The objective of this research is to identify and systematize the medical conditions generated by SARS-CoV-2 on the optic nerve and retina of young, adult, and elderly adults who suffered from COVID-19 in the period 2019−2022. A theoretical documentary review (TDR) was conducted within the framework of an investigation to determine the current state of knowledge of the subject under study. The TDR includes the analysis of publications in the scientific databases PubMed/Medline, Ebsco, Scielo and Google. A total of 167 articles were found, of which 56 were studied in depth, and these evidence the impact of COVID-19 infection on the retina and optic nerve of infected patients, both during the acute phase and in subsequent recovery. Among the reported findings, the following stand out: anterior and posterior non-arteritic ischemic optic neuropathy, optic neuritis, central or branch vascular occlusion, paracentral acute medial maculopathy, neuroretinitis, as well as concomitant diagnoses such as possible Vogt-Koyanagi-Harada disease, multiple evanescent white dot syndrome (MEWDS), Purtscher-like retinopathy, among others.
El objetivo de la presente investigación es identificar y sistematizar las afectaciones generadas por el SARS-CoV-2 en el nervio óptico y en la retina de pacientes jóvenes, adultos y adultos mayores que padecieron COVID-19 en el período 2019al 2022. Se realizó una Revisión Teórica Documental (RTD) en el marco de una investigación para determinar el estado actual del conocimiento del tema objeto de estudio. La RTD contempla el análisis de publicaciones en las bases de datos científicas PubMed/Medline, Ebsco, Scielo y Google. Se encontraron un total de 167 artículos de los cuales se estudiaron a profundidad 56 artículos, se evidencia el impacto de la infección por COVID-19 en la retina y el nervio óptico de los pacientes contagiados, tanto durante la fase aguda como en la recuperación posterior. Entre los hallazgos reportados sobresalen: Neuropatía óptica isquémica no arterítica anterior y posterior, neuritis óptica, oclusión vascular central o de rama, maculopatía medial aguda paracentral, neurorretinitis, así como también diagnósticos concomitantes como Enfermedad posible de Vogt Koyanagi Harada, Síndrome de Múltiples Puntos Blancos Evanescentes (MEWDS), Retinopatía Purtscher-like, y otros.
December 2019 saw the outbreak of severe acute respiratory syndrome caused by the SARS-CoV-2 virus in Wuhan Province, China, which was subsequently declared a pandemic on 30 January 2020 by Dr Tedros Adhanom Ghebreyesus, head of the World Health Organization (WHO), and became a major global public health problem.
SARS-CoV-2 is an enveloped β-coronavirus, with a genetic sequence very similar to SARS-CoV-1 (80 %) and bat coronavirus RaTG13 (96,2 %).1 Its viral coat is covered by spike (S) glycoprotein, envelope (E) and membrane (M) proteins. The first step in the infectious process is the binding of the virus to a host cell via its target receptor. The S1 subunit of the S protein contains the receptor-binding domain that binds to the peptidase domain of angiotensin-converting enzyme 2 (ACE 2). In SARS-CoV-2, the S2 subunit is highly conserved and is considered a potential antiviral target. According to the WHO, COVID-19 is a disease caused by the coronavirus known as SARS-CoV-2 whose uncertain behaviour and diverse clinical course with an as yet poorly understood mechanism of invasion has created an urgent need for global multi-centre and multidisciplinary clinical studies to understand and evaluate its origin, methods of diagnosis, disease course, methods of prevention, treatment and management of post-infection sequelae.
SARS-CoV-2 is now known to penetrate host cells via the angiotensin-converting enzyme receptor 2 (ACE2), which manifests in a variety of tissues, including vascular endothelium and neurosensory retina.2,3 Although its original symptomatology is associated with the development of a respiratory syndrome that includes fever, cough, odynophagia, rhinorrhoea, general malaise, among others, an extensive and varied spectrum of clinical manifestations affecting other anatomical structures has also been reported and described. Studies have reported an increase in arterial and venous thromboembolism in individuals infected with COVID-19, which could be associated with direct viral invasion and secondary inflammation generated in vascular endothelial cells.4
After infection with SARS-CoV-2, approximately 30% of patients also have ocular involvement such as "conjunctivitis, conjunctival hyperemia, conjunctival follicular nodulations, red or dry eye, chemosis, lacrimation, ocular pain, epiphora, photophobia, blurred vision, keratoconjunctivitis, microhemorrhages, summarised as changes affecting both the anterior and posterior segments",5 which have been verified by biomicroscopic examination and complementary examinations such as optical coherence tomography.
Retinal ischaemic changes such as flame hemorrhages, cotton-wool spots and sectoral pallor have been reported in patients post SARS-CoV-2 infection.6 Several studies on the microvascular manifestations in the retina secondary to SARS-CoV-2 infection have recently been published, showing that mean macular capillary vessel density was significantly lower and with low levels of peripapillary perfusion density in patients with COVID-19 compared to age-matched normal controls, but without evidence of infection.7,8 On the other hand, the scientific literature has reported central nervous system (CNS) involvement by COVID-19 infection and the neurotropic potential of SARS-CoV-2.9 The neurological symptoms and complications in COVID-19, however, are few and inconclusive, and no long-term follow-up has been performed to date, and it has been reported that it can cause optic nerve edema.10 and it is known that when the nerve sheath is dilated, from a neurological perspective, COVID-19 has an impact on the alterations of this structure.11,12
In view of the diversity of clinical findings reported caused by this virus, the need and importance of this literature review is justified. The aim of this review is to compile and unify the findings of studies and clinical cases published in the scientific literature. This review aims to alert ophthalmologists to the need to evaluate these ophthalmological sequelae following SARS-CoV2 infection, which could contribute to preventing the possible progression of pre-existing ophthalmological pathologies affecting the retina and optic nerve.
