Technology Enhanced Medical Education International Conference (THEME 2019)
Más datosThis study aimed to analyze the differences in the expression of genes PRLR and STAT5, body mass index, and the volume of milk in nursing mothers.
MethodThis study uses the approach of observational studies that were carried out from April to December 2019 in Makassar, Indonesia. Subjects consisted of 80 third trimester pregnant women (32–36 weeks) selected based on specific criteria. The mother will be followed up until the baby is six months old. The technique of analyzing differences in the expression of PRLR and STAT5 genes uses qPCR with sub-classification of optimal and sub-optimal milk volume groups.
ResultThere is a different gene expression PRLR (<0.001) between the two groups, but not with the expression of STAT5 (0078). The more volume of milk shows an increase in genetic expression, although STAT5 is not statistically significant, the average volume of milk tends to increase with the level of expression (>median). Based on simultaneous tests, there is a strong interaction between body mass index with PRLR gene expression (<0.003), and STAT5 (<0.023), mothers who have healthy body mass index tend to have more volume of breastmilk.
ConclusionThis study provides an understanding that the nutritional factor is a biological underlying that supports the process of transcription and translation of milk-coding genes in order to work optimally. Thus, it is essential to intervene with excess weight pregnant women with the right antenatal care midwifery approach.
Endocrine regulation largely determines the process of transcription and secretion of breast milk in the breast glands. The amount of milk volume depends on the hormone prolactin and oxytocin.1 The involvement of genetic variation enables to modify the physiology of the mammary gland, hormone production, and milk production.2 Prolactin is an essential hormone in the production of milk produced by the anterior pituitary and secreted into the blood. Prolactin is secreted since pregnancy, but the concentration of prolactin in milk reaches its peak at 36h after giving birth (125ng/ml) and decreases to 50ng/ml on day 42 after giving birth.3 The amount of prolactin in the blood serum of nursing mothers is 251±8ng/ml,4 and this value increases tenfold compared to normal conditions. Prolactin will affect the growth and development of mammary glands (mammogenesis), milk synthesis (lactogenesis), and maintenance of milk secretion (galactopoietic). In mammogenesis, prolactin regulates the development of lobuloalveolar production in the mammary gland during pregnancy. Whereas lactogenesis prolactin stimulates the uptake of several amino acids, synthesizes casein milk protein and α-lactalbumin, lactose, milk fat and takes glucose.5
The role of prolactin as a milk production hormone and the development of the mammary gland will bind to prolactin receptors on the surface of the milk gland epithelial cells.6 Prolactin will pass through blood vessels to target organs to carry out their biological functions. Prolactin requires receptors to reach the target gene in the alveoli.7 This interaction activates the JAK 2/STAT5A transduction signal that plays a role in alveologenesis during pregnancy and leads to the expression of milk protein-forming genes.8
Several studies in dairy cows found that mutations that occur in genes that encode milk production can affect the amount of milk volume.9,10 In humans, research related to genes that encode milk production is still identified to identify genetic changes that have a role in milk production.2 These findings emphasize not only the amount but also the genetic variation role in each individual.
Transcription and epi-genetic studies will be critical to identify and understand the regulation of viral genes in the lactation process.2 Data that can explain the influence of genetic variation on the physiology of lactation are still limited to mammals. Therefore further research is needed to understand the implications of epigenetics in humans better.5 Studies have shown that changes in the prolactin signalling pathway can affect the function, yield, and also milk volume because recombinant prolactin has succeeded in increasing milk volume, lactose concentration, calcium, and oligosaccharides in some women, but with limited success. Pharmacogenomics in this pathway can be an alternative approach to improve lactation results in some women.2
The main cascade of prolactin signalling via PRLR (prolactin receptor), which binds to JAK2 (Janus kinase) and STAT (signal transducer and activator of transcription).6 Prolactin binding induces receptor dimerization and activation of JAK2, a kinase that is constitutively related to PRLR. JAK2 phosphorylates some tyrosine residues from PRLR and allows binding of STAT5.11
STAT5 is phosphorylated by tyrosine so that it is released from the receptor, polymerizes and translocates into the nucleus, and then binds to the target gene promoter.12 Some of the target genes targeted are related to milk protein, especially beta-casein and lactoglobulin.13,14 However, no data have been found that indicate how much this pathway is phenotypically expressed and whether it is related to the amount of milk volume in nursing mothers.2
Obesity, on the other hand, is a metabolic syndrome which mostly influences the body's physiological adaptations. In the process of breastfeeding, obesity can delay the initiation of lactation, reduce the duration, and encourage mothers to switch to formula milk due to lack of milk volume.15,16 Several hypotheses explain the success of lactation is lower in obese mothers, including a weak prolactin response to breastfeeding, changes in breastfeeding patterns due to pregnancy metabolic imbalances, and mechanical problems due to excessive deposition of adipose tissue in milk tissue.17 The function of gene transcription relevant to any biological process, often recognized differently, either temporally or permanently, on the physiological state of the change. Therefore, this study aims to analyze differences in the expression of the PRLR and STAT5 genes based on body mass index, and milk volume in nursing mothers.
