Depression is considered one of the ten most disabling conditions on the planet, causing physical, personal and social limitations. Antidepressants are the most commonly used drug treatment for eliminating or controlling the symptoms of depression. A variety of factors can contribute to treatment abandonment, particularly severe side effects. The present article is aimed to provide information and guidelines on the development of a pharmacotherapy follow-up program for patients treated with antidepressant medication. According to this practice, the pharmacist evaluates whether the three following requirements of a proper pharmacotherapy are being observed: need, effectiveness and safety. If one of these requirements is not met, the pharmacist seeks to solve the problem, by intervening in the pharmacotherapy, within the scope of his/her skills. Pharmaceutical care is aimed to improve adherence to treatment and minimize side effects, as well as the occurrence of drug interactions. Thus, the present article presents and discusses the main strategies of pharmaceutical care to achieve the proposed objectives.
La depresión es considerada una de las diez principales causas de incapacitación en el mundo, con limitación del funcionamiento físico, personal y social. Los antidepresivos son el tratamiento administrado con más frecuencia para la remisión o el control de los síntomas de depresión. Muchos factores pueden contribuir al abandono del tratamiento, entre los que destacan los efectos adversos. El objetivo de este artículo es ofrecer información y recomendaciones para la formulación de programas de seguimiento farmacoterapéutico de pacientes tratados con antidepresivos. De acuerdo con esta estrategia, el farmacéutico valora si se cumplen los tres requisitos de una farmacoterapia apropiada: necesidad y efectividad terapéutica y tolerabilidad. Si no se satisface uno de dichos requisitos, el farmacéutico tratará de resolver el problema, con una intervención en la farmacoterapia de acuerdo con su ámbito de actuación profesional. La atención farmacéutica trata de mejorar el cumplimiento, reducir los efectos adversos y las interacciones farmacológicas. En este sentido, este artículo presenta y describe las principales estrategias de atención farmacéutica para alcanzar los objetivos propuestos.
Depression is one of the ten most disabling conditions on the planet, causing physical, personal and social limitations. However, few patients receive proper treatment and they are heavily stigmatized. The way the population identified depression symptoms and their beliefs on the etiology of depression may influence the pursuit of recovery, adherence to treatment, as well as society's attitudes and behavior towards the people who suffer with this disorder.1
Prevalence studies in several Western countries have demonstrated that depression is a frequent disorder which annual prevalence rate of the disorder in the population varies between 3% and 11%. In primary care setting the prevalence is over 10%, whereas in specific populations, such as patients with recent myocardial infarction, it is 33%, and may reach 47% in cancer patients.2
The World Health Organization identified depression as the fourth leading cause of total disease burden and the leading cause of disability worldwide. In the United States, recent samples estimate a lifetime depression prevalence of 16.2% and a 12-month prevalence of 6.6%.3 These drugs improve the depressive symptoms in 60–70%, within a month, whereas only 30% of improvement is obtained with the use of placebo. This is hardly obtained with other therapeutic approaches, except for electroconvulsive therapy.4
Some factors may adversely affect antidepressant therapy and significantly interfere with patient's adherence to treatment. These include patient's level of education, the extent to which a patient understands the information contained in the prescription, as well as information provided by the physician who prescribes the antidepressants, the conflict between the therapeutic proposal and patient's own behavior, based on personal or cultural choices, long-term treatments and undesirable side effects.5 In this context, an intervention of pharmacist and a multi-professional team is indispensable.
Many studies support the idea that the depressive patient seeks advice from his/her pharmacist concerning antidepressant medication. In Spain, issues related to central nervous system (CNS) disorders are among the eighth most frequent consultations in community pharmacies. In the United States, depression-related aspects are among the fifth most frequent consultations to the pharmacist, including effectiveness of medication and adverse effects of antidepressant drugs. On the other hand, when a pharmacotherapy follow-up program is implemented to manage depression, most patients believe that the pharmacist helps solving drug-related problems, improving adherence to treatment.6 Therefore, concerning the follow-up of patients with depression, the medical literature highlights the importance of the pharmacist's role in solving drug-related problems (DRP) or Drug-related negative clinical outcomes (DNO) increasing patient's satisfaction with treatment, improving adherence.