MethodsA Theoretical Documentary Review (TDR) was carried out as part of a research project to determine the current state of knowledge on the subject under study. The TDR contemplates the analysis of publications on alterations in the optic nerve and retina in patients with COVID-19, published in the period from 2019 to 2022, in the scientific databases PubMed/Medline, Ebsco, Scielo and Google Scholar, considering clinical cases, review articles, clinical trials and studies of epidemiological approaches that allow establishing the different affectations of the optic nerve and retina in patients with the clinical entity studied.
The descriptors used for TDR are: SARS-CoV-2, COVID-19, retina and optic nerve. The following MESH terms and Boolean operators were used for the information selection process:
(COVID-19) AND (OPTIC NERVE) NOT (VACCINE) NOT (CHILDREN)
(COVID-19) AND (RETINA) NOT (VACCINE) NOT (CHILDREN)
The languages used for consultation in the selected databases are English and Spanish.
Inclusion criteriaThe information was selected with reference to the criteria mentioned below:
- -
Articles containing information on young, adult and older adult patients with a confirmed diagnosis of COVID-19 or post COVID-19
- -
Articles describing alterations in the optic nerve and retina
- -
Information described in the medical literature between 2019 and 2022 including clinical case reports, review articles, case series, observational, cross-sectional, prospective, retrospective, cohort, case-control, and case-control studies.
- -
Pediatric patients or under 18 years of age
- -
Patients with retinal and optic nerve alterations secondary to other pathologies not related to COVID-19.
- -
Conference abstracts, letters, duplicate publications, unfinished or in-progress research
- -
Patients with COVID-19 post-vaccine manifestations
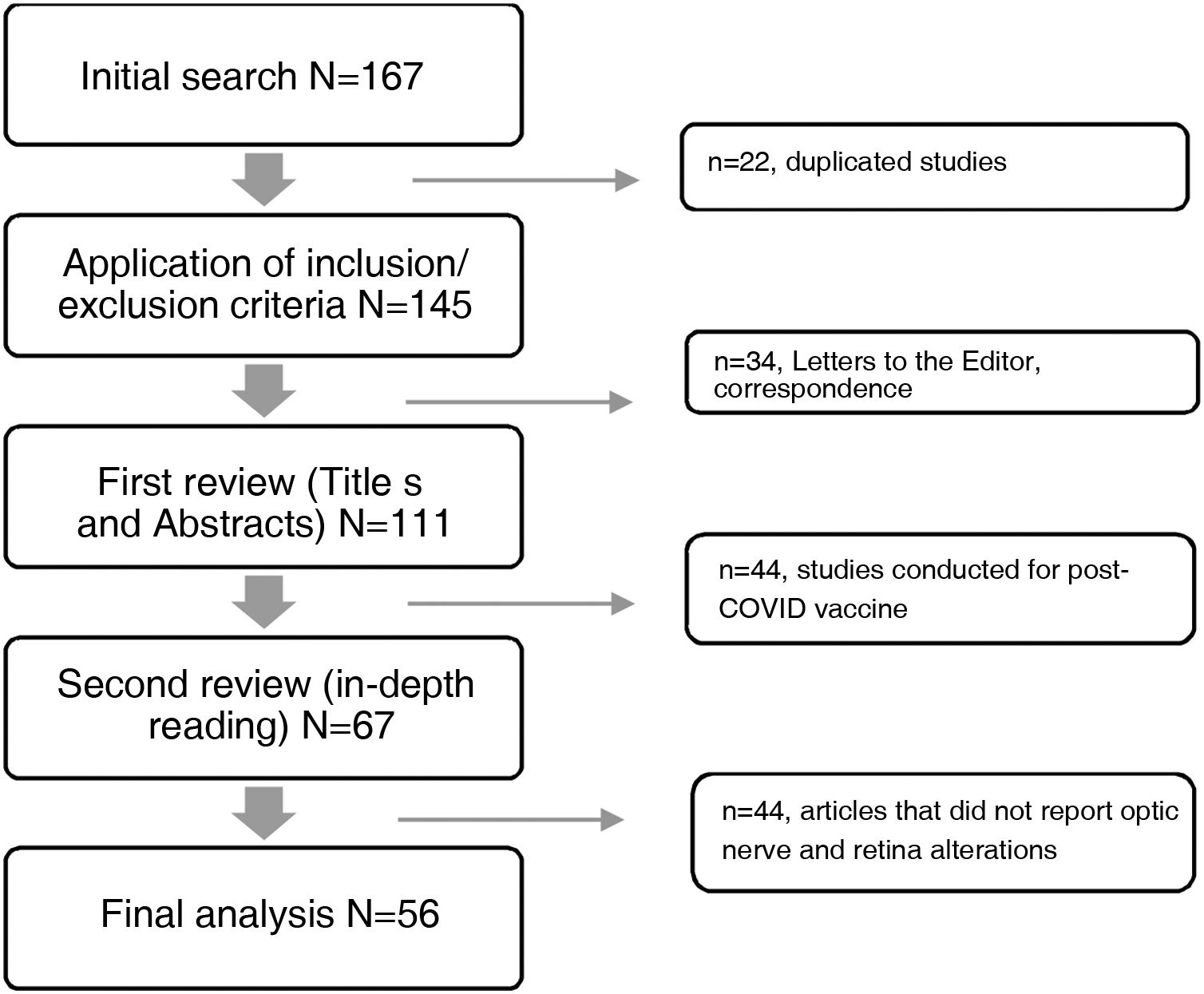
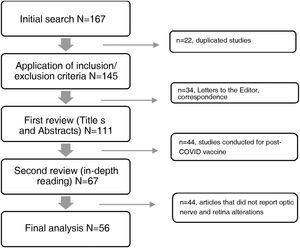
The databases selected for consultation published a total of 167 articles in the period under study, 111 of which were excluded for not meeting the selection criteria, while 56 articles met the criteria and were included (Fig. 1). A data matrix was created with the following aspects evaluating the following variables: year, title of the study, objective of the research and results, which allows the presentation of the most relevant findings.
