Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosPostpartum depression and breastfeeding are two complex situations regulated by neuroendocrine system, primarily cortisol and prolactin. These two hormones play a role in different ways through stress environment. Thus, this study aims to analysed cortisol and prolactin levels, milk volume, and weaning time in breastfeeding mothers with depressive symptoms.
MethodsA longitudinal study conducted to 92 mothers in Makassar, South Sulawesi, Indonesia. Baseline information related to socio-demography, parity, body mass index, tobacco exposure, trauma history collected at enrolment, later depressive symptoms, cortisol and prolactin levels, milk volume, collected at postpartum. Follow-up ended at the time of each subject's weaning. This study performed Chi-square test for baseline data, Mann–Whitney U-Test for cortisol, prolactin, milk volume, and Survival Test Cox Proportional Hazard Model for weaning time.
Resultsshowed that low cortisol (p=0.973) and prolactin (p<0.040) levels were higher in mothers with depressive symptoms. The mean volume of milk (p<0.001) was higher, and the weaning time (p<0.001) was longer in mothers without depressive symptoms. The Cox proportional hazard regression test results p<0.000, OR: 0.134, 95% CI 0.07–0.25, showed that mothers with symptoms of depression in the second week had the potential to wean 13.4% faster.
ConclusionsThis study confirms the difference between prolactin and postpartum depression symptoms. Milk volume produced at second week postpartum highly related to longer duration of breastfeeding.
Further study need to consider in understanding transcription pathway of prolactin and cortisol in breastfeeding mothers with acute and chronic stress symptoms. Primary depression screening should be performed prenatal and postpartum more frequently, to prevent the possibility of early weaning.
Depression is a mental disorder that affects almost everyone, at least once in their lifetime, and is multi-faceted. Postpartum depression is similar to general depression, with the only different onset of events, during pregnancy up to four weeks postpartum,1 especially in primiparous mothers.2 Postpartum depression and breastfeeding are two complex situations that are regulated by the neuroendocrine system, notably cortisol and prolactin. Postpartum depression is a pathological disorder of the Hypothalamus–Pituitary–Adrenal (HPA) axis pathway.3 Physiological conditions of pregnancy occur a surge in the secretion of various hormones, including cortisol and prolactin.4 Cortisol increases physiologically at the beginning of conception and continues to surge very typically, up to three times in the third trimester; however, this increase is also followed by the binding hormone Corticotrophine Releasing Hormone-Binding Protein (CRH-BP) so that it is not recognized by the receptor and transcribes.5 Towards the end of pregnancy, the CRH-BP level decreases so that cortisol begins to express its function to childbirth.6,7 Cortisol function in early pregnancy suppresses the mother's inflammatory system through the adrenal zone fasiculata pathway to not attack the foetus.8,9 Helps supply the placenta, ripens fetal organs (brain and lungs), thus enabling pregnant women to be less responsive to acute stress.3,10 Cortisol also stimulates the readiness of a motherhood function.11 Cortisol secretion increases according to the circadian rhythm, tends to be high in the morning to noon, and has the lowest decrease at night; changes in sleep habits also change cortisol production.12
In most cases, a decrease of cortisol concentration levels in saliva will be derived within two weeks after delivery.13,14 Different conditions are signs of persistent HPA-axis suppression and hypocortisolemia, which are believed to be susceptible factors to suffering from postpartum depression. Although it has been proven that perinatal depression can be triggered by various internal disorders that show different symptoms, both before and after delivery, it can be ascertained, that hypo and hypersensitivity of HPA-axis activity are associated with postpartum depression.15
Prolactin is one of the most adaptive hormones because of its role in breastfeeding and modulation of stress responses during pregnancy and lactation.16 Basal prolactin increases during pregnancy, and breastfeeding, and returns to average relative to three weeks postpartum.17 Women who maintain lactation have basal prolactin levels higher, and decreases in the postpartum months.18 Prolactin has a role in regulating mood swings and shaping maternal parenting behavior.19 Prolactin stimulates the secretion of milk in mammary epithelial cells via the paraventricular nucleus activation pathway,20 then activates the main pathway of synthesis lactation proteins through the Janus Kinase-Signal Transducer and Activation of Transcription (JAK-STAT) such as casein, lactoglobulin, and lactoferrin.21,22 Deficiency in the amount of prolactin or excessive activation pathway blockade increases maternal anxiety and impairs maternal behavior after delivery.23 Prolactin-induced stress response reduces milk supply and perinatal mood disorders.24 Long-term stress in animal studies tends to reduce the effect of endogenous opioids on prolactin secretion, which showed that HPA tonic activation could inhibit prolactin development.25 Obesity and a history of alcoholism correlate with the slow response of prolactin to breastfeeding in human studies.26,27 Hypothalamic amenorrhea can potentially decrease the response and release of Thyrotropin Releasing Hormone (TRH).28 Increased reactivity of stress pathways may correlate with maternal mood and prolactin development, resulting in unwanted weaning due to reduced milk supply.4,29 So it can be concluded that these two hormones experience an increase in levels at the end of pregnancy and a gradual decrease after delivery.30 Both of these hormones play a role in the stress response in different ways, but whether they can be a marker of longer breastfeeding duration. Thus, this study aims to analysed differences in cortisol, prolactin, and milk volume, and weaning time in mothers with postpartum depressive symptoms, also measured these parameters in predict lactation duration.