The important role of the clinical pharmacist in the management and follow-up of patients with depression has been demonstrated in several studies.7–10 Rosen and Holmes (1978) report that the activities of a pharmacist trained in mental health can monitor psychiatric patients stabilized at a cost of 40% of the cost of providing that service by a psychiatrist.11 Finley and colleagues (2002) conducted a follow-up study of 6 months for patients treated with antidepressants. There was no statistically significant difference regarding gender, age and chronic disease scores. However, adherence to treatment was significantly higher in the intervention group who received regular visits and phone calls from the pharmacist. There was also a reduction in the number of medical appointments in the intervention group.12 Brook and colleagues (2003) conducted a clinical follow-up of 3 months for patients with depression who used a non-tricyclic antidepressant. The control group (n=79) received no type of intervention and the case group (n=69) participated in three visits to the pharmacy and received a 25-min video about depression and treatment. At the end of follow-up there was no statistically significant difference between groups with regard to demographic variables, health status or clinical symptoms. However, patients found the video useful and the training provided by the pharmacist as well.13 In 2009, Rubio Valera et al. published a follow-up protocol for patients with depression Pharmacotherapeutic in order to evaluate the effectiveness and cost-effectiveness of the pharmacist's role in improving treatment adherence and clinical outcomes.14 In 2011, the same group published a systematic review and meta-analysis that evaluated six randomized controlled trials on the impact of pharmaceutical intervention on adherence to antidepressants. The results showed that the intervention of a pharmacist in increasing compliance is effective, but the authors suggest that further studies are conducted, especially outside the United States.15
In Brazil, only four studies have been carried out which concern pharmaceutical care. All of them were published over the past 5 years. Two studies concern exploratory descriptive epidemiology studies of patients admitted at the Psychosocial Care Centers (CAPS).16,17 However, none of the referred studies comprised pharmaceutical care per se. A third study that effectively involved pharmacotherapy follow-up had a limited scope (report of one case).18 The most comprehensive article on pharmaceutical care in Brazil was carried out by Ceresér and collaborators and included patients with bipolar disorder. In this study, 28 patients with bipolar disorder were randomly selected from an outpatient setting of a tertiary hospital in Porto Alegre. The follow-up method used was the Dáder method and the assessment was made with the Hamilton rating scale for depression and YMRS (Young Mania Rating Scale). According to the authors, 32.14% of the patients had low adherence to the treatment and half of them had better adherence to treatment after the pharmacotherapy follow-up program.19
Due to the lack of information on pharmaceutical care, as well as the growing interest on the subject in Brazil and other Latin American countries, the present article is aimed to provide information and guidelines for the development of pharmacotherapy follow-up of patients treated with antidepressants.
Pharmacotherapy follow-up program of patients treated with antidepressantsThe national consensus in Brazilian Pharmaceutical Care published in 2002 sought to define the elements of the practice of pharmaceutical care. Six macro components were defined in this consensus, as follows: health care education, pharmaceutical guidance and assistance, dispensing, systematic records of the activities, measurement and assessment of results, and pharmacotherapy follow-up.20
According to the Dáder method, the main reference upon which the present article is based, Pharmacotherapy Follow-up is: “a clinical practice aimed to the continuous monitoring and assessment of patient's pharmacotherapy in order to improve the health system performance”.21