MethodsResearch sitesThis study uses an observational analytic study approach conducted from April to December 2019 in Makassar City and has received a recommendation of ethical approval from committee of the faculty of medicine, University of Hasanuddin Makassar, number: 171/UN4.6.4.5.31/PP36/2019 March 11, 2019, has approved the entire study procedure. The study was conducted in four Primary Health Care in Makassar, namely Primary Health Care, Kassi-Kassi, Jongaya, Jumpandang Baru, Bara-Baraya.
Data types and sourcesSubjects consisted of 80 third trimester pregnant women (32–36 weeks) selected based on specific criteria. The criteria for selecting subjects were primipara/multipara, age range 18–40 years, vaginal delivery, healthy mothers, and babies during the data collection period and no history of postpartum complications.
The mother will be followed up until the baby is six months old. The technique of analyzing differences in the expression of PRLR and STAT5 genes uses qPCR with sub-classification of optimal and sub-optimal milk volume groups.
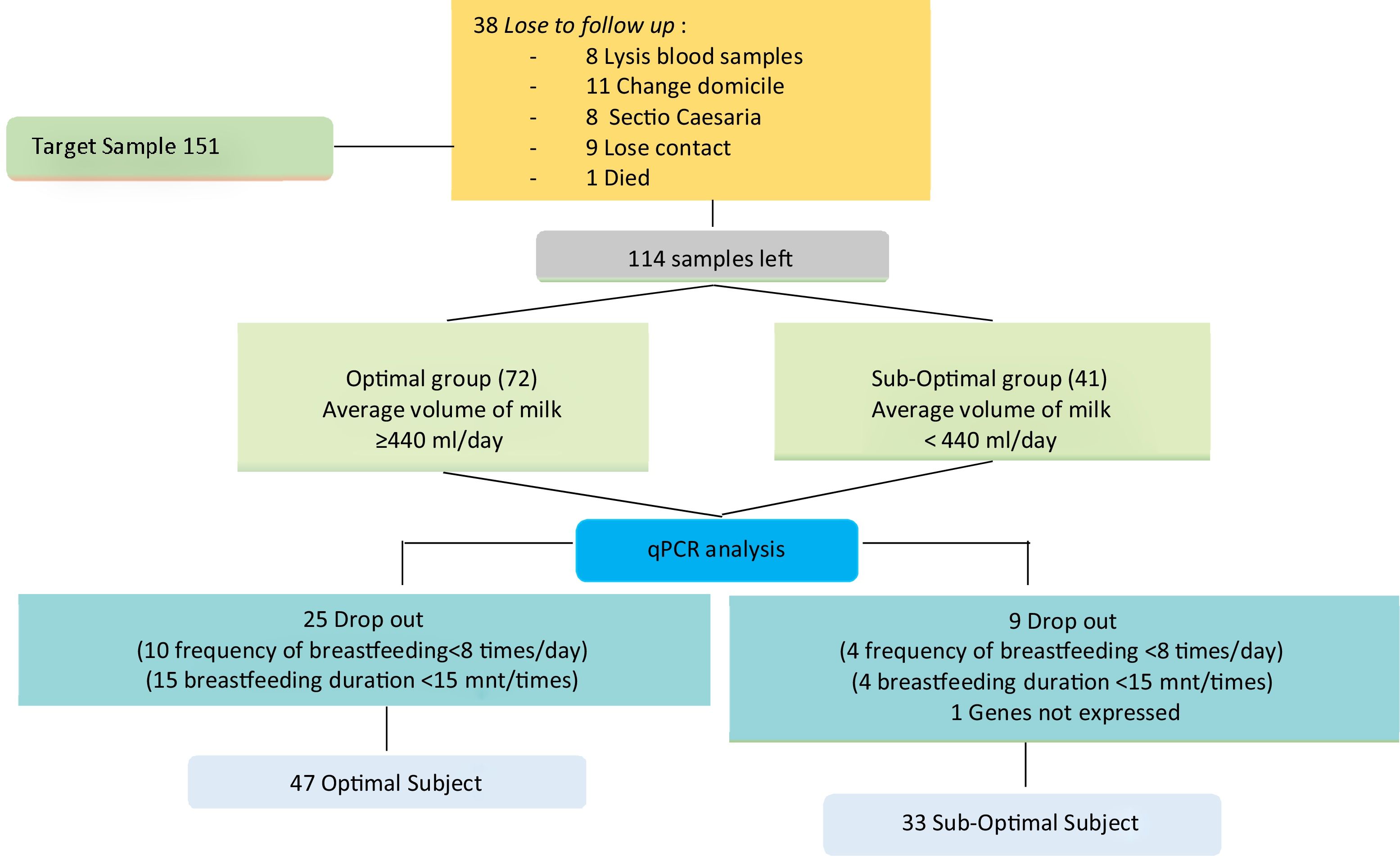
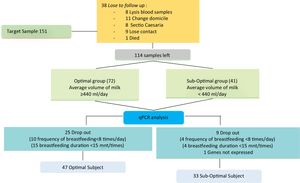