Ethical considerationsThe present research is a TDR on publications that meet the ethical criteria of the Helsinki declaration on digital platforms circulated on the web for open and unrestricted consultation.
ResultsFrom the information collected, 56 articles were analysed in detail in two matrices describing the findings in retina and optic nerve. The first refers to the 22 case reports, and the second details the information collected from the 34 review articles, case series, observational, cross-sectional, prospective, retrospective, cohort, case-control and retrospective studies.
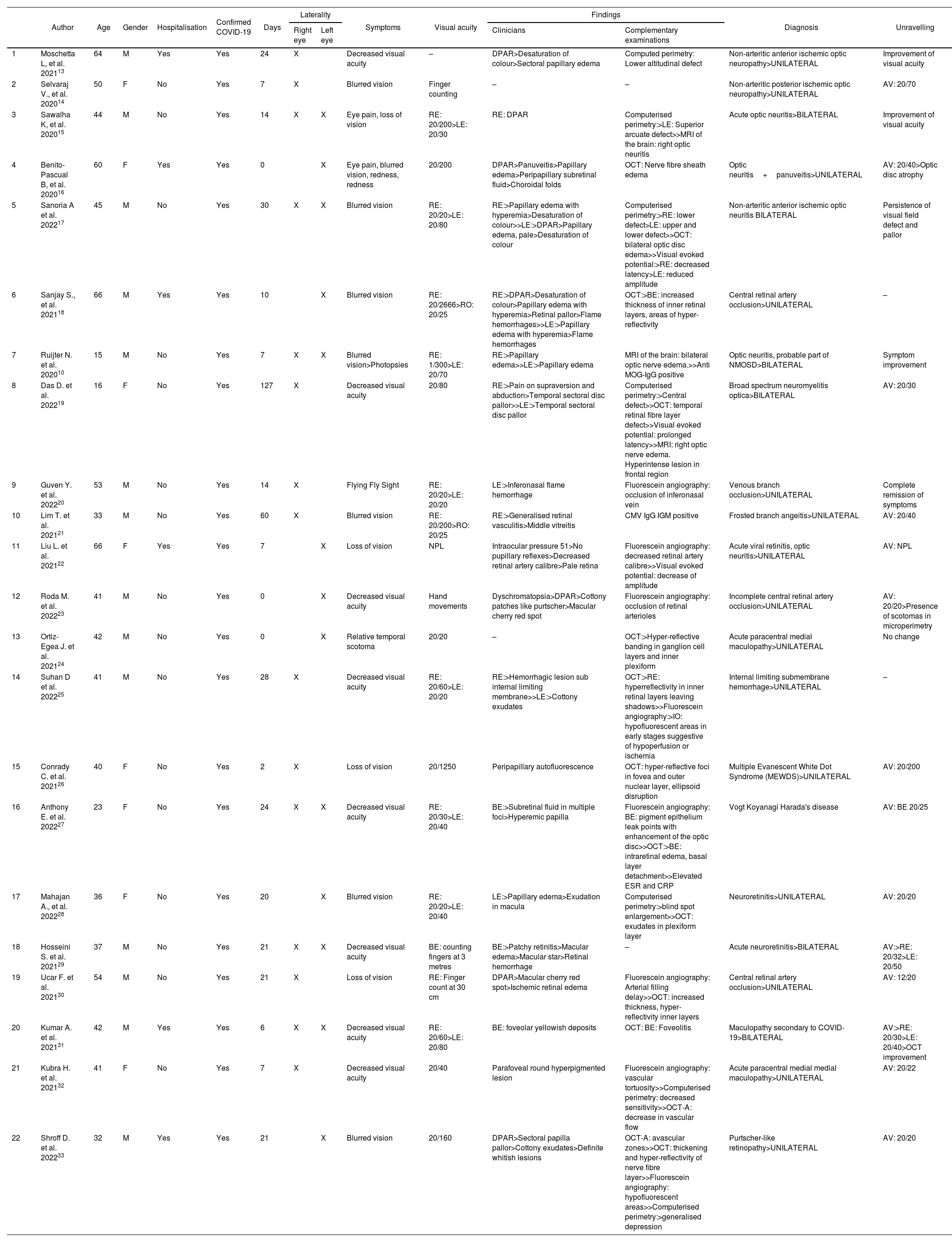
In Table 1, of the 22 case reports with a total of 28 affected eyes, the mean age was 42 years ± 14.32, with a predominance of male patients 63.6% (n=14). Of the total number of cases, 27.3% required hospitalisation, with an average of 28.11±6.39 days between the onset of COVID-19 symptoms and the onset of ophthalmological pathology. With regard to laterality, a higher incidence of involvement in the right eye was determined with 9 cases, 7 cases in the left eye and 6 cases bilaterally. Among the symptomatology expressed by the patients, decreased visual acuity and ocular pain were found. Visual acuity at the time of initial evaluation was less than 20/70, with 3 cases presenting vision of 20/40. Clinical findings included, in order of frequency: relative afferent pupillary defect, altered colour vision, sectorial or total papilla edema, optic atrophy, flame hemorrhages, generalised retinal vasculitis, decreased retinal vascular calibre, cotton-wool spots, sub-retinal fluid, pale retina, macular cherry red spot. In the visual field, campimetric defects were found such as altitudinal defects, arcuate, central, increased blind spot, and decreased sensitivity, without a predilection for laterality of the visual field. Optical coherence tomography showed nerve fibre layer edema, loss of nerve fibre layer in the temporal area, hyper-reflectivity of the retinal layers, presence of exudates, decreased vascular flow and avascular areas. Fluorescein angiography showed decreased vascular calibre and tortuosity, vascular occlusions, areas of hypoperfusion or ischaemia, leakage points in the pigment epithelium.