The main purpose of pharmacotherapy follow-up is to ensure the effectiveness and safety of the treatment through the detection, prevention and/or resolution of DRPs and DNOs. According to the III Consensus of Granada (2007). The DRPs are “those situations where the use of the drug causes or may cause an negative clinical outcomes associated with drug”. The DRPs may have several causes: drug misadministration, improper maintenance, contraindication, dose, dosage or duration of the improper treatment, duplicity, dispensing errors, non adherence to treatment, other health problems that interfere with the treatment, probability of severe side effects, health problem insufficiently treated, among others. The DNOs are situations associated with the use or misuse of drugs. These are classified into six categories according to the III Consensus of Granada (2007): (a) untreated health problem: the patient requires medication to treat a health condition but does not take this medication; (b) effect of unnecessary treatment: the patient suffers from a health condition associated with unnecessary treatment; (c) ineffectiveness of drug (not for dosing): the patient suffers from a health condition associated with ineffectiveness of drug that is not related to the dosing; (d) quantitative ineffectiveness of drug: the patient suffers from a health condition associated with quantitative (dosing) ineffectiveness of drug; (e) unsafety of drugs (not for dosing): the patient suffers from a health problem associated with the lack of safety of the drug (not for dosing); (f) quantitative unsafety of the drug. The patient suffers from a health problem associated with quantitative (dosing) lack of safety of the drug.22
According to Fridman (2001) and Fridman and Filinger (2002), pharmaceutical care to patients with neuropsychiatric problems should focus on the following aspects: (a) management of the most frequent adverse drug reactions (ADRs); (b) concomitant diseases that may influence the proper treatment of the mental disorder; (c) adherence to the treatment; (d) possible occurrence of drug–drug or drug–food interactions that may harm the effectiveness of the pharmacotherapy; (e) provide guidance on specific care concerning the prescribed medication; (f) the use of psycho-active drugs by risk groups (pregnant women, lactating women, elderly, and children); (g) use of over-the-counter (OTC; because abbreviation for over the counter, pertaining to a drug available without a prescription) drugs that may interact with the prescribed drugs; (h) monitoring and follow-up of the drug treatment; (i) technical factors: need to change the route of drug administration, pharmaceutical form the drug cannot be supplied in a given pharmaceutical form; (j) provide information to the patient, family members and those who provide care.23,24
Thus, the objectives to be met by pharmaceutical care for this type of patient concern the improvement of the quality of life of this patient. So, a better physician-patient collaboration is essential, as well as observing and analyzing the possible complications of the different neuropsychiatric disorders and emphasizing to the patient the need to observe those aspects related to increased treatment efficiency.23
Assessment of the needThe first assumption to be met in pharmaceutical care is that pharmacotherapy must be necessary. Therefore, there are two DNOs that the pharmacist must seek in patients with depression receiving drug treatment.
The first DNO concerns the patient who does not take medication needed to treat a real health condition.21 In a one to one conversation between pharmacist and patient, the former can identify health problems that are not being treated, such as, e.g. pyrosis caused by the SSRIs. On the other hand, the patient may have a health problem associated with administration of unnecessary treatment.21 This situation occurs in self-medication, e.g. the use of phytotherapeutic medication, such as Valeriana officinallis, Kawa kawa, Hypericum perforatum, or even allopathic drugs, although restricted by ordinance no. 344/98 (National Agency of Sanitary Surveillance – ANVISA, Brazil; note that resolution that sets standards for prescription and dispensing of medicines containing psychotropic substances), are sold in many drug stores without prescription. In such cases the pharmacist shall immediately suspend the treatment, inform the patient on the risks of using such drugs without medical advice, and refer the patient to a physician.