Data collection techniqueSupporting data collection includes sociodemographic history, obstetrics, lactation, and body mass index, at the beginning of the study conducted after the mother filled out informed consent. In the third trimester of pregnancy during an antenatal care visit >38 weeks, a 5cm3 brachial venous blood is taken. On days 7, 8, 9, postpartum done pumping to measure the volume of milk produced by the mother. Pumped milk will be given to the baby little by using a spoon. The volume of milk for three days will be calculated based on the concept of daily estimates for three consecutive days. In order to obtain the average volume of milk that is grouped into sub-optimal <440 and optimal ≥440ml. Se continued to do the analysis qPCR series touch CFX96 (Bio-Rad Laboratories, Inc.) (Fig. 1).
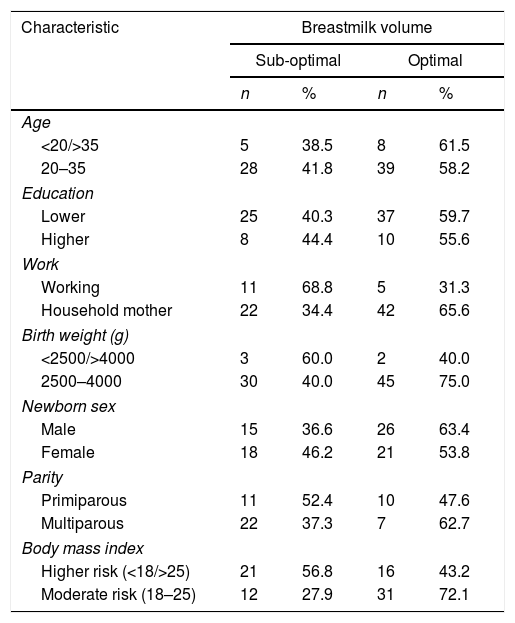
ResultsTable 1 shows the majority of mothers of healthy reproductive age that is 20–35 years, with the characteristics of low education, more multipara, and housewives. The lagging weight of infants is generally healthy, with a nearly balanced gender, and a body mass index that differs slightly between high and moderate risk. High risk includes mothers with body mass index categories under, over, and obese. The weight that is an indicator is the pre-pregnancy weight.