Characteristics of COVID-19 clinical cases with alterations in the optic nerve and retina.
| Author | Age | Gender | Hospitalisation | Confirmed COVID-19 | Days | Laterality | Symptoms | Visual acuity | Findings | Diagnosis | Unravelling | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right eye | Left eye | Clinicians | Complementary examinations | |||||||||||
| 1 | Moschetta L, et al. 202113 | 64 | M | Yes | Yes | 24 | X | Decreased visual acuity | – | DPAR>Desaturation of colour>Sectoral papillary edema | Computed perimetry: Lower altitudinal defect | Non-arteritic anterior ischemic optic neuropathy>UNILATERAL | Improvement of visual acuity | |
| 2 | Selvaraj V., et al. 202014 | 50 | F | No | Yes | 7 | X | Blurred vision | Finger counting | – | – | Non-arteritic posterior ischemic optic neuropathy>UNILATERAL | AV: 20/70 | |
| 3 | Sawalha K, et al. 202015 | 44 | M | No | Yes | 14 | X | X | Eye pain, loss of vision | RE: 20/200>LE: 20/30 | RE: DPAR | Computerised perimetry:>LE: Superior arcuate defect>>MRI of the brain: right optic neuritis | Acute optic neuritis>BILATERAL | Improvement of visual acuity |
| 4 | Benito-Pascual B, et al. 202016 | 60 | F | Yes | Yes | 0 | X | Eye pain, blurred vision, redness, redness | 20/200 | DPAR>Panuveitis>Papillary edema>Peripapillary subretinal fluid>Choroidal folds | OCT: Nerve fibre sheath edema | Optic neuritis+panuveitis>UNILATERAL | AV: 20/40>Optic disc atrophy | |
| 5 | Sanoria A et al. 202217 | 45 | M | No | Yes | 30 | X | X | Blurred vision | RE: 20/20>LE: 20/80 | RE:>Papillary edema with hyperemia>Desaturation of colour>>LE:>DPAR>Papillary edema, pale>Desaturation of colour | Computerised perimetry:>RE: lower defect>LE: upper and lower defect>>OCT: bilateral optic disc edema>>Visual evoked potential:>RE: decreased latency>LE: reduced amplitude | Non-arteritic anterior ischemic optic neuritis BILATERAL | Persistence of visual field defect and pallor |
| 6 | Sanjay S., et al. 202118 | 66 | M | Yes | Yes | 10 | X | Blurred vision | RE: 20/2666>RO: 20/25 | RE:>DPAR>Desaturation of colour>Papillary edema with hyperemia>Retinal pallor>Flame hemorrhages>>LE:>Papillary edema with hyperemia>Flame hemorrhages | OCT:>BE: increased thickness of inner retinal layers, areas of hyper-reflectivity | Central retinal artery occlusion>UNILATERAL | – | |
| 7 | Ruijter N. et al. 202010 | 15 | M | No | Yes | 7 | X | X | Blurred vision>Photopsies | RE: 1/300>LE: 20/70 | RE:>Papillary edema>>LE:>Papillary edema | MRI of the brain: bilateral optic nerve edema.>>Anti MOG-IgG positive | Optic neuritis, probable part of NMOSD>BILATERAL | Symptom improvement |
| 8 | Das D. et al. 202219 | 16 | F | No | Yes | 127 | X | Decreased visual acuity | 20/80 | RE:>Pain on supraversion and abduction>Temporal sectoral disc pallor>>LE:>Temporal sectoral disc pallor | Computerised perimetry:>Central defect>>OCT: temporal retinal fibre layer defect>>Visual evoked potential: prolonged latency>>MRI: right optic nerve edema. Hyperintense lesion in frontal region | Broad spectrum neuromyelitis optica>BILATERAL | AV: 20/30 | |
| 9 | Guven Y. et al. 202220 | 53 | M | No | Yes | 14 | X | Flying Fly Sight | RE: 20/20>LE: 20/20 | LE:>Inferonasal flame hemorrhage | Fluorescein angiography: occlusion of inferonasal vein | Venous branch occlusion>UNILATERAL | Complete remission of symptoms | |
| 10 | Lim T. et al. 202121 | 33 | M | No | Yes | 60 | X | Blurred vision | RE: 20/200>RO: 20/25 | RE:>Generalised retinal vasculitis>Middle vitreitis | CMV IgG IGM positive | Frosted branch angeitis>UNILATERAL | AV: 20/40 | |
| 11 | Liu L. et al. 202122 | 66 | F | Yes | Yes | 7 | X | Loss of vision | NPL | Intraocular pressure 51>No pupillary reflexes>Decreased retinal artery calibre>Pale retina | Fluorescein angiography: decreased retinal artery calibre>>Visual evoked potential: decrease of amplitude | Acute viral retinitis, optic neuritis>UNILATERAL | AV: NPL | |
| 12 | Roda M. et al. 202223 | 41 | M | No | Yes | 0 | X | Decreased visual acuity | Hand movements | Dyschromatopsia>DPAR>Cottony patches like purtscher>Macular cherry red spot | Fluorescein angiography: occlusion of retinal arterioles | Incomplete central retinal artery occlusion>UNILATERAL | AV: 20/20>Presence of scotomas in microperimetry | |