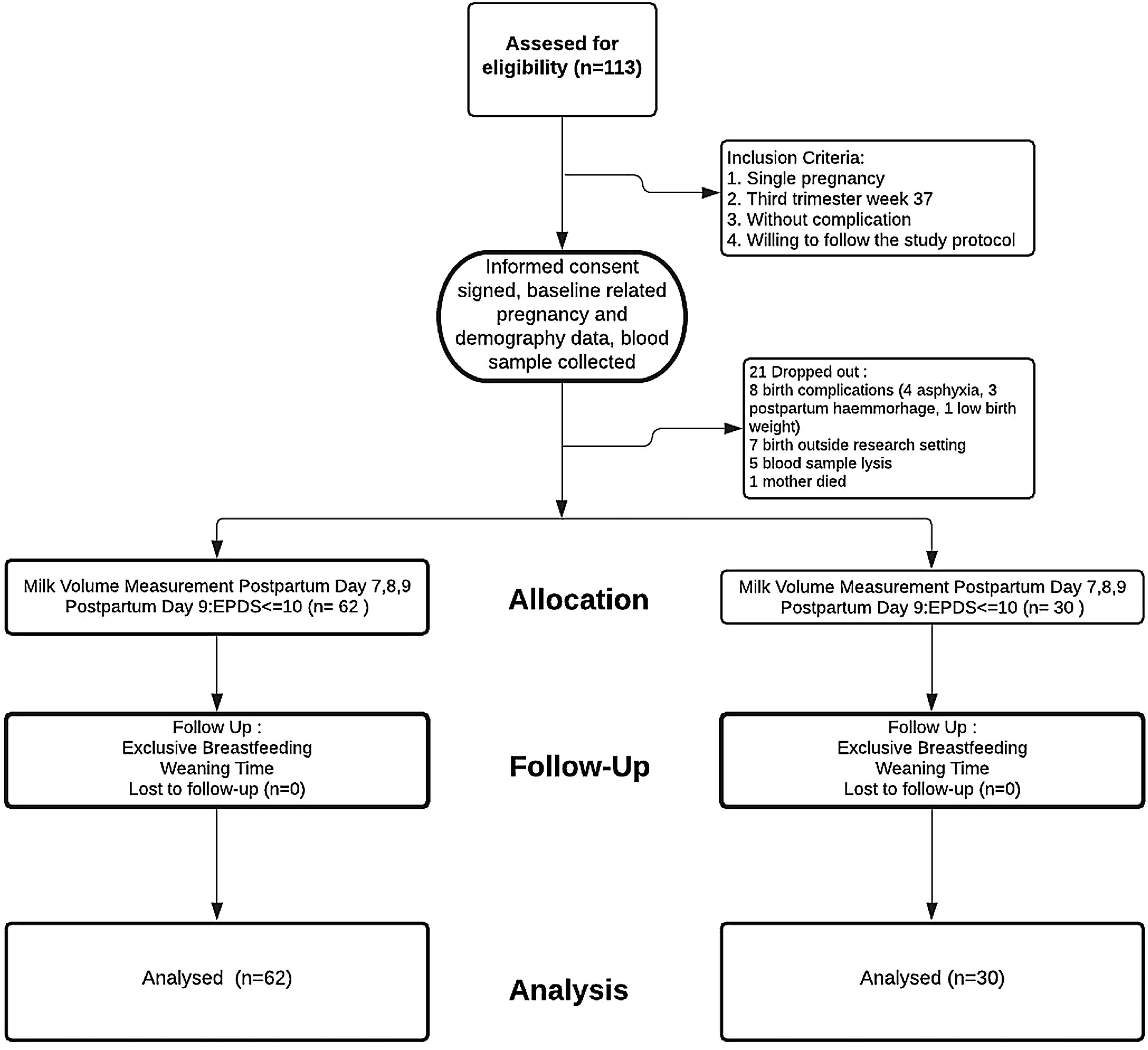
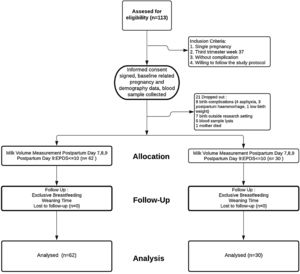
Materials and methodsResearch design and subject selectionThis study used a longitudinal approach to third-trimester pregnant women. The research subjects came from three clusters for primary maternal and child care clinics in Makassar City, Indonesia. A total of 113 women who met the criteria (singleton pregnancy, 37th week gestational age, without pregnancy complications, willing to be followed up to six months of breastfeeding, or until weaning) were selected purposively. A total of 21 subjects dropped out, with details; 8 mothers experienced labor complications (4 cases of asphyxia, 3 bleeding, 1 low birth weight), 7 gave birth not at the study location (leaving their domicile), 5 damaged blood specimens during the analysis process, 1 mother died during childbirth. The remaining 92 subjects were analyzed and followed up for up to six months, or until weaning time (see Fig. 1. Enrollment flow). Mothers were asked to participate in this study during their antenatal care visits. After received sufficient explanation, those willing to sign the informed consent form were accompanied by their families and midwives who were on duty at the clinic. All procedures in this study approved by the research ethics committee of Makassar Health Polytechnic No. 321KEPK-PTKMKS/IV/2019.
