal imaging examinations (either CT or MRI) performed every 2 months. Complete resection with microscopic clear margins (R0) was achieved in four cases, whereas macroscopic clearance with microscopic infiltration of resection margins (R1) was obtained in five cases. One patient underwent extensive tumor debulking procedure with macroscopically incomplete resection (R2). Half of the patients required concomitant resection of adjacent organs (left nephrectomy in two cases; right nephrectomy, left hemicolectomy, and left salpingo–oophorectomy in one case each). The mean duration of hospital stay at initial surgery was 22.3±14.4 days. Three patients received postoperative adjuvant therapy with either anthracycline-based chemotherapy or radiotherapy at some stage during disease progression (Table 2).
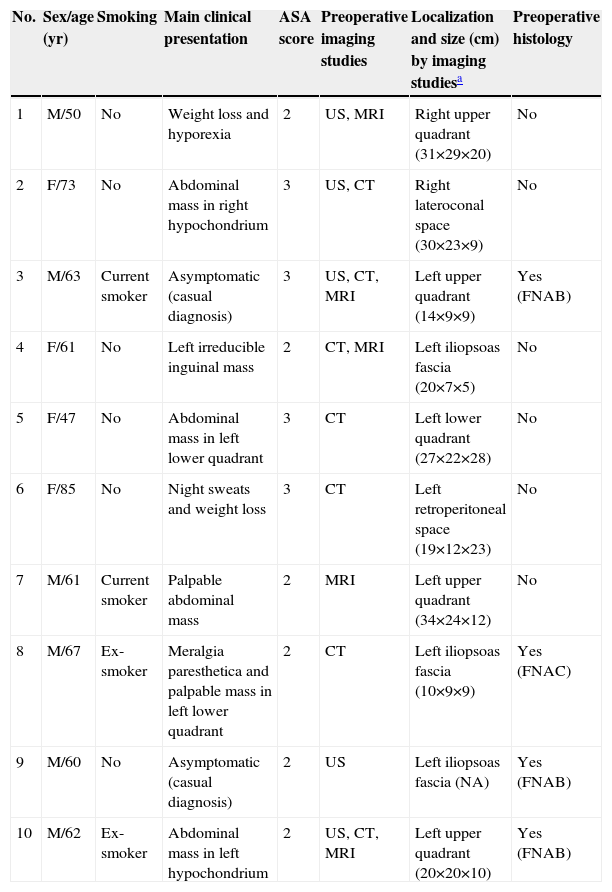
Clinical features and preoperative evaluation in 10 patients with RPLS
| No. | Sex/age (yr) | Smoking | Main clinical presentation | ASA score | Preoperative imaging studies | Localization and size (cm) by imaging studiesa | Preoperative histology |
| 1 | M/50 | No | Weight loss and hyporexia | 2 | US, MRI | Right upper quadrant (31×29×20) | No |
| 2 | F/73 | No | Abdominal mass in right hypochondrium | 3 | US, CT | Right lateroconal space (30×23×9) | No |
| 3 | M/63 | Current smoker | Asymptomatic (casual diagnosis) | 3 | US, CT, MRI | Left upper quadrant (14×9×9) | Yes (FNAB) |
| 4 | F/61 | No | Left irreducible inguinal mass | 2 | CT, MRI | Left iliopsoas fascia (20×7×5) | No |
| 5 | F/47 | No | Abdominal mass in left lower quadrant | 3 | CT | Left lower quadrant (27×22×28) | No |
| 6 | F/85 | No | Night sweats and weight loss | 3 | CT | Left retroperitoneal space (19×12×23) | No |
| 7 | M/61 | Current smoker | Palpable abdominal mass | 2 | MRI | Left upper quadrant (34×24×12) | No |
| 8 | M/67 | Ex-smoker | Meralgia paresthetica and palpable mass in left lower quadrant | 2 | CT | Left iliopsoas fascia (10×9×9) | Yes (FNAC) |
| 9 | M/60 | No | Asymptomatic (casual diagnosis) | 2 | US | Left iliopsoas fascia (NA) | Yes (FNAB) |
| 10 | M/62 | Ex-smoker | Abdominal mass in left hypochondrium | 2 | US, CT, MRI | Left upper quadrant (20×20×10) | Yes (FNAB) |
ASA: American Society of Anesthesiologists; CT: computed tomography; F: female; FNAB: fine-needle aspiration biopsy; FNAC: fine-needle aspiration cytology; M: male; MRI: magnetic resonance imaging; NA: not available; RPLS: retroperitoneal liposarcoma; US: ultrasonography.