Assessment of effectivenessA drug is considered effective when it meets the therapeutic objective intended for patient's clinical situation. For example, an antihypertensive treatment administered to a hypertensive patient with type 2 diabetes is effective when the values for arterial blood pressure are kept below 130/80mmHg during the 24h of the day. Nevertheless, in some circumstances, a treatment can be considered effective, even when the therapeutic objectives are not met, e.g., when it is difficult to control the health problem, and the drug administered is significantly improving patient's health.21
After assessing the need for the drug, the pharmacist shall also assess the effectiveness of the adopted treatment. The pharmacist may identify two types of DNO: Ineffectiveness of drug (not for dosing), where the drug does not produce the desired effect on the patient. The second DNO concerns the quantitative ineffectiveness of the drug. This can only occur due to low dosage, drug–drug or drug–food interactions.21
According to Nieto and Manrique (2005) and Morente and Gastelurrutia (2003), prior to assessing the effectiveness of the recommended antidepressant treatment, and infer the ineffectiveness of this treatment, the following information must be checked: (a) find out if the patient has actually been diagnosed with depression; (b) find out the amount of information on the treatment that was given to the patient; (c) find out if the latency period has elapsed. All antidepressants take two or three weeks to take effect; (d) find out if dosing alterations occurred recently; (e) find out the level of patient adherence to the proposed treatment.6,25
There are several causes of poor adherence to treatment; usually, factors such as live alone, have low socioeconomic status, complex dosing regimen, negative attitude of the patient towards the pathology and treatment are the main conditions of poor adherence to the drug treatment.23,26
Other factors that influence adherence to treatment are: the relationship between the patient and the healthcare team; the degree of sanitary information received.23
It is important to inform the patient on symptomatic improvement and on the need to continue the treatment even after the symptoms have disappeared. Many patients affirm they ignored the fact that they should resume the treatment.25,27 Ribeiro and Poço, 2006 in their study titled: Reasons for abandoning the treatment in a public mental healthcare system found that 57.7% of the patients did not seek treatment again. Of these, 35% reported improvement and/or that the treatment was no longer necessary, whereas 19% considered the treatment improper or ineffective.28
One of the instruments that the pharmacist can use to assess the effectiveness of the treatment during the pharmacotherapy follow-up program are the depression assessment scales such as the Beck and Hamilton scales.29 Once a DNO of ineffectiveness is identified, the pharmacist may take steps to solve it with the help of a prescribing physician or multidisciplinary team.21
According to the guidelines for depression treatment, the strategies of interventions that must be used when a patient does not respond to antidepressant treatment are: (a) dose increase; (b) potentialization; (C) association of antidepressant drugs; (d) change of antidepressant medication; (e) electroconvulsive therapy; (f) associated psychotherapy.2
Safety assessmentAfter assessing the need and effectiveness of the treatment, the pharmacist shall assess the safety of the proposed treatment. The pharmacist may identify two types of DNO: non quantitative (not for dosing) unsafety, where the patient has a health problem related to the occurrence of adverse drug reactions (ADRs); or quantitative unsafety where the patient has a health problem caused by high dose of the medication or due to drug–drug or drug–food interactions.21
It is important that the patient and/or family members understand the nature of the disorder and what can be expected with the use of the proposed treatments; many patients that take antidepressants feel like suspending the treatment because they are not adequately informed on the undesired effects that may follow.6,25
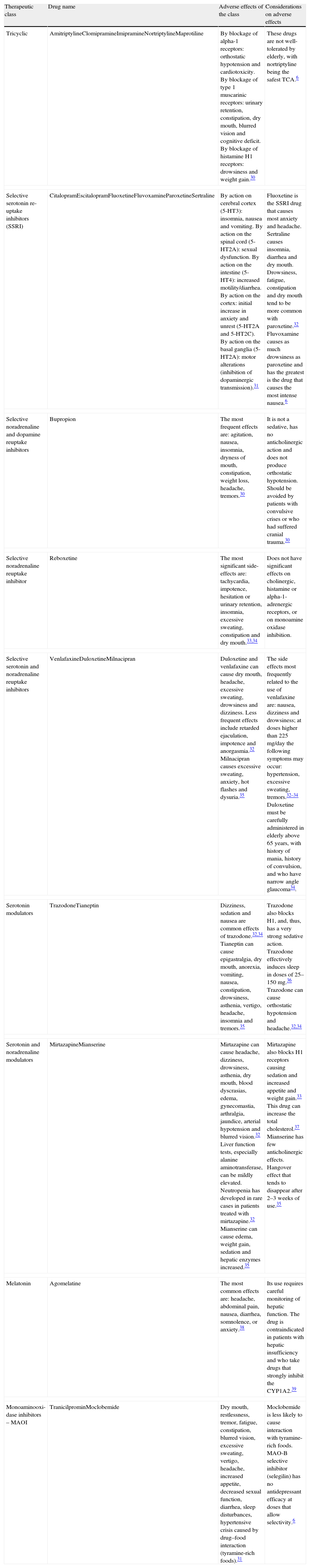
The most common troublesome side-effects of antidepressants are: dry mouth, constipation, sedation, nausea, double vision, restlessness or insomnia, and alterations in sexual function. The knowledge of the most frequent undesired side effects makes it possible for the pharmacist to perform the actions necessary to eliminate or minimize such effects.6 Some of these effects are listed in Tables 1 and 2.