| 13 | Ortiz-Egea J. et al. 202124 | 42 | M | No | Yes | 0 | X | Relative temporal scotoma | 20/20 | – | OCT:>Hyper-reflective banding in ganglion cell layers and inner plexiform | Acute paracentral medial maculopathy>UNILATERAL | No change | |
| 14 | Suhan D et al. 202225 | 41 | M | No | Yes | 28 | X | Decreased visual acuity | RE: 20/60>LE: 20/20 | RE:>Hemorrhagic lesion sub internal limiting membrane>>LE:>Cottony exudates | OCT:>RE: hyperreflectivity in inner retinal layers leaving shadows>>Fluorescein angiography:>IO: hypofluorescent areas in early stages suggestive of hypoperfusion or ischemia | Internal limiting submembrane hemorrhage>UNILATERAL | – | |
| 15 | Conrady C. et al. 202126 | 40 | F | No | Yes | 2 | X | Loss of vision | 20/1250 | Peripapillary autofluorescence | OCT: hyper-reflective foci in fovea and outer nuclear layer, ellipsoid disruption | Multiple Evanescent White Dot Syndrome (MEWDS)>UNILATERAL | AV: 20/200 | |
| 16 | Anthony E. et al. 202227 | 23 | F | No | Yes | 24 | X | X | Decreased visual acuity | RE: 20/30>LE: 20/40 | BE:>Subretinal fluid in multiple foci>Hyperemic papilla | Fluorescein angiography: BE: pigment epithelium leak points with enhancement of the optic disc>>OCT:>BE: intraretinal edema, basal layer detachment>>Elevated ESR and CRP | Vogt Koyanagi Harada's disease | AV: BE 20/25 |
| 17 | Mahajan A., et al. 202228 | 36 | F | No | Yes | 20 | X | Blurred vision | RE: 20/20>LE: 20/40 | LE:>Papillary edema>Exudation in macula | Computerised perimetry:>blind spot enlargement>>OCT: exudates in plexiform layer | Neuroretinitis>UNILATERAL | AV: 20/20 | |
| 18 | Hosseini S. et al. 202129 | 37 | M | No | Yes | 21 | X | X | Decreased visual acuity | BE: counting fingers at 3 metres | BE:>Patchy retinitis>Macular edema>Macular star>Retinal hemorrhage | – | Acute neuroretinitis>BILATERAL | AV:>RE: 20/32>LE: 20/50 |
| 19 | Ucar F. et al. 202130 | 54 | M | No | Yes | 21 | X | Loss of vision | RE: Finger count at 30 cm | DPAR>Macular cherry red spot>Ischemic retinal edema | Fluorescein angiography: Arterial filling delay>>OCT: increased thickness, hyper-reflectivity inner layers | Central retinal artery occlusion>UNILATERAL | AV: 12/20 | |
| 20 | Kumar A. et al. 202131 | 42 | M | Yes | Yes | 6 | X | X | Decreased visual acuity | RE: 20/60>LE: 20/80 | BE: foveolar yellowish deposits | OCT: BE: Foveolitis | Maculopathy secondary to COVID-19>BILATERAL | AV:>RE: 20/30>LE: 20/40>OCT improvement |
| 21 | Kubra H. et al. 202132 | 41 | F | No | Yes | 7 | X | Decreased visual acuity | 20/40 | Parafoveal round hyperpigmented lesion | Fluorescein angiography: vascular tortuosity>>Computerised perimetry: decreased sensitivity>>OCT-A: decrease in vascular flow | Acute paracentral medial medial maculopathy>UNILATERAL | AV: 20/22 | |
| 22 | Shroff D. et al. 202233 | 32 | M | Yes | Yes | 21 | X | Blurred vision | 20/160 | DPAR>Sectoral papilla pallor>Cottony exudates>Definite whitish lesions | OCT-A: avascular zones>>OCT: thickening and hyper-reflectivity of nerve fibre layer>>Fluorescein angiography: hypofluorescent areas>>Computerised perimetry:>generalised depression | Purtscher-like retinopathy>UNILATERAL | AV: 20/20 | |
RAPD: Relative Affterent Pupillary Defect AV: visual acuity RE: right eye LE: left eye BE: both eyes MRI: Magnetic Resonance Imaging OCT: optical coherence tomography OCT-A: angiographic optical coherence tomography NMOSD: Neuromyelitis optica spectrum disorders ESR: erythrocyte sedimentation rate CRP: C-Reactive protein.
Days between COVID-19 symptoms and onset of ophthalmologic symptoms/findings.
A wide spectrum of diagnoses were reported in the analysis: anterior and posterior non-arteritic ischemic optic neuropathy, optic neuritis, central or branch vascular occlusion, acute paracentral medial maculopathy, neuroretinitis, as well as less frequently concomitant diagnoses such as possible Vogt Koyanagi Harada disease, Multiple Evanescent White Dot Syndrome (MEWDS), Purtscher-like retinopathy.