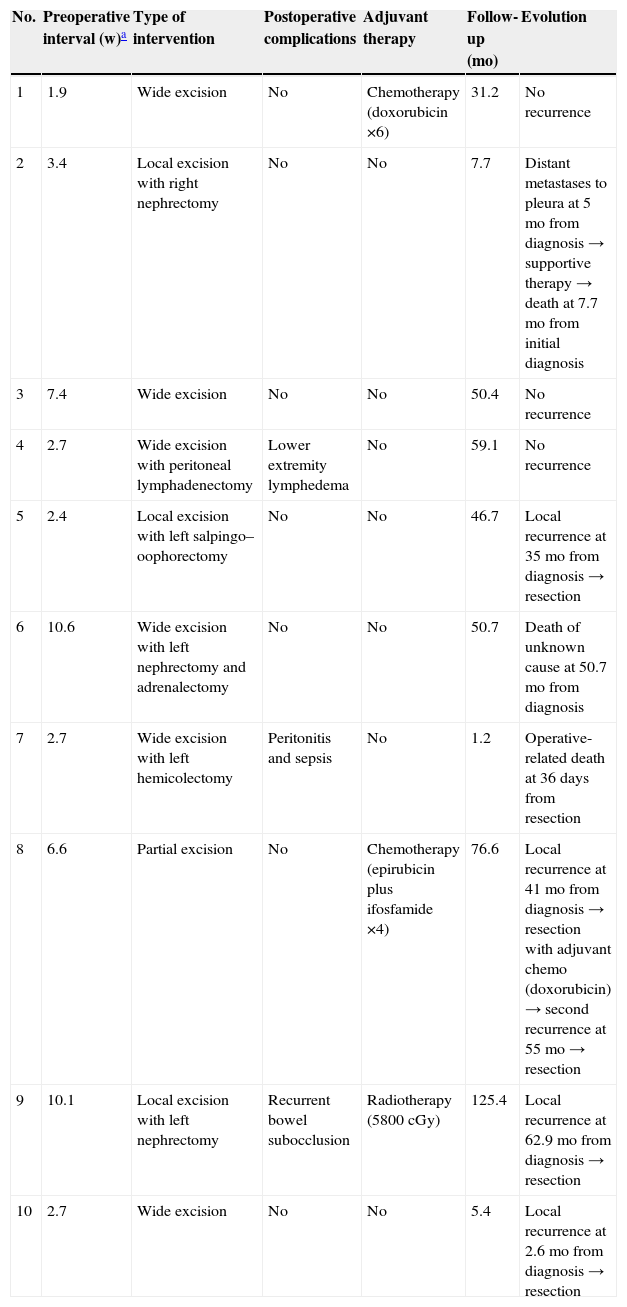
Type of therapeutic approach and clinical outcomes
| No. | Preoperative interval (w)a | Type of intervention | Postoperative complications | Adjuvant therapy | Follow-up (mo) | Evolution |
| 1 | 1.9 | Wide excision | No | Chemotherapy (doxorubicin ×6) | 31.2 | No recurrence |
| 2 | 3.4 | Local excision with right nephrectomy | No | No | 7.7 | Distant metastases to pleura at 5mo from diagnosis → supportive therapy → death at 7.7mo from initial diagnosis |
| 3 | 7.4 | Wide excision | No | No | 50.4 | No recurrence |
| 4 | 2.7 | Wide excision with peritoneal lymphadenectomy | Lower extremity lymphedema | No | 59.1 | No recurrence |
| 5 | 2.4 | Local excision with left salpingo–oophorectomy | No | No | 46.7 | Local recurrence at 35mo from diagnosis → resection |
| 6 | 10.6 | Wide excision with left nephrectomy and adrenalectomy | No | No | 50.7 | Death of unknown cause at 50.7mo from diagnosis |
| 7 | 2.7 | Wide excision with left hemicolectomy | Peritonitis and sepsis | No | 1.2 | Operative-related death at 36 days from resection |
| 8 | 6.6 | Partial excision | No | Chemotherapy (epirubicin plus ifosfamide ×4) | 76.6 | Local recurrence at 41mo from diagnosis → resection with adjuvant chemo (doxorubicin) → second recurrence at 55mo → resection |
| 9 | 10.1 | Local excision with left nephrectomy | Recurrent bowel subocclusion | Radiotherapy (5800cGy) | 125.4 | Local recurrence at 62.9mo from diagnosis → resection |
| 10 | 2.7 | Wide excision | No | No | 5.4 | Local recurrence at 2.6mo from diagnosis → resection |
Chemo: chemotherapy; mo: months; w: weeks.