Undesirable side effect profile of classes of antidepressants and particularities concerning the undesired side effects of each drug.
| Therapeutic class | Drug name | Adverse effects of the class | Considerations on adverse effects |
| Tricyclic | AmitriptylineClomipramineImipramineNortriptylineMaprotiline | By blockage of alpha-1 receptors: orthostatic hypotension and cardiotoxicity. By blockage of type 1 muscarinic receptors: urinary retention, constipation, dry mouth, blurred vision and cognitive deficit. By blockage of histamine H1 receptors: drowsiness and weight gain.30 | These drugs are not well-tolerated by elderly, with nortriptyline being the safest TCA.6 |
| Selective serotonin re-uptake inhibitors (SSRI) | CitalopramEscitalopramFluoxetineFluvoxamineParoxetineSertraline | By action on cerebral cortex (5-HT3): insomnia, nausea and vomiting. By action on the spinal cord (5-HT2A): sexual dysfunction. By action on the intestine (5-HT4): increased motility/diarrhea. By action on the cortex: initial increase in anxiety and unrest (5-HT2A and 5-HT2C). By action on the basal ganglia (5-HT2A): motor alterations (inhibition of dopaminergic transmission).31 | Fluoxetine is the SSRI drug that causes most anxiety and headache. Sertraline causes insomnia, diarrhea and dry mouth. Drowsiness, fatigue, constipation and dry mouth tend to be more common with paroxetine.32 Fluvoxamine causes as much drowsiness as paroxetine and has the greatest is the drug that causes the most intense nausea.6 |
| Selective noradrenaline and dopamine reuptake inhibitors | Bupropion | The most frequent effects are: agitation, nausea, insomnia, dryness of mouth, constipation, weight loss, headache, tremors.30 | It is not a sedative, has no anticholinergic action and does not produce orthostatic hypotension. Should be avoided by patients with convulsive crises or who had suffered cranial trauma.30 |
| Selective noradrenaline reuptake inhibitor | Reboxetine | The most significant side-effects are: tachycardia, impotence, hesitation or urinary retention, insomnia, excessive sweating, constipation and dry mouth.33,34 | Does not have significant effects on cholinergic, histamine or alpha-1-adrenergic receptors, or on monoamine oxidase inhibition. |
| Selective serotonin and noradrenaline reuptake inhibitors | VenlafaxineDuloxetineMilnacipran | Duloxetine and venlafaxine can cause dry mouth, headache, excessive sweating, drowsiness and dizziness. Less frequent effects include retarded ejaculation, impotence and anorgasmia.32 Milnacipran causes excessive sweating, anxiety, hot flashes and dysuria.35 | The side effects most frequently related to the use of venlafaxine are: nausea, dizziness and drowsiness; at doses higher than 225mg/day the following symptoms may occur: hypertension, excessive sweating, tremors.32–34 Duloxetine must be carefully administered in elderly above 65 years, with history of mania, history of convulsion, and who have narrow angle glaucoma34. |
| Serotonin modulators | TrazodoneTianeptin | Dizziness, sedation and nausea are common effects of trazodone.32,34 Tianeptin can cause epigastralgia, dry mouth, anorexia, vomiting, nausea, constipation, drowsiness, asthenia, vertigo, headache, insomnia and tremors.35 | Trazodone also blocks H1, and, thus, has a very strong sedative action. Trazodone effectively induces sleep in doses of 25–150mg.36 Trazodone can cause orthostatic hypotension and headache.32,34 |
| Serotonin and noradrenaline modulators | MirtazapineMianserine | Mirtazapine can cause headache, dizziness, drowsiness, asthenia, dry mouth, blood dyscrasias, edema, gynecomastia, arthralgia, jaundice, arterial hypotension and blurred vision.32 Liver function tests, especially alanine aminotransferase, can be mildly elevated. Neutropenia has developed in rare cases in patients treated with mirtazapine.32 Mianserine can cause edema, weight gain, sedation and hepatic enzymes increased.35 | Mirtazapine also blocks H1 receptors causing sedation and increased appetite and weight gain.33 This drug can increase the total cholesterol.37 Mianserine has few anticholinergic effects. Hangover effect that tends to disappear after 2–3 weeks of use.35 |
| Melatonin | Agomelatine | The most common effects are: headache, abdominal pain, nausea, diarrhea, somnolence, or anxiety.38 | Its use requires careful monitoring of hepatic function. The drug is contraindicated in patients with hepatic insufficiency and who take drugs that strongly inhibit the CYP1A2.39 |
| Monoaminooxi-dase inhibitors – MAOI | TranicilprominMoclobemide | Dry mouth, restlessness, tremor, fatigue, constipation, blurred vision, excessive sweating, vertigo, headache, increased appetite, decreased sexual function, diarrhea, sleep disturbances, hypertensive crisis caused by drug–food interaction (tyramine-rich foods).31 | Moclobemide is less likely to cause interaction with tyramine-rich foods. MAO-B selective inhibitor (selegilin) has no antidepressant efficacy at doses that allow selectivity.6 |
Main drug–drug interactions of antidepressants.