Subject characteristic.
| Characteristic | Breastmilk volume | |||
|---|---|---|---|---|
| Sub-optimal | Optimal | |||
| n | % | n | % | |
| Age | ||||
| <20/>35 | 5 | 38.5 | 8 | 61.5 |
| 20–35 | 28 | 41.8 | 39 | 58.2 |
| Education | ||||
| Lower | 25 | 40.3 | 37 | 59.7 |
| Higher | 8 | 44.4 | 10 | 55.6 |
| Work | ||||
| Working | 11 | 68.8 | 5 | 31.3 |
| Household mother | 22 | 34.4 | 42 | 65.6 |
| Birth weight (g) | ||||
| <2500/>4000 | 3 | 60.0 | 2 | 40.0 |
| 2500–4000 | 30 | 40.0 | 45 | 75.0 |
| Newborn sex | ||||
| Male | 15 | 36.6 | 26 | 63.4 |
| Female | 18 | 46.2 | 21 | 53.8 |
| Parity | ||||
| Primiparous | 11 | 52.4 | 10 | 47.6 |
| Multiparous | 22 | 37.3 | 7 | 62.7 |
| Body mass index | ||||
| Higher risk (<18/>25) | 21 | 56.8 | 16 | 43.2 |
| Moderate risk (18–25) | 12 | 27.9 | 31 | 72.1 |
*Chi-square continuity correction.
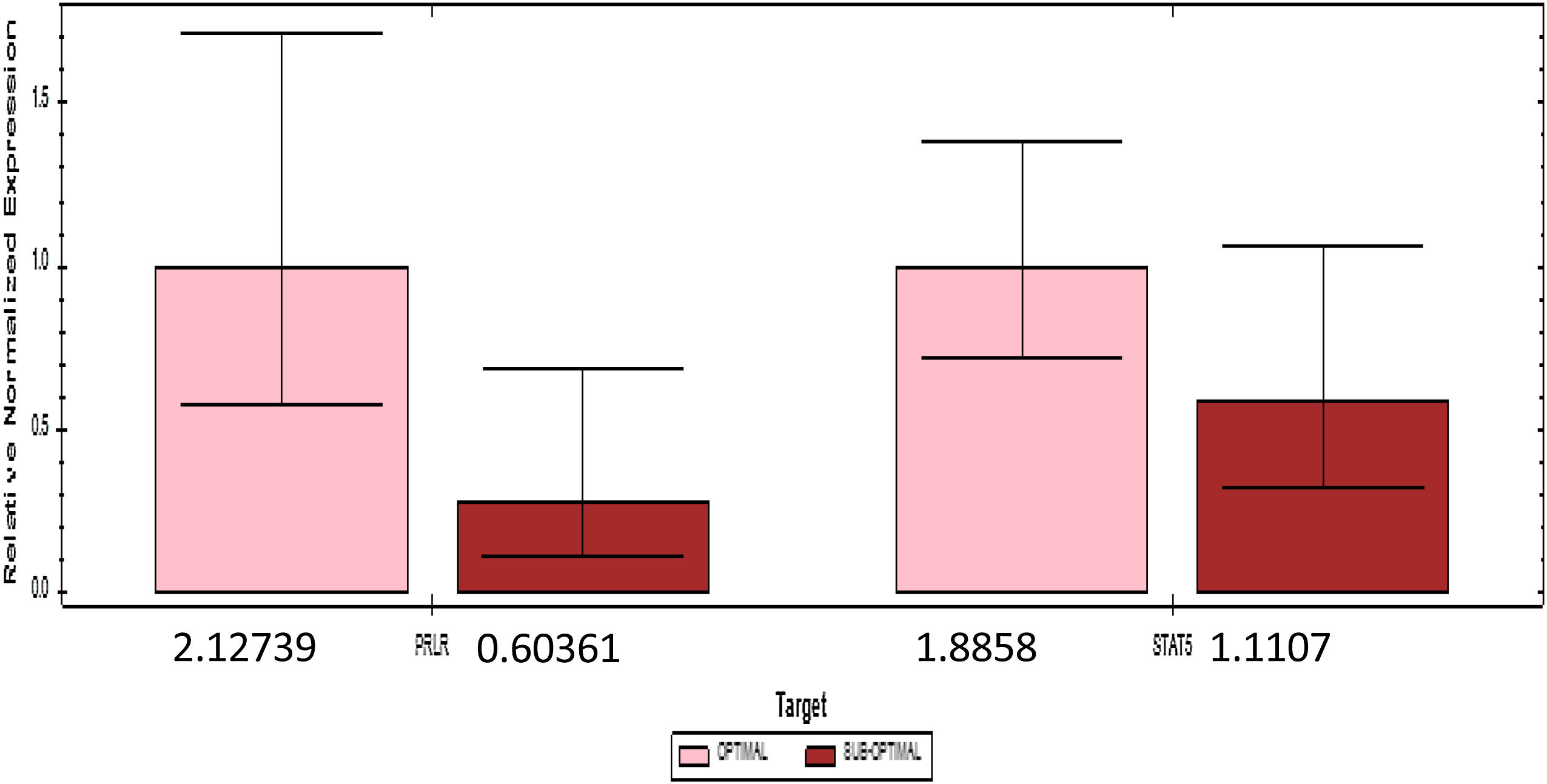
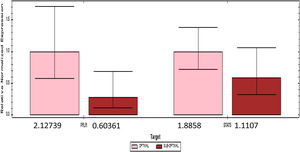
PRLR and STAT5 gene expression are divided by the median cutoff distributed. Gene expression decreases if it is below the median (1.73) PRLR and (1.43) STAT5. The number of mothers who had a gene expression £ median comparable with gene expression>median (Fig. 2).