Data collection and analysis of blood specimensMeasurement of supporting data such as maternal demographic, parity, body mass index carried out at the time of recruitment using the interview form after the mother signed the informed consent. At the same time, 3 cc of blood specimens were taken from the brachial vein by clinical laboratory staff where the mother had routine antenatal care visits, then analysed using the ELISA test.
ELISA kitProlactin and cortisol concentrations from canine serum measured using an EIA (enzyme immunoassay) kit (Demeditec Diagnostics GmBH, Kiel, Germany for Cortisol ELISA DEH3388; Prolactin ELISA DE1291), according to the manufacturer's instructions.
Postpartum depression score measurementPostpartum depression was measured in the second week, precisely on a ninth day after pumping milk. The potential for postpartum depression was identified using the Indonesian version of the Edinburgh Postnatal Depression Scale (EPDS), which had been translated and validated by a translation expert.
Measurement of milk volume and follow-upMeasurement of the volume of milk using the Medela Swing Double pump. Pumping is done once a day for 20minutes for each breast, on days seven, eight, and nine, in the mornings between 07.00 and 09.00AM. The mean results of pumping milk were then multiplied by the mean frequency of breastfeeding for three days so that the estimated results of the milk volume in second week postpartum obtained. Follow-up of each subject was carried out until the weaning period via telephone, and chat via WhatsApp to monitor the daily milk feeding process. The termination of observation ends at weaning for each subject. This research took place from February 2019 to April 2020.
Statistic analysisThe data distribution normality test was carried out on four parameters (prolactin, cortisol, milk volume, and weaning time), all of which showed the Kolmogorov–Smirnov value (p<0.005), means the statistical test used the Mann–Whitney U-Test. The relationship between subjects’ characteristics and the category of postpartum depression tested through the Chi-Square test to show the presence or absence of this characteristic as a potential confounder (p<0.05). To examine the relationship between cortisol and prolactin levels and second-week milk volume with weaning time, Spearman's rank correlation test was used (p<0.05). To see the survival rate between postpartum depression and the weaning time of each subject, a survival analysis using the Cox Proportional Hazard Model was applied.
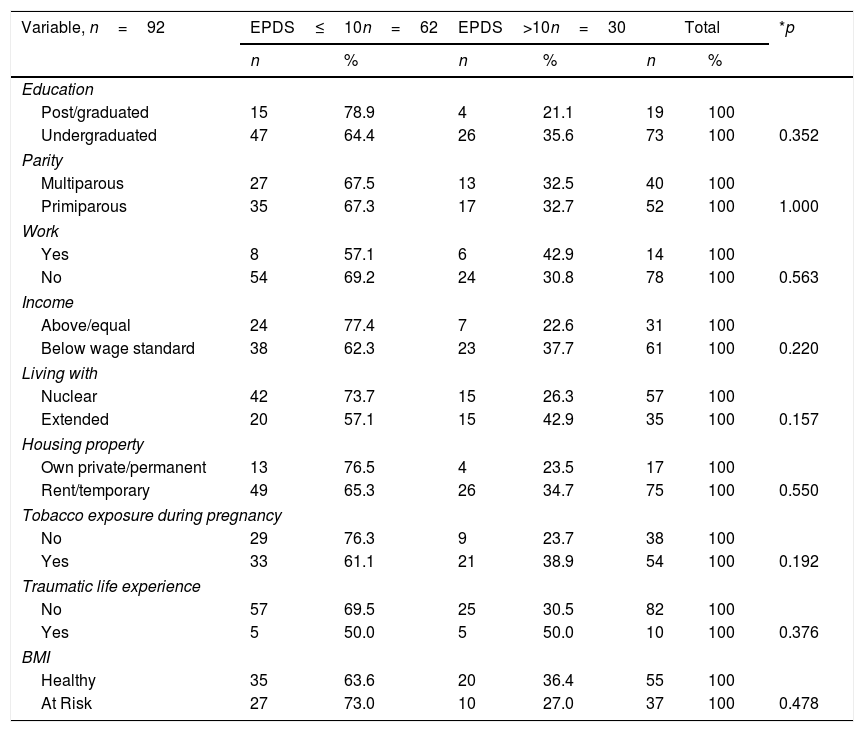
ResultThis study aims to determine differences in hormone cortisol levels, prolactin, milk volume, and weaning time in mothers with symptoms of postpartum depression using EPDS. The ten-cut point was used to screen for potential primordial depression for early screening. A chi-square continuity correction test was performed to measured homogeneity between groups (EPDS score cut-off 10), as shown in Table 1. The prevalence of mothers with depression symptoms (score above or equal to 10) was 32.6% of the total of 92 mothers. The results of the analysis identified that the characteristics between categories are similar. The underserved mother's demographic condition describe along with basic education level of 12 years (p=0.352). More than half of primiparous women had more significant potential for depression than multiparous (p=1.000). They are mostly housewives (p=0.563) who live on an income below the local minimum wage standard, 3 million Rupiahs or equivalent to $ 214.28 (p=0.220).