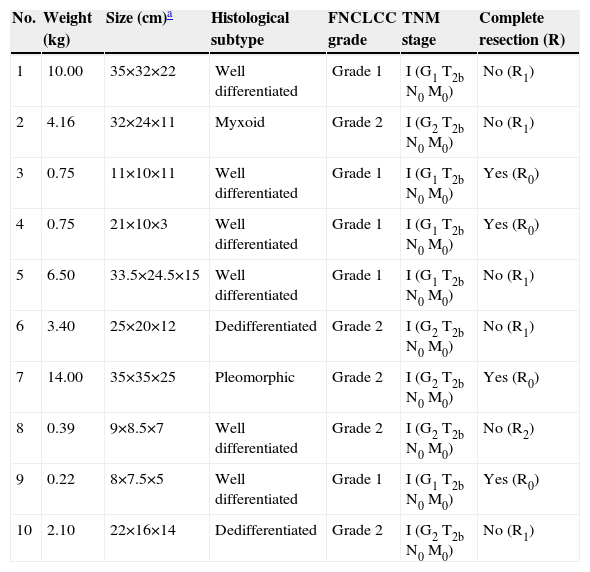
Histopathologic findings, including tumor grade, histological subtype, and TNM stage, are reported in detail in Table 3. The median weight of resected tumor was 2.7kg (range: 0.2–14). The mean maximum tumor size in patients with casual diagnosis (9.7±3.2cm) was lower compared with those symptomatic at diagnosis (26.3±9.3cm). Histological analysis revealed the following subtypes: well-differentiated (6 cases), dedifferentiated (two cases), pleomorphic, and myxoid/round cell (one case each). Most of the tumors arose from the left side of the abdominal cavity or the retroperitoneum, including the left iliopsoas fascia (three cases) and the left hemipelvis (one case). RPLS was predominantly located in the upper abdomen in four cases, and in the lower abdomen in the remaining six cases.
Histopathologic characteristics and tumor stage at diagnosis
| No. | Weight (kg) | Size (cm)a | Histological subtype | FNCLCC grade | TNM stage | Complete resection (R) |
| 1 | 10.00 | 35×32×22 | Well differentiated | Grade 1 | I (G1 T2b N0 M0) | No (R1) |
| 2 | 4.16 | 32×24×11 | Myxoid | Grade 2 | I (G2 T2b N0 M0) | No (R1) |
| 3 | 0.75 | 11×10×11 | Well differentiated | Grade 1 | I (G1 T2b N0 M0) | Yes (R0) |
| 4 | 0.75 | 21×10×3 | Well differentiated | Grade 1 | I (G1 T2b N0 M0) | Yes (R0) |
| 5 | 6.50 | 33.5×24.5×15 | Well differentiated | Grade 1 | I (G1 T2b N0 M0) | No (R1) |
| 6 | 3.40 | 25×20×12 | Dedifferentiated | Grade 2 | I (G2 T2b N0 M0) | No (R1) |
| 7 | 14.00 | 35×35×25 | Pleomorphic | Grade 2 | I (G2 T2b N0 M0) | Yes (R0) |
| 8 | 0.39 | 9×8.5×7 | Well differentiated | Grade 2 | I (G2 T2b N0 M0) | No (R2) |
| 9 | 0.22 | 8×7.5×5 | Well differentiated | Grade 1 | I (G1 T2b N0 M0) | Yes (R0) |
| 10 | 2.10 | 22×16×14 | Dedifferentiated | Grade 2 | I (G2 T2b N0 M0) | No (R1) |
FNCLCC: French Federation of Cancer Centers grading system; mo: months; TNM: Tumor-Node-Metastases staging system.