The results of qPCR quantification, changes in the relative expression level (fold change) >1 indicate an increase in expression; conversely, changes in expression level <1, a decrease in expression. The number 1 is the quantity agreed upon for determining the target gene.
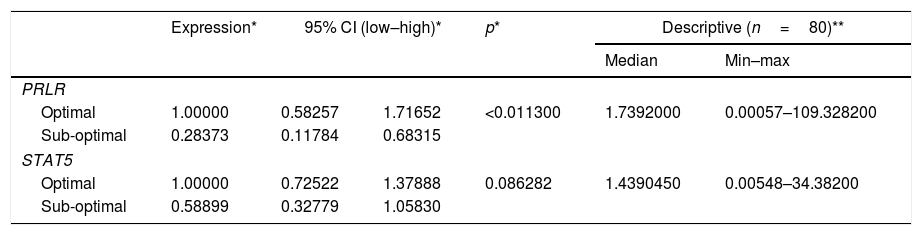
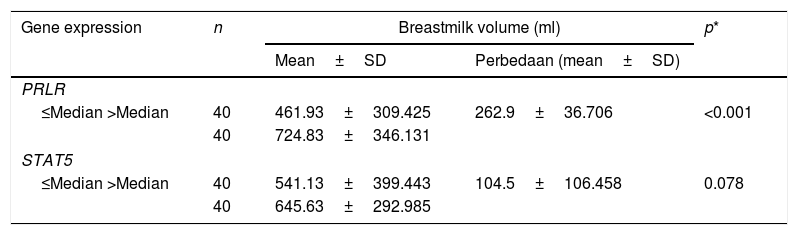
Table 2 shows the quantification value of the PRLR gene in the sub-optimal group decreased by 2% compared to the optimal group, while the STAT5 quantification value in the sub-optimal group decreased by 8% compared to the optimal group. Based on the results of the qPCR significance test, there were significant differences in the expression of PRLR, but not on STAT5. The median value of PRLR gene expression was higher than that of the STAT5 gene. The range of variations in the individual values of the PRLR gene expression is extensive, this shows that the data obtained has high heterogeneity. In contrast to PRLR, the expression of gene STAT5 shows a narrower range or has a tendency of lower variability than the data expression of the PRLR gene.
PRLR gene STAT5 expression value.
These findings gave rise to the assumption that the data PRLR gene and STAT5 were more expressed, tends to produce the volume of milk is higher (Table 3). Statistical test results showed no relationship between STAT5 expression and milk volume. This insignificant value is made possible by the difference in the average volume of milk produced by the mother does not differ much in both criteria (/>median), with a value of SD (106,485ml) is narrower in the average volume of milk category>median.