| Therapeutic class | Drug name | Most important interactions |
| Tricyclics | AmitriptylineClomipramineImipramineNortriptylineMaprotiline | With direct-acting sympathomimetic agents: it may cause arrhythmias and hypertension. Associated to clonidine, it may reduce the antihypertensive effect of this drug. With phenotiazines: psychosis and/or agitation may occur due to additive anticholinergic effects.31 Barbiturates may increase and cimetidine may reduce TCA metabolism. The concomitant use of phenytoin and TCA may result in increased phenytoin toxicity. Associated to warfarin, it increases the anticoagulant action and may cause bleedings.31 |
| Selective serotonin re-uptake inhibitors (SSRI) | CitalopramEscitalopramFluoxetineFluvoxamineParoxetineSertraline | Plasma levels increase when associated with TCA and cimetidine. Associated to benzodiazepines, hepatic depuration is decreased. If associated with insulin, glycemia alterations occur; with digoxin, side-effects are increased, and with warfarin, the coagulation time is increased.31 Increased plasma levels of carbamazepine or phenytoin by metabolism inhibition with fluoxetine and fluvoxamine, raising the level of phenytoin by sertraline. Increased nausea with fluvoxamine and carbamazepine. Increase in level of valproate (>50%) with fluoxetine. Valproate may increase plasma levels of fluoxetine.33 |
| Selective noradrenaline and dopamine reuptake inhibitors | Bupropion | Bupropion, in association with MAOI may cause hypertensive crisis. With fluoxetine, confusion and convulsion of the major epileptic type may occur. With valproate, plasma levels of sodium valproate are increased.31 The bupropion with medications that lower seizure limial: TCAs, clozapine, fluoxetine, haloperidol, lithium, maprotiline, trazodone may be increased risk of seizure.33 |
| Selective serotonin and noradrenaline reuptake inhibitors | VenlafaxineDuloxetineMilnacipran | Duloxetine inhibits the cytochrome P450 2D6 and is metabolized by 1A2. Drugs like quinolones that inhibit the cytochrome 1A2 increase the levels of duloxetine, and may cause toxicity.34 Venlafaxine associated to MAOI may result in neuroleptic malignant syndrome, hypertensive crisis or serotonin syndrome.31 |
| Serotonin modulators | TrazodoneTianeptin | Trazodone associated to paroxetine or buspirone or MAOI+methylfenidate may result in serotonin syndrome. The concomitant use of antihypertensive medication may increase the risk of hypotension.31 Tianeptin should not be administered concomitantly to mianserine and no selective MAOI.35 |
| Selective noradrenaline reuptake inhibitor | Reboxetine | Should not be associated to MAOI. Does not inhibit hepatic enzymes and has does not have significant interactions.34 |
| Serotonin and noradrenaline modulators | MirtazapineMianserine | Mirtazapine associated to alcohol or diazepam results in subjective and objective effects that affect motor and cognitive performance.31 Mianserine may interact with warfarin. Carbamazepine and fenitoin may interact with mianserine decreasing its plasma level.35 |
| Monoaminooxi-dase inhibitors – MAOI | TranicilprominMoclobemide | Tyramine-rich foods may generate a hypertensive crisis and even death. Association with direct-acting sympathomimetic agents may also cause hypertensive crisis. Associated to pethidine it may cause over excitation, hypertension, pyrexia, coma and death. The concomitant use of methylfenidate or buspirone may increase arterial blood pressure. Association with meperidine should be avoided since it may lead to death. Associated with SSRIs, serotonin syndrome may occur. There are reports of brief psychotic episodes in its use associate to dextromethorfan.31 |
Once any of these undesired side-effects or drug–drug or drug–food interaction is identified in the depressive patient receiving drug treatment, and provided this effect or interaction might have a negative impact on the treatment, the pharmacist must attempt to solve or minimize these effects with or without the assistance of a prescribing physician.21
Pharmaceutical care for the resolution or minimization of undesired side-effects of antidepressantsAccording to the literature, there is a set of recommendations (shown in Table 3) to be implemented to minimize or eliminate some undesirable side-effects.