Characteristics of research subjects.
| Variable, n=92 | EPDS≤10n=62 | EPDS>10n=30 | Total | *p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Education | |||||||
| Post/graduated | 15 | 78.9 | 4 | 21.1 | 19 | 100 | |
| Undergraduated | 47 | 64.4 | 26 | 35.6 | 73 | 100 | 0.352 |
| Parity | |||||||
| Multiparous | 27 | 67.5 | 13 | 32.5 | 40 | 100 | |
| Primiparous | 35 | 67.3 | 17 | 32.7 | 52 | 100 | 1.000 |
| Work | |||||||
| Yes | 8 | 57.1 | 6 | 42.9 | 14 | 100 | |
| No | 54 | 69.2 | 24 | 30.8 | 78 | 100 | 0.563 |
| Income | |||||||
| Above/equal | 24 | 77.4 | 7 | 22.6 | 31 | 100 | |
| Below wage standard | 38 | 62.3 | 23 | 37.7 | 61 | 100 | 0.220 |
| Living with | |||||||
| Nuclear | 42 | 73.7 | 15 | 26.3 | 57 | 100 | |
| Extended | 20 | 57.1 | 15 | 42.9 | 35 | 100 | 0.157 |
| Housing property | |||||||
| Own private/permanent | 13 | 76.5 | 4 | 23.5 | 17 | 100 | |
| Rent/temporary | 49 | 65.3 | 26 | 34.7 | 75 | 100 | 0.550 |
| Tobacco exposure during pregnancy | |||||||
| No | 29 | 76.3 | 9 | 23.7 | 38 | 100 | |
| Yes | 33 | 61.1 | 21 | 38.9 | 54 | 100 | 0.192 |
| Traumatic life experience | |||||||
| No | 57 | 69.5 | 25 | 30.5 | 82 | 100 | |
| Yes | 5 | 50.0 | 5 | 50.0 | 10 | 100 | 0.376 |
| BMI | |||||||
| Healthy | 35 | 63.6 | 20 | 36.4 | 55 | 100 | |
| At Risk | 27 | 73.0 | 10 | 27.0 | 37 | 100 | 0.478 |
Most subject are small families (p=0.157), live together in one house with a lease or temporary status (p=0.550). Many subject reported being exposed to cigarette during pregnancy because their husbands smoked (p=0.192). More than two-thirds of the subjects stated that they had experienced traumatic conditions terribly disturbing in their childhood (p=0.376). Of the 92 mothers studied, only one third had unhealthy Body Mass Index (BMI). BMI (p=0.478) measured from body weight before pregnancy; the risk group comprises the underweight, overweight, and obese mother. In conclusion, all of the subject characteristics negatively correlated with postpartum depression symptoms.
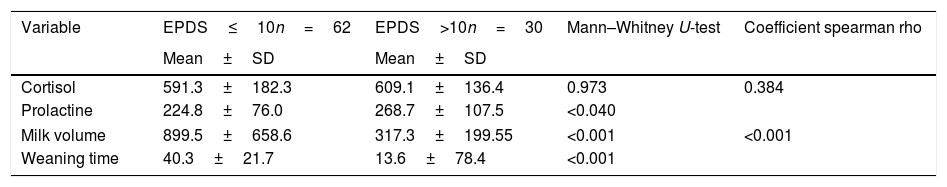
In Table 2, the test results show the difference between levels of the cortisol, prolactin, milk volume, and weaning time towards depressive symptoms. Mean cortisol levels noted slightly higher in mothers with depressive symptoms and reported non-significant difference between the two groups (p=0.973). Like cortisol, prolactin showed a similar rhythm and significant differences in mothers with depressive symptoms (p<0.040). However, there was no positive correlation between cortisol and prolactin levels amongst subject (p=0.384).
Results of the analysis of differences in hormone levels, milk volume, and weaning time.