Breastmilk volume, PRLR, and STAT5 expression.
| Gene expression | n | Breastmilk volume (ml) | p* | |
|---|---|---|---|---|
| Mean±SD | Perbedaan (mean±SD) | |||
| PRLR | ||||
| ≤Median >Median | 40 | 461.93±309.425 | 262.9±36.706 | <0.001 |
| 40 | 724.83±346.131 | |||
| STAT5 | ||||
| ≤Median >Median | 40 | 541.13±399.443 | 104.5±106.458 | 0.078 |
| 40 | 645.63±292.985 | |||
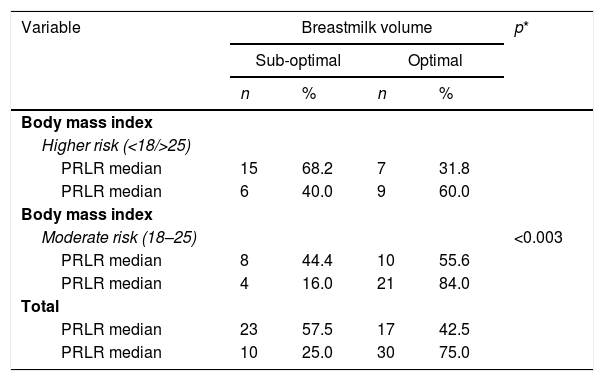
Results test simultaneously (Table 3) at variable body mass index with PRLR expression and STAT5, showed the risk factors of negative and positive categories dispersed normative. Based on the value of p<0.003 is proven, there is a significant relationship between the nutritional status of mothers with PRLR expression and milk production.
Mothers who have a body mass index are at risk (Table 4), tend to have low STAT5 expression, and vice versa. A p-value <0.023, means that mothers with functional nutritional status early in pregnancy, have increased STAT5 expression and more milk volume. So that there are mutually supporting interactions between internal variables (transcriptomic pathways) and external (nutritional status) in the regulation of milk secretion in nursing mothers.
Body mass index, breastmilk volume, PRLR expression.
| Variable | Breastmilk volume | p* | |||
|---|---|---|---|---|---|
| Sub-optimal | Optimal | ||||
| n | % | n | % | ||
| Body mass index | <0.003 | ||||
| Higher risk (<18/>25) | |||||
| PRLR median | 15 | 68.2 | 7 | 31.8 | |
| PRLR median | 6 | 40.0 | 9 | 60.0 | |
| Body mass index | |||||
| Moderate risk (18–25) | |||||
| PRLR median | 8 | 44.4 | 10 | 55.6 | |
| PRLR median | 4 | 16.0 | 21 | 84.0 | |
| Total | |||||
| PRLR median | 23 | 57.5 | 17 | 42.5 | |
| PRLR median | 10 | 25.0 | 30 | 75.0 | |
This study shows differences in the expression of the PRLR gene among mothers who have an optimal compared to sub-optimal milk volume. Of course, this assumption supports the empirical statement that for the primary process of induction of lactation in mammals, many hormonal necessary ingredients of prolactin needed. Process of binding of prolactin are both in cell membranes allow the transfer of signals to multiple activation pathways that deliver the transcription process to the cell nucleus and the target gene of interest. The amount of PRLR determines the interaction between prolactin (PRL) and prolactin receptor (PRLR) in the lactation process, not by the number of PRL.15 Mice that do not have PRLR reveal the formation of alveoli structures with a small lumen.16 In mammary glands, found an increasing number of mRNA PRLR genes at the beginning of lactation and decreased at the end of lactation.10 In a similar study, it was reported that the expression of the PRLR and STAT5A genes in breast milk increased sharply on the 4th day after delivery.13
PRLR signalling activates several signalling cascades, such as JAK2, STAT5 (STAT5a and STAT5b), Src kinase, mitogen-activated protein kinase, and the phosphoinositide three kinase pathway in normal and neoplastic mammary epithelial cells (MEC).17 The transcription pathway that occurs in alveoli cells encodes STAT through Janus kinase (JAK). The first bind between JAK and STAT is the immune response function of the target DNA. STAT transcription pathways are also divided into several directions with different target cell expressions. Some literature shows that STAT, in addition to being responsible for lactation, also plays a role in regulating the process of progressive cycles, cell proliferation, and immune response.18,20
The STAT5 gene plays a vital role in regulating cell proliferation, increasing differentiation, forming milk protein, and delaying cell apoptosis during involution.19 Some of these proteins are Cyclin D1 (which regulates cell cycle development) and BCL2-lik1 (Bcl-XL) anti-apoptotic factor during the lactation process.20 STAT5A gene expression increases up to 2.5-fold in mammary gland epithelial cells found in milk in the first 72h after delivery.18 Without STAT5A, the number of luminal progenitors decreases, the ductus develops, but alveoli unformed during pregnancy, this study also shows that mice lacking the STAT5A gene, the alveolar number is reduced by 50%, and the expression of WAP milk genes and alpha-lactalbumin decrease by 32% and 23%.21