The median postoperative follow-up for the entire cohort was 45.4 months (range: 1.2–125.4). At the end of this period, three of the patients (30%) had deceased, with a mean overall survival of 85.02 months (95%CI: 48.4–121.6). The overall 3- and 5-year survival rates were 79% and 61%, respectively. There was one postoperative death due to severe sepsis following suture dehiscence with peritonitis at 36 days from resection. Two patients died at 7.7 and 50.7 months from RPLS diagnosis, respectively. The mean recurrence-free survival was 43.3 months (95%CI: 25.7–60.8), with five of the patients developing at least one tumor recurrence; in four cases the first recurrence was local (limited to the retroperitoneum or abdominal cavity). Only one patient developed distant dissemination (pleural metastases). Patients who underwent complete resection (R0) exhibited a borderline significant trend toward longer recurrence-free survival than those who did not undergo such treatment (62.9 vs. 29.3 months, respectively; P=0.06). Regarding the histological subtypes of RPLS, the mean recurrence-free survival in patients with well-differentiated tumors was longer than that with other histologies (55.9 vs. 24.8 months), although the difference did not attain statistical significance (P=0.13). Finally, patients who received postoperative adjuvant therapy also showed a longer recurrence-free mean survival compared with that of the remaining patients (51.8 vs. 36.6 months), but again not reaching significance (P=0.65).
DiscussionRPLS consitutes an uncommon malignancy that exhibits an indolent pattern of growth in the clinically silent retroperitoneum. Due to the lack of specific anatomic compartments in this space, the presenting symptoms usually appear late in the course of the disease and depend on the structure or organ that is displaced, and whether stretched or compressed by the tumor bulk.3,4,8 These malignancies usually arise from the perinephric fat and they may attain huge dimensions.13 The tumor was in continuity with the iliopsoas muscle in three of the analyzed patients. Similarly to previous studies, most of the patients in the present series noticed gradual abdominal enlargement over several months, often accompanied by vague abdominal or pelvic pain, suggesting significant tumor growth at the time of diagnosis.2,3,8 It has been proposed that an upper abdominal tumor location could act as a good prognostic factor, with a lower local recurrence rate3. Interestingly, only one of the four patients with RPLS located in the upper abdominal region developed tumor recurrence in our series, compared to four of six patients with lower abdominal location. Both CT and MRI may provide an accurate assessment of the characteristics of soft tissue sarcomas and the involvement of adjacent structures.1 The presence of macroscopic fat usually leads to the presumptive diagnosis of liposarcoma; high-grade tumors appear as a dense, heterogeneous mass, enhancing with intravenous contrast (Figure 1). Lahat et al.14 have recently suggested that CT is an accurate and specific tool for identifying most of the dedifferentiated forms of RPLS, with no further diagnostic procedures required. Nonetheless, a preoperative histological diagnosis was obtained in four patients of the present series. Despite the increased use of abdominal imaging, most retroperitoneal tumors are still diagnosed at locally advanced stages.4,13 None of our patients had a tumor mass less than 5cm in diameter, and the mean maximum tumor size either by preoperative imaging (22.8±8.1cm) or by operative findings (23±10.8cm) was slightly larger than those reported by other authors.3,15 Local progression associated to progressive cachexia and multifocal intestinal obstruction is almost invariably responsible for mortality in patients with RPLS2. Liposarcoma appears to have less of a predilection for distant metastases than other retroperitoneal soft tissue sarcomas.2,16 In previously published large series of RPLS the incidence of distant metastatic disease ranged from 5% to 37%, mainly located in the lungs and liver.2,4–6,17 In the present study, we have found only one case of myxoid RPLS with well-documented metastatic dissemination to the pleural space (patient 2).