| Variable | EPDS≤10n=62 | EPDS>10n=30 | Mann–Whitney U-test | Coefficient spearman rho |
|---|---|---|---|---|
| Mean±SD | Mean±SD | |||
| Cortisol | 591.3±182.3 | 609.1±136.4 | 0.973 | 0.384 |
| Prolactine | 224.8±76.0 | 268.7±107.5 | <0.040 | |
| Milk volume | 899.5±658.6 | 317.3±199.55 | <0.001 | <0.001 |
| Weaning time | 40.3±21.7 | 13.6±78.4 | <0.001 | |
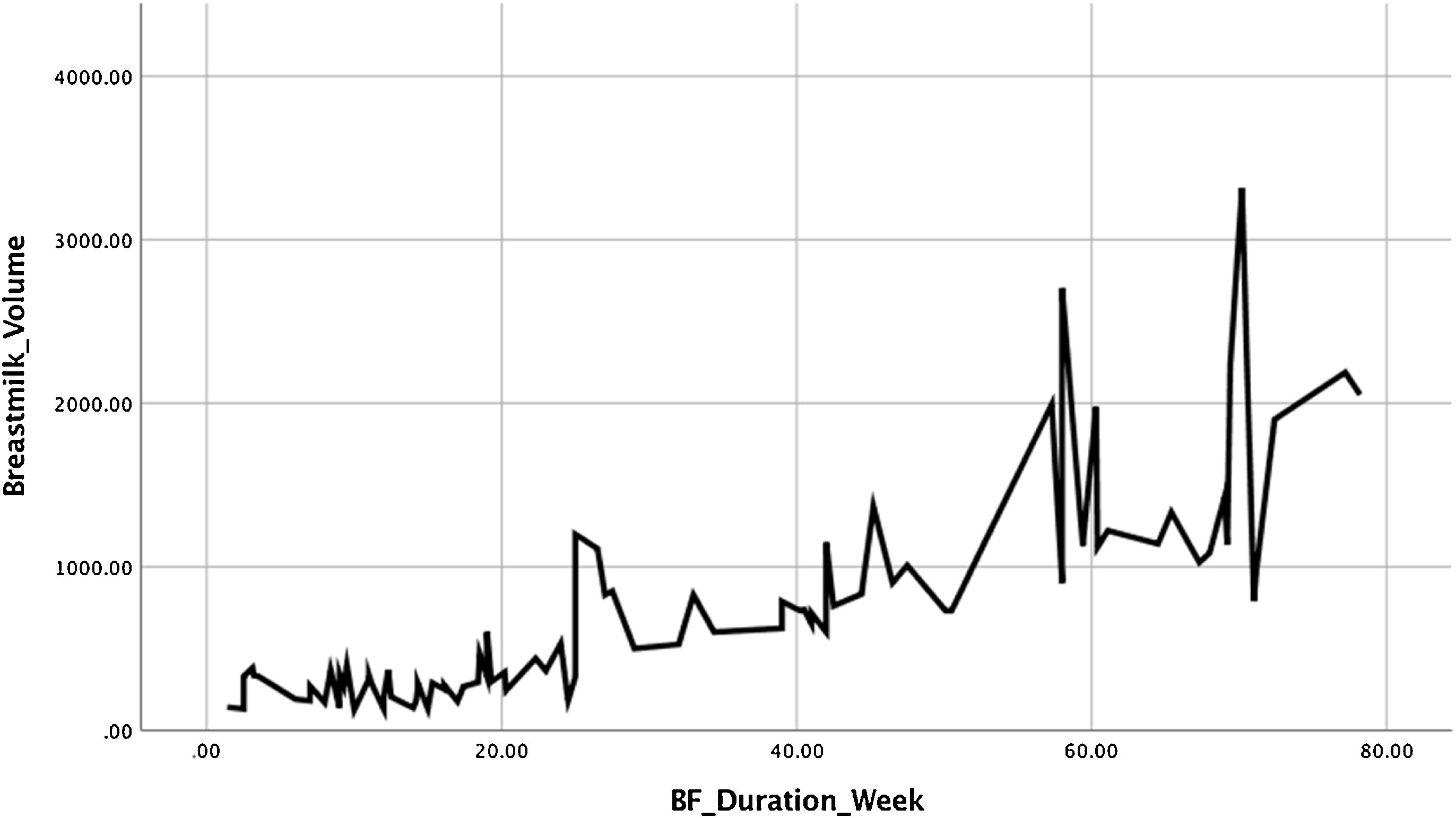
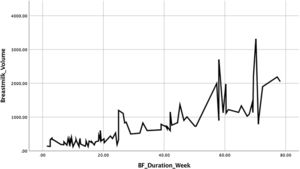
The milk volume, demonstrated higher mean in mothers without symptoms, also with high significant difference (p<0.001). Weaning time reveal the similar fact; mothers with depressive symptoms 13.6 weeks earlier experienced weaning time, compared to mothers without symptoms 40.3, and statistically significant (p<0.001), there also strong correlation between milk volume and weaning period (p<0.001). The linearity relationship between the milk volume in second week (day 7,8,9) and weaning time (weeks) illustrated in Fig. 3.
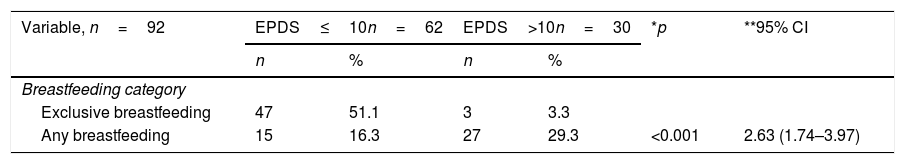
After a follow-up subject, this study found that the prevalence of exclusively breastfeeding mothers was 54.3%. In Table 3, mothers with postpartum depression symptoms encounter 2.63 times higher risk of failing exclusive breastfeeding. Mothers with postpartum depression symptoms tend to have lower milk volumes and shorter breastfeeding durations.