Management of the main undesirable side-effects of antidepressants.
| Undesirable effect | Management |
| Dryness of mouth | Use of sugarless chewing gum, artificial saliva products, fluor mouth washes or gargling with pilocarpine (eye drops) given as mouth drops). The latest strategy may significantly contribute to increase saliva production. Salivation can also be increased with the use of C vitamin tablets. However, this resource should not be used for a long period because the acidity of the vitamin may cause tooth erosion.40 |
| Constipation | Advise the patient to immediately respond to the defecation reflex, establish regular bowel habits, relax, perform regular physical activity, for it increases intestinal motility and strengthens the abdominal muscles, and eat fibers and drink a lot of liquids. If these measures do not achieve the desired results, the use of laxatives to increase the mass of fecal material is recommended.6,41 |
| Sedation | If the patient develops excessive sedation, he (she) may be advised to take the medication at night. If this measure does not achieve the desired result, the pharmacist may suggest to the physician that he/she might change (reduce) the dose of the medication.6 |
| Nausea | Ingest frozen foods or foods at room temperature. Avoid sweet, salty or fat foods. Get some sleep and try to have fun when feeling sick, perform physical activities. Take the medication after the meal and eat several small meals throughout the day.41 |
| Restlessness or insomnia | The patient can be advised to take the medication in the morning. If this measure alone does not achieve the desired result, the pharmacist may suggest to the physician the insertion of a benzodiazepine, or else, if insomnia is caused by a SSRI, a tricyclic antidepressant or tradozone (that has a strong sedative effect) can be administered.5,6,41 |
| Sexual dysfunctions | The drugs that cause this problem are the SSRIs. The pharmacist may suggest to the physician that he/she might reduce the dose, drug holidays or change the medication. To maintain dopamine levels thought necessary for optimal sexual functioning, the strategies consist of adding to the SSRI regimen either high daily doses of buspirone (60mg/day), an anxiolytic that prevents serotonin's damping effect on dopamine, or therapeutic daily doses of bupropion (300–450mg/day), a dopaminergic AD in its own right36. Neostigmine at the 7.5–15mg dose taken 30min before the sexual intercourse may increase libido; and ciproeptadina at a dose of 4mg/day may decrease anorgasmia. Sildenafil can be considered. Tradozone prolongs the erection and penile turgidity of some men and can be taken at a dose of 150–200mg.34,41–43 |
| Hypertension by venlafaxine | Follow-up the arterial blood pressure and suggest to the physician dose reduction. If this measure does not reach the desired result, the medication should be changed.6 |
| Tachycardia caused by tricyclic antidepressants | Suggest dose reduction or change of the medication to the physician.6 |
| Weight gain | Suggest dose reduction or change of the antidepressant drug.6 |
| Orthostatic hypotension | Advise the patient to avoid sudden changes in posture (such as stand up fast from a bed or couch) and reduce the caffeine intake, ingest 2–2.5l of liquid and have a salt-rich diet, perform physical exercises to strengthen the legs and abdominal legs, e.g. swimming. Use of elastic compression stockings.6,44. |
| Urinary retention | Take bethanechol.41 |
| Headache | Reduce the dose or discontinue treatment and give another antidepressant.45 |
| Increase in cholesterol | Suggest to the physician dose reduction or change of antidepressant drug.45 |
| Photosensitization | Tricyclic antidepressants, SSRIs, venlafaxine, maprotiline, trazodone and tranicilpromine are photosensitizers. Thus, it is recommended that the patient applies sun cream every 2h, avoid exposure to sun between 10 and 16 and wear sunglasses.46 |