With regard to visual acuity in the 22 cases reported in this matrix, the vast majority had improved visual acuity compared to that at diagnosis.
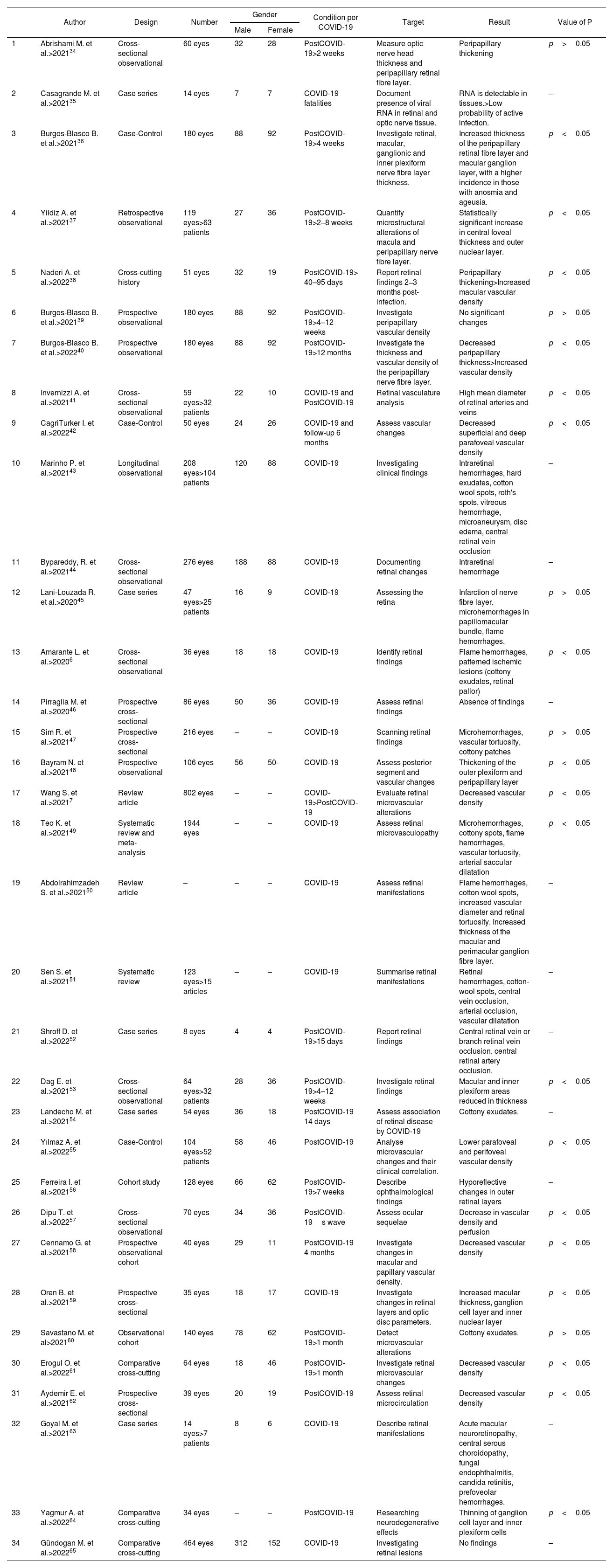
In Table 2, 34 articles were reviewed, including a total of 6177 eyes analysed, highlighting the information from the articles that reported statistical significance (p<0.05) during the symptomatic picture of COVID-19, in the ocular fundus they reported flame hemorrhages and microhemorrhages, ischemic lesions in pattern (cotton wool exudates, retinal pallor), vascular tortuosity and arterial saccular dilatation. Additionally, clinical studies that performed complementary ophthalmological diagnostic tests on patients during the active infectious stage and the recovery phase between the second week and 12 months after COVID-19 diagnosis are incorporated; most reported increased thickness of the outer retinal plexiform layer, peripapillary area, macular ganglion cell layer, and inner and outer nuclear retinal layer, as well as increased total macular thickness; other reported findings were an increase in the mean diameter of retinal arteries and veins, and a decrease in superficial and deep vascular density. Contrary to the above mentioned reports, 3 studies observed a decrease in peripapillary and macular thickness as well as an increase in vascular density.
Characteristics of included studies.