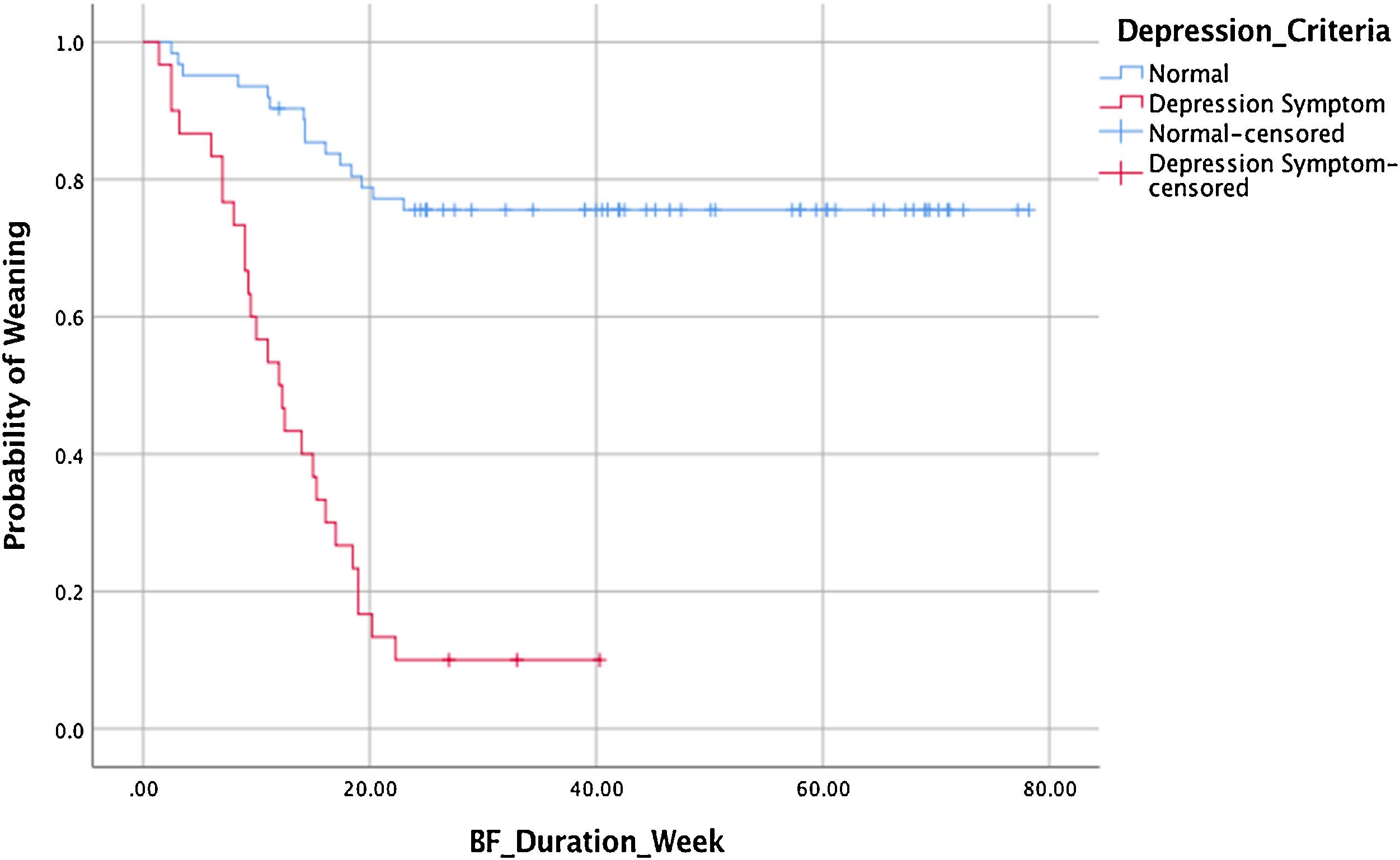
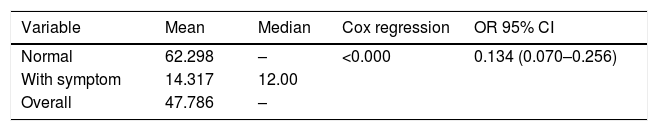
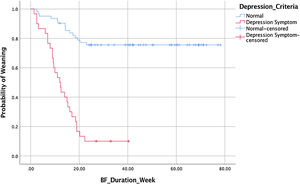
This study also collected data on time to stop giving milk or known as weaning. This data was demonstrated by a survival analysis to see the difference between the weaning time based on the presence or absence of postpartum depression symptoms. Of the 92 observations, 42 mothers experienced a weaning event, 50% of mothers with symptoms of postpartum depression weaned at week 12 (Table 4).
Fig. 2 shows the survival rate for weaning in the asymptomatic group was higher than with symptoms. As many as 50% of all study subjects experienced an onset of weaning at week 47.7. The results of the Cox proportional hazard regression test p<0.000, OR: 0.134, 95% CI 0.07–0.25, showed that mothers with depressive symptoms in the second week fall on weaning 13.4% faster.