The high expression of STAT5 much supports the cell's need for transcription of target genes, especially STAT5A in the transcriptomic pathways of beta-casein and alpha-lactoglobulin, where these two protein compounds are the main elements of milk protein composition. In studies that examine the amount and production of milk in mammals noted that the amount and composition of protein in milk determined by its genetics, and it difficult to modify with nutrition. However, due to the high need for protein synthesis for energy, the results of milk protein can be influenced by the energy content in food.22
Excessive energy intake, especially in obese conditions, reduces the success of breastfeeding. In female rats were exposed to a high-fat diet showed a reduction in milk production by 33%; mammopoiesis occurs only a third of the area of animal mammary glands compared with control. Mice are obese induced by diet showed higher levels of basal pSTAT5 in the network and the hypothalamus, and the stimulus of acute prolactin can increase levels of pSTAT5 above basal levels. Conversely, obese women who have low levels of leptin (appetite-suppressing hormone), genetically show a normal prolactin response. Besides, the expression of leptin receptors in this study observes in the basal/myoepithelial cells of the mammary glands.23
A study of 2288 mothers with different body mass index showed that mothers who were overweight or obese had shorter duration and less milk production.24 The under nutrient (starving) condition will decrease the synthesis of the mRNA gene that codes for milk protein in mice.25,26 Based on research on 50 obese mothers (pregestational BMI) vs. 50 normal mothers (sample match) observed until the age of one month postpartum, found differences in high leptin levels in obese mothers (4.8ng/ml vs. 2.5ng/ml). Also found differences in the volume of milk on days three to five, with a lower volume of milk in obese mothers.27 PRLR, JAK2, and STAT5 signalling are essential for the healthy development of breast tissue during the pregnancy and lactation phase. However, excessive expression of PRLR in pre and postmenopausal women increases the risk of developing breast cancer. More than 95% of cases of breast cancer are found to be overexpressed by PRLR. JAK2 is very important for tyrosine phosphorylation and STAT5 activation in response to PRL signalling. The phenotype abnormalities observed in the mammary glands of conventional and conditional knockout mice that lack PRL, their receptors, or both STAT5 isoforms (i.e., STAT5a and STAT5b) are very similar.28 JAK2/STAT5 signalling cascade is critical to mediate the primary biological responses initiated by the PRL. In murine (rat family), PRLR signalling through JAK2 and STAT5 is needed for the proliferation and differentiation of alveolar progenitors during pregnancy and lactation. Phosphorylated STAT5 in tyrosine residues 694 or 699 in STAT5a and STAT5b was also found in a subset of human breast cancer cases.29,30 Similar to the increased levels of PRL that circulate or stimulate the local synthesis of this hormone in the mammary glands, overexpression of wild type STAT5a and constitutionally active JAK2/STAT5 fusion proteins can induce neoplastic transformation of MECs in vivo.19 These findings prove that the manifestation of excessive circulation of PRL through the hyperactive JAK2/STAT5 signalling cascade may mediate the growth of breast cancer in humans.
So the underlying assumption formed in this research is that PRLR supports milk production through the physiological activation pathway of the JAK2/STAT5 cascade. However, further tracing is needed on another path transcribed by STAT5. Including the role that should be observed are mothers who enter the involution phase through the expression of cyclin D1. In addition to further exploring the role of STAT5 in lactation activation and transduction, it is also recommended to be able to analyze the expression of STAT1 and STAT3 through JAK2, taking into account other contributing variables.
ConclusionThis study successfully strengthened the findings in experimental animals (mice and mammals) that, along with the increased expression of breast milk encoding genes, also phenotypically increased milk volume. This formulation may occur with the support of functional nutritional status in early pregnancy. This study provides an understanding that the nutritional factor is a biological underlying that supports the process of transcription and translation of milk-coding genes in order to work optimally. Thus, it is essential to intervene with excess weight pregnant women with the right antenatal care midwifery approach.
Conflict of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the Technology Enhanced Medical Education International Conference (THEME 2019). Full-text and the content of it is under responsibility of authors of the article.