| Author | Design | Number | Gender | Condition per COVID-19 | Target | Result | Value of P | ||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
| 1 | Abrishami M. et al.>202134 | Cross-sectional observational | 60 eyes | 32 | 28 | PostCOVID-19>2 weeks | Measure optic nerve head thickness and peripapillary retinal fibre layer. | Peripapillary thickening | p>0.05 |
| 2 | Casagrande M. et al.>202135 | Case series | 14 eyes | 7 | 7 | COVID-19 fatalities | Document presence of viral RNA in retinal and optic nerve tissue. | RNA is detectable in tissues.>Low probability of active infection. | – |
| 3 | Burgos-Blasco B. et al.>202136 | Case-Control | 180 eyes | 88 | 92 | PostCOVID-19>4 weeks | Investigate retinal, macular, ganglionic and inner plexiform nerve fibre layer thickness. | Increased thickness of the peripapillary retinal fibre layer and macular ganglion layer, with a higher incidence in those with anosmia and ageusia. | p<0.05 |
| 4 | Yildiz A. et al.>202137 | Retrospective observational | 119 eyes>63 patients | 27 | 36 | PostCOVID-19>2–8 weeks | Quantify microstructural alterations of macula and peripapillary nerve fibre layer. | Statistically significant increase in central foveal thickness and outer nuclear layer. | p<0.05 |
| 5 | Naderi A. et al.>202238 | Cross-cutting history | 51 eyes | 32 | 19 | PostCOVID-19> 40–95 days | Report retinal findings 2−3 months post-infection. | Peripapillary thickening>Increased macular vascular density | p<0.05 |
| 6 | Burgos-Blasco B. et al.>202139 | Prospective observational | 180 eyes | 88 | 92 | PostCOVID-19>4–12 weeks | Investigate peripapillary vascular density | No significant changes | p>0.05 |
| 7 | Burgos-Blasco B. et al.>202240 | Prospective observational | 180 eyes | 88 | 92 | PostCOVID-19>12 months | Investigate the thickness and vascular density of the peripapillary nerve fibre layer. | Decreased peripapillary thickness>Increased vascular density | p<0.05 |
| 8 | Invernizzi A. et al.>202141 | Cross-sectional observational | 59 eyes>32 patients | 22 | 10 | COVID-19 and PostCOVID-19 | Retinal vasculature analysis | High mean diameter of retinal arteries and veins | p<0.05 |
| 9 | CagriTurker I. et al.>202242 | Case-Control | 50 eyes | 24 | 26 | COVID-19 and follow-up 6 months | Assess vascular changes | Decreased superficial and deep parafoveal vascular density | p<0.05 |
| 10 | Marinho P. et al.>202143 | Longitudinal observational | 208 eyes>104 patients | 120 | 88 | COVID-19 | Investigating clinical findings | Intraretinal hemorrhages, hard exudates, cotton wool spots, roth's spots, vitreous hemorrhage, microaneurysm, disc edema, central retinal vein occlusion | – |
| 11 | Bypareddy, R. et al.>202144 | Cross-sectional observational | 276 eyes | 188 | 88 | COVID-19 | Documenting retinal changes | Intraretinal hemorrhage | – |
| 12 | Lani-Louzada R. et al.>202045 | Case series | 47 eyes>25 patients | 16 | 9 | COVID-19 | Assessing the retina | Infarction of nerve fibre layer, microhemorrhages in papillomacular bundle, flame hemorrhages, | p>0.05 |
| 13 | Amarante L. et al.>20206 | Cross-sectional observational | 36 eyes | 18 | 18 | COVID-19 | Identify retinal findings | Flame hemorrhages, patterned ischemic lesions (cottony exudates, retinal pallor) | p<0.05 |
| 14 | Pirraglia M. et al.>202046 | Prospective cross-sectional | 86 eyes | 50 | 36 | COVID-19 | Assess retinal findings | Absence of findings | – |
| 15 | Sim R. et al.>202147 | Prospective cross-sectional | 216 eyes | – | – | COVID-19 | Scanning retinal findings | Microhemorrhages, vascular tortuosity, cottony patches | p>0.05 |
| 16 | Bayram N. et al.>202148 | Prospective observational | 106 eyes | 56 | 50- | COVID-19 | Assess posterior segment and vascular changes | Thickening of the outer plexiform and peripapillary layer | p<0.05 |
| 17 | Wang S. et al.>20217 | Review article | 802 eyes | – | – | COVID-19>PostCOVID-19 | Evaluate retinal microvascular alterations | Decreased vascular density | p<0.05 |
| 18 | Teo K. et al.>202149 | Systematic review and meta-analysis | 1944 eyes | – | – | COVID-19 | Assess retinal microvasculopathy | Microhemorrhages, cottony spots, flame hemorrhages, vascular tortuosity, arterial saccular dilatation | p<0.05 |
| 19 | Abdolrahimzadeh S. et al.>202150 | Review article | – | – | – | COVID-19 | Assess retinal manifestations | Flame hemorrhages, cotton wool spots, increased vascular diameter and retinal tortuosity. Increased thickness of the macular and perimacular ganglion fibre layer. | – |
| 20 | Sen S. et al.>202151 | Systematic review | 123 eyes>15 articles | – | – | COVID-19 | Summarise retinal manifestations | Retinal hemorrhages, cotton-wool spots, central vein occlusion, arterial occlusion, vascular dilatation | – |
| 21 | Shroff D. et al.>202252 | Case series | 8 eyes | 4 | 4 | PostCOVID-19>15 days | Report retinal findings | Central retinal vein or branch retinal vein occlusion, central retinal artery occlusion. | – |
| 22 | Dag E. et al.>202153 | Cross-sectional observational | 64 eyes>32 patients | 28 | 36 | PostCOVID-19>4–12 weeks | Investigate retinal findings | Macular and inner plexiform areas reduced in thickness | p<0.05 |
| 23 | Landecho M. et al.>202154 | Case series | 54 eyes | 36 | 18 | PostCOVID-19 14 days | Assess association of retinal disease by COVID-19 | Cottony exudates. | – |
| 24 | Yılmaz A. et al.>202255 | Case-Control | 104 eyes>52 patients | 58 | 46 | PostCOVID-19 | Analyse microvascular changes and their clinical correlation. | Lower parafoveal and perifoveal vascular density | p<0.05 |
| 25 | Ferreira I. et al.>202156 | Cohort study | 128 eyes | 66 | 62 | PostCOVID-19>7 weeks | Describe ophthalmological findings | Hyporeflective changes in outer retinal layers | – |
| 26 | Dipu T. et al.>202257 | Cross-sectional observational | 70 eyes | 34 | 36 | PostCOVID-19s wave | Assess ocular sequelae | Decrease in vascular density and perfusion | p<0.05 |
| 27 | Cennamo G. et al.>202158 | Prospective observational cohort | 40 eyes | 29 | 11 | PostCOVID-19 4 months | Investigate changes in macular and papillary vascular density. | Decreased vascular density | p<0.05 |