Fig. 3 shows the graph between the mean volume of milk on the seventh, eighth, nine-day postpartum with the weaning time of each study subject. The higher the volume of milk produced by a nursing mother, the longer the weaning time is experienced. These findings emphasized that the higher production of milk in second week postpartum, the more prolonged weaning occurs.
DiscussionThis research is a mother with a dominant deprived character (low-income family) which doubled potential for postpartum depression. Although statistical tests do not show any significance for maternal characteristics, the comparative rates between these two groups suggest an adverse socio-demographic prevalence may increase the susceptibility to depression during pregnancy and breastfeeding. Among the demographic vulnerabilities, economic factors are still the main focus of concern. So that awareness of mood changes during pregnancy and breastfeeding placed at secondary attention. The struggle to fulfilled the primary needs itself is a source of depression for most middle-lower society.31 In the previous study, it was reported that for low-income families, the main factor of postpartum depression was poverty.32 So it is difficult to discriminate against the source of depression-related problems—pregnancy and childbirth from the economic burden. Depression also lacks consultation space, especially essential clinical services conducted by midwife. This issue related to their insufficient knowledge in assessing mental health in pregnant women.33 Public awareness of depression remain low,34 when mother complained about the physiological and psychological pressures during childbirth, it reflected their in-capabilities to managed childcare issue, as it attached to the role of motherhood. On the other hand, stigmatized mental health issue prevented them to be more exposed to their volatile mood state. While mood swing may be the initial symptoms of anxiety due to stress. Excessive, hidden, untreated stress tends towards compulsive behavior; self-harm, hurting a baby, or even suicidal ideation.35
Depression biologically recognized by dysregulation of HPA axis.28 Cortisol is a steroid hormone that is regulated by the HPA Axis. In this study, serum cortisol and prolactin were examined during the third trimester of pregnancy (37th week). The statistical test showed that there was no significant difference in the mean cortisol levels between mothers with postpartum depression symptoms compared to other group. However, the mean cortisol level reported higher in mothers with depressive symptoms. Similar findings stated by Evan et al., in observing the gestational age of 36 weeks. Salivary cortisol was collected at three time points, women with comorbid major depression and anxiety had higher cortisol levels at multiple time points, but cortisol levels did not differ between women diagnosed with major depression or anxiety alone vs. healthy controls.36 Rouse and Goodman also compared urinary cortisol (from waking to noon) of pregnant women diagnosed with major depression (n=23) with healthy pregnant women (n=54). Cortisol was collected every month; during pregnancy, there was an increase, but the levels did not differ between groups.9 Animal studies have shown the role of lactogenic hormone, decreased progesterone, adrenal stress reactivity, and thyroid homeostasis in lactation physiology and maternal behavior, serving as a proxy for postpartum mood disorders.28 Clinical studies also provide evidence for this pathway in the pathophysiology of lactation failure and perinatal depression.30
While prolactin shows a more promising relationship, some of the previous study have also been proven successful in this study's findings. Mothers with depressive symptoms had higher mean serum prolactin and significant correlation with depressive symptoms. Parry et al. studied serum prolactin in pregnant women (3 with major depression, 2 healthy) and postpartum women (13 major depression, 2 healthy), had higher prolactin levels in depressed vs. non-depressed subjects, both in the antepartum and postpartum periods. Among depressed women, prolactin is higher in breastfeeding women than non-breastfeeding women.7 In Levine, Higuchi and Glasow show the direct activity of prolactin in adrenal steroidogenesis to increase adrenal androgens, dehydroepiandrosterone, and dehydroepiandrosterone sulfate and cortisol and aldosterone.37 Prolactin is an immunomodulatory factor that functions to maintain homeostasis under stressful conditions; prolactin balances the adverse effects of glucocorticoid and other immune and inflammatory mediators.38 Prolactin prevents glucocorticoid-induced lymphocytes cell death and also increases the effect of cellular macrophages. Prolactin can also reverse the antagonistic effect of the adrenocorticotropic hormone on inflammatory physiology by suppressing glucocorticoid release.39 Prolactin has also been found to enhance the recovery effects of the hematopoietic system.40
Physiologically, these two hormones will increase and decrease in some of the body's responses to stress. However, baseline protein lipid soluble cortisol and water soluble prolactin make them different in regulation and transcription.41 Prolactin requires receptors to communicate on mammary cells, while cortisol penetrates the cell wall and binds to cytosolic glucocorticoid receptors.42,43 Cortisol and prolactin are required for milk production. In mammary epithelial cells, glucocorticoids circulate and bind to cytosol receptors, and act synergistically with prolactin initiating transcription and translation of target proteins.44 At the same time, cortisol levels decrease during a breastfeeding episode, and the HPA axis response to physical stress is reduced in women who are direct breastfeeding rather than bottle-fed.45 The attenuated stress response in mammals is mediated by oxytocin and prolactin.46 A marked transition in the HPA axis function occurs during the puerperium. During pregnancy, the placenta produces CRH increasing the mother's HPA axis, thereby increasing circulating cortisol and suppressing CRH secretion in the hypothalamus. This suppression of the hypothalamus gradually decreases after birth.24 Mothers with prenatal depression, experience a transition to a significantly decreased adrenocorticotropic hormone (ACTH) response to CRH at the same time in the anterior pituitary and hypocortisolism.42 Deviation of the HPA axis reactivity is hypothesized to play a role in postpartum depression and lactation failure. Lack of circulating cortisol affects milk production in mammary epithelial cells directly. Oxytocin disorders and prolactin weaken the stress response, interest in breastfeeding mothers, faster weaning occurs.30