According to Nieto and Manrique (2005), some recommendations should be provided to patients treated with antidepressants: (a) inform the patient on the existence of a latent period before the therapeutic effects of the drug; (b) start treatment at low doses of medication to minimize the occurrence of undesirable side-effects; (c) monitor the patient to reduce the possibility of suicide attempt; (d) an antidepressant drug previously administered to a patient with success should be considered in case of recurrence of the depressive episode; (e) the therapy should not last less than 6 months to avoid recurrence. This information should be disclosed to the patient, in order to prevent him/her to abandon the treatment when there is improvement in the symptoms.25
Antidepressants and patient profileAccording to Souza (1999), the choice of the antidepressant drug should take into consideration the patient profile, because all classes of antidepressants have similar efficacy. Thus, this choice must be based on the characteristics of a depression, side-effects, risk of suicide, other clinical disturbances, concomitant therapy, tolerability, cost, cognitive data.4,5,42
PregnancyApproximately 10% of pregnant women suffer from depression. Untreated depression during pregnancy may have serious consequences to the mother and the fetus. TADs and SSRIs are not contraindicated during pregnancy because there is a favorable benefit-to-risk ratio for the patient. The decision of treating depression shall depend on the severity of the condition. The FDA recommends fluoxetine, maprotiline or sertraline.6 Nomura e Pinto-Silva, 2007 suggest it is necessary to seek alternative treatments to depression during pregnancy, although some women might require drug treatment. The use of antidepressants should consider the severity of the condition, the consequences of the disease for the newborn and the family, and other clinical aspects of the patient.47
LactationIt is important to consider that all antidepressants are secreted in breast milk. However, if the mother is not treated, the relationship between mother and son can be very much affected, and, thus, depending on the severity of the disorder, the following drugs can be administered to the patient: amitriptiline, nortriptiline, clomipramine and sertraline.6 Patients under antidepressants treatment should avoid breastfeeding and give commercialized infant milk formulas.
ElderlyIn the elderly the prevalence of depression is approximately 15%. TDAs should not be administered. Elderly usually do not tolerate the undesired effects of these drugs (dry mouth, urinary retention, constipation, postural hypotension, among others). The TDAs most widely used by elderly are nortriptyline and desipramine due to their lower risk of causing postural hypotension, less anticholinergic, sedative and cardiovascular effects.30 The SSRIs are the most commonly prescribed treatment, always taking into account the profile of each patient.32 Bupropion has also demonstrated to be as efficient as other antidepressants. Trazodone and mirtazapine, due to their strong sedative effects, can be useful for elderly depressed patients with agitation.30
Depression secondary to other disordersDiabetesDrugs that have little effect on glycemia such as venlafaxine, or with hypoglycemiant effects such as the SSRIs are recommendable to patients with depression and antidiabetic drugs user. Bupropion can also be given since it does not interfere with glycemia. The TDAs are contraindicated because they produce hyperglycemia, increase appetite leading to weight gain, which is not desirable in a diabetic patient.6,48
ObesityThe antidepressants less likely to cause weight gain are: SSRIs, trazodone and bupropion.6
Peptic ulcer diseaseAvoid the SSRIs because they increase gastric acid secretion. The TDAs tend to reduce the acid secretion, but they can retard gastrointestinal motility. Thus, they are not recommended for those patients who suffer from dyspepsia related to dysmotility.6 Schatzberg and Nemeroff (2002) recommend the use of doxepin.31
ConclusionsSeveral international studies demonstrate the effectiveness of pharmaceutical care (PC) in patients with cardiac insufficiency, diabetes, hypertension and dyslipidemia, but there are few studies on such effectiveness on depression. Pharmaceutical care in Brazil is a recent practice, and, consequently, there are few studies demonstrating its effectiveness on depressed patients. All things considered, the present article highlights the need for further research into the impact of Phamaceutical Care in patients with mental disorders.
Conflict of interestsThe authors declare no conflicts of interest.