| 28 | Oren B. et al.>202159 | Prospective cross-sectional | 35 eyes | 18 | 17 | COVID-19 | Investigate changes in retinal layers and optic disc parameters. | Increased macular thickness, ganglion cell layer and inner nuclear layer | p<0.05 |
| 29 | Savastano M. et al>202160 | Observational cohort | 140 eyes | 78 | 62 | PostCOVID-19>1 month | Detect microvascular alterations | Cottony exudates. | p>0.05 |
| 30 | Erogul O. et al.>202261 | Comparative cross-cutting | 64 eyes | 18 | 46 | PostCOVID-19>1 month | Investigate retinal microvascular changes | Decreased vascular density | p<0.05 |
| 31 | Aydemir E. et al.>202162 | Prospective cross-sectional | 39 eyes | 20 | 19 | PostCOVID-19 | Assess retinal microcirculation | Decreased vascular density | p<0.05 |
| 32 | Goyal M. et al.>202163 | Case series | 14 eyes>7 patients | 8 | 6 | COVID-19 | Describe retinal manifestations | Acute macular neuroretinopathy, central serous choroidopathy, fungal endophthalmitis, candida retinitis, prefoveolar hemorrhages. | – |
| 33 | Yagmur A. et al.>202264 | Comparative cross-cutting | 34 eyes | – | – | PostCOVID-19 | Researching neurodegenerative effects | Thinning of ganglion cell layer and inner plexiform cells | p<0.05 |
| 34 | Gündogan M. et al.>202265 | Comparative cross-cutting | 464 eyes | 312 | 152 | COVID-19 | Investigating retinal lesions | No findings | – |
Based on the known information on the pathophysiological mechanisms used by COVID-19 to produce alterations, direct interaction between the virus and the host has been described, as well as theories suggesting indirect involvement, in which the virus may trigger an autoimmune process, vasculopathies or inflammation mediated by the viral response, mechanisms that occur individually or together at the time of infection, converging in the structural alterations detected in the structures of the posterior segment of the eyeball and in the vascular structures.48
One theory describes viral tropism for angiotensin-converting enzyme 2 receptors present in neurons, vascular endothelium and choroids.16 These are tissues present in ocular structures that, when infected by the virus, can trigger inflammatory conditions due to their direct relationship with neuronal tissue, which could explain the reported cases of optic neuritis. This is confirmed by the increased thickness of the retinal layers that would cause trans-synaptic damage which, when the inflammatory process subsides, could result in tissue atrophy observed in patients one year after suffering from the disease, as reported in the studies analysed in the present review.
With respect to the vasculopathy theory, a set of prothrombotic effects originating from endothelial dysfunction due to direct involvement of vascular tissue, associated with a state of hypercoagulability, platelet activation and stasis, results in ischemic optic neuropathy which can consequently manifest as optic nerve atrophy. Importantly, thrombotic events due to COVID-19 were evidenced in 30% of patients.18 This makes the occurrence of the event a probability of presentation due to the high incidence of the disease at present.
Another theory of endotheliopathy states a process of vasoconstriction inducing vasoplegia with transient hypoperfusion.13 which has been demonstrated by decreased vascular density and findings of vascular hypoperfusion. It is widely known that hypoperfusion of the optic nerve and retina triggers a loss of nerve fibres which, in pathologies with a similar pathophysiology, would constitute an aggravating factor of the disease, as in the case of retinopathies or glaucoma. Similarly, by affecting the receptors present in the choroid, which are known to be the mechanism of action of some drugs used for the treatment of glaucoma, the possibility of a transient or definitive poor response to the medication must be considered, leading to optic nerve damage.
SARS-CoV-2 infection is also known to cause a cytokine storm with consequent elevation of proinflammatory cytokines that provoke an exaggerated immune response causing tissue damage directly or indirectly by activating the coagulation cascade leading to the hypercoagulable state described above.28 Thus, taking into account the history of COVID-19 viral infection 1–4 weeks previously31 may present with neuroretinitis and maculopathy secondary to COVID-19.
With all this information, it is important to keep in mind that COVID-19 infection could contribute to aggravate pre-existing diseases in these structures that already maintained a previous inflammatory state, such as hypertensive retinopathy, diabetic retinopathy, age-related macular degeneration. Similarly, the alteration of vascular flow to the optic nerve generates the possibility of glaucoma progression, as well as other neurodegenerative affectations, and to evaluate the microvasculature as a non-invasive alternative for the prognosis of the underlying pathologies. Although it is also important to mention that there are other studies that do not report alterations in the retina and optic nerve secondary to COVID-19 infection, so more studies and evidence are needed to help us support these findings.
With the analysis of the existing evidence at the time of this review, it could be recommended that it is important in patient assessment to know the time elapsed since the diagnosis of SARS-CoV-2 infection to guide towards the presence of inflammatory signs or sequelae secondary to the infection.
ConclusionsAfter analysing the scientific information obtained, the importance of conducting a detailed anamnesis prior to the evaluation of a patient, inquiring about a history of COVID infection is highlighted, as this factor could be considered as a possible cause of the evolution or progression of an underlying ophthalmological pathology or a poor response to an administered treatment. In addition, the time elapsed since the infection should be analysed to confirm the ophthalmological findings obtained in the physical examination or complementary examinations. Consequently, this review allows us to establish strategies for prevention and timely management of possible alterations that may develop into sequelae, by optimising their treatment through close monitoring and follow-up of the patient.