In addition to the significant difference in the hormone prolactin, this study shows a lower weaning survival rate in mothers with depressive symptoms. Even the volume of milk in the second week postpartum shows linearity with weaning time, which is indicated by a positive intercorrelation value. The higher the volume of milk a mother has at the beginning of postpartum, the longer the weaning occurs. If it is not mediated by mood disorders, anxiety, and depression, then breastfeeding duration is undoubtedly prolonged. Many findings are in line with this research. The duration of breastfeeding is inversely related to the symptoms of postpartum depression. A study confirmed this relationship after controlling for several cofactors such as; socioeconomic status, age, and education level, as well as a history of past depression, increased social stress, and the use of psychoactive drugs.47,48 Several studies have also reported an association between postpartum depression symptoms and early weaning.49–51 McLean et al. reported that mothers with depressive symptoms were less likely to continue breastfeeding for up to two to four months than mothers without depressive symptoms (AOR=0.73, p<0.001).52 Our study also confirmed that mothers with depressive symptoms experienced weaning 13% faster than healthy breastfeeding mothers. Several studies have noted that postpartum depression symptoms precede the cessation of breastfeeding.47,51 Another study found that having higher depressive symptoms at two weeks postpartum was associated with cessation of breastfeeding at twelve weeks postpartum.53 Similar results also showed that early postpartum depressive symptoms were associated with a prescription of eight weeks of breastfeeding.54 It was also reported that subjects discontinued breastfeeding. Breastfeeding experienced dissatisfaction with the way they breastfed, difficulties with lactation techniques, and low self-efficacy scores.55 Our findings also confirm that depressive symptoms correlate strongly with weaning at week twelve postpartum. Over time, the symptoms of postpartum depression will affect the psychological and physiological breastfeeding outcomes.
The relationship between postpartum depression and breastfeeding was conceptualized as a one-way relationship in which postpartum depression would determine the shorten period of breastfeeding.50 However, the natural aspect of causality stated that while postpartum depression leads to a cessation of breastfeeding, not breastfeeding increases the risk of postpartum depression. Some evidence suggests that breastfeeding exerts a protective effect against postpartum depression by speeding up recovery from mood disorders.56
Cortisol and prolactin appear to be both involved in the stress response, and levels tend to be related. However, the response of the hormones prolactin and cortisol to stressful situations may be different. It is widely reported that basal cortisol levels will return to normal in acutely stressful situations, such as postpartum situations require a short role adjustment time. Nevertheless, the situation will be different in chronic stress conditions, which tend to have a suppressive effect on the HPA axis, reducing the body's response to stress. Some authors hypothesize that prolactin release may act as an alternative response to cortisol in stressful situations. Prolactin secretion from the pituitary gland through the lactotroph axis activation pathway is a marker of acute stress in some species.57 Prolactin also plays a direct role in oxytocin secretion in conditions modulated by acute stress in rats during pregnancy.58 Given a large number of prolactin-mediated functions in the body, even inhomogeneous, genetically controlled animal-based studies, including hormonal and behavioral repetitive stress measurements (prolactin and cortisol associated with stress and fear), have not shown a positive correlation. However, prolactin can be a more stable alternative as a marker of chronic stress in humans.59 Therefore, further research is suggested to include the observation of the transcription and translation pathways of these two hormones in breastfeeding mothers with repeated serum measurements and control of subjects through definitive screening of clinical diagnosis acute and chronic stress.
LimitationThis study did not match and control the subject selection based on a clinical diagnosis of depression. Also we did not perform repeated measurements of basal serum cortisol at different times, so there is insufficient evidence to show that EPDS scores correlate conclusively with cortisol levels.
ConclusionWe managed to complete the cohort on the target research subjects until they passed the weaning period of each. Our findings confirm a significant difference in serum prolactin levels with symptoms of postpartum depression. We also managed to identify a correlation between depressive symptoms and weaning at week twelve. The higher the volume of milk produced by the mother, the longer the duration of breastfeeding. Our study recommends more specific observations in the transcription and translation pathways of prolactin and cortisol in breastfeeding mothers, especially those with acute and chronic stress symptoms, to better understand the differences in their responses to stress. We recommend that baseline depression screening be performed more frequently in the prenatal and postpartum periods to prevent possible weaning. So that understanding this situation can help mothers achieve more satisfying breastfeeding goals and feeding outcomes.
Conflict of interestThe authors declare no conflict of interest.
Our appreciation to the Indonesian Government's Higher Education, Science and Technology Ministry for fully granting this research project. Our appreciation to all our fellow mothers and family for their patience and commitment to follow our study. Lastly, to all primary healthcare workers to support the study site.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.