To date, surgery remains the mainstay of therapy for retroperitoneal sarcomas, and all patients with potentially respectable RPLS should undergo laparotomy with intention to cure.3 However, the rate of complete resection varies widely in the literature from 65% to 95%.4,9,18 Several factors that may make complete resection difficult or impossible include major vasculature involvement, peritoneal spread, or distant metastasis. Microscopically negative resection margins (R0) were achieved in 40% of our patients, similarly to previous studies.8,9,15,17 In most of the series concerning retroperitoneal sarcomas no survival benefit had been observed in patients undergoing partial excision with gross positive margins (R2).16 However, Shibata et al.2 recently suggested that incomplete surgical resection with a debulking procedure may provide prolongation in survival with successful symptomatic palliation in select patients with unresectable RPLS, compared with those who had only biopsy. “En bloc” resection of the tumoral mass with the adjacent involved organs (kidney, hemicolon, or pelvic adnexa) was performed in half of the patients in this study. This rate is consistent with those reported by other authors, such as Doglietto et al.9 (45%) or Neuhaus et al.5 (57%).
Although the addition of adjuvant therapy to surgical excision appears to provide a modest improvement in the recurrence-free survival rate for sarcomas arising in the extremities, the role of chemotherapy for soft tissue sarcomas at other locations remains controversial.2,5,16 Only two drugs, doxorubicin and ifosfamide, have demonstrated a relatively consistent single-agent activity, with response rates ranging from 10% to 25%. In the present series, two patients with R1−R2 incomplete resection received adjuvant chemotherapy (including doxorubicin, epirubicin, ifosfamide, and mesna) as part of the initial therapeutic approach. Both cases had a good performance status and no major comorbid conditions. One of them had undergone partial tumor resection with macroscopically positive surgical margins and suffered from local recurrence at 41 months (patient 8). Preoperative or neoadjuvant chemotherapy may have some advantages over postoperative chemotherapy, including the possibility to monitor response and alter or terminate therapy in patients who do not appear to be deriving any benefit. Extrapolating from evidence of improved local disease control for sarcomas of the extremities, adjuvant radiotherapy has been occasionally used in RPLS.7 However, substantial morbidity is associated in this setting because of the proximity of dose-limiting structures, such as the small intestine, spleen and bone marrow.5,16 Not surprisingly, only one patient in our experience received postoperative radiotherapy in spite of achieving complete surgical resection (patient 9). In view of the available evidence, the FNCLCC concluded in the 2006 updated recommendations for the radiotherapeutic management of soft tissue sarcoma that no systematic irradiation should be done in patients with retroperitoneal sarcoma.19 Successful treatment of recurrent RPLS using CT-guided radiofrequency ablation has been recently reported and appears to be a feasible therapeutic approach for patients with previous surgery.20
RPLS is characterized by a high rate of local recurrence even when all macroscopic disease has been apparently removed, and ranged from 41% to 80% according to previous reports.5,15 Most tumor recurrences occur within a few years of initial surgery, as we found in the present study (with a median time of recurrence of 34.8 months), although local failure can continue to occur beyond 5–10 years following resection.16 The role of complete resection of retroperitoneal sarcomas has been well established as a major determinant of tumor recurrence and patient prognosis.3,8,17,21 Accordingly, the patients in the present study who underwent complete resection with negative surgical margins exhibited a near-significant trend toward longer recurrence-free survival than those who did not undergo such treatment. Higher tumor grade4,5,8,9 or dedifferentiated histology15,17are also prognostic variables reported in the literature. In a large cohort of 72 patients with primary RPLS, Neuhaus et al.5 identified low tumor grade and macroscopic clearance of tumor as the only variables significantly associated with a decrease in the rate of local recurrence. In a recent study, Lahat et al.15 demonstrated that well-differentiated and dedifferentiated subtypes of RPLS have distinct biological behaviours, with significant differences in resectability rate, recurrence-free survival, and overall survival. Therefore, the authors emphasize the need for different therapeutic approaches, treating both subtypes as two distinct disease entities. Finally, tumor size has not been identified as predictor of survival since virtually all retroperitoneal sarcomas are diagnosed at a considerable size (usually larger than 5cm), as exemplified by the present study and others.2,4,5,8
In conclusion, RPLS constitutes an uncommon malignancy often diagnosed at an advanced stage due to the lack of early symptoms. Complete surgical excision is likely to offer the best chance for long-term survival, and concomitant organ resection is frequently required to achieve tumor clearance. However, the high recurrence rate makes the prognosis of these patients poor.
Conflict of interestThe authors declare that they do not have any conflict of interests.






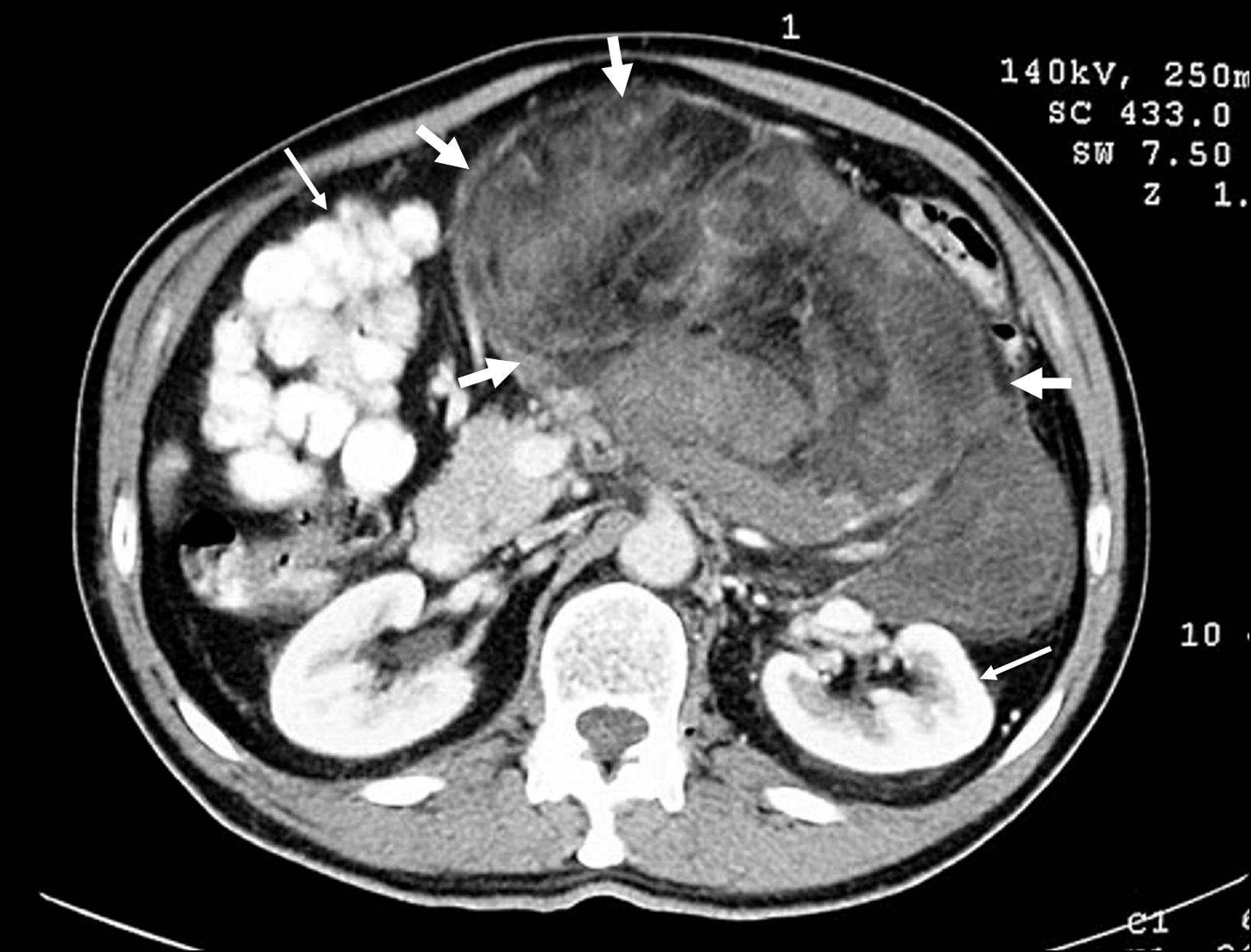
![Contrast-enhanced axial CT scan showing a large, high-grade retroperitoneal liposarcoma (thick arrows) with areas of low density due to degeneration or necrosis, and extreme displacement of adjacent organs (thin arrows) [patient 10]. Contrast-enhanced axial CT scan showing a large, high-grade retroperitoneal liposarcoma (thick arrows) with areas of low density due to degeneration or necrosis, and extreme displacement of adjacent organs (thin arrows) [patient 10].](https://static.elsevier.es/multimedia/02105705/0000003300000005/v1_201305141258/S0210570510000129/v1_201305141258/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

