69 - LONG-TERM (5-YEAR) MAINTENANCE OF CLINICALLY MEANINGFUL IMPROVEMENT IN HEALTH- RELATED QUALITY OF LIFE (HRQoL) IN PATIENTS WITH MODERATE TO SEVERE CROHN’S DISEASE (CD) TREATED WITH USTEKINUMAB IN THE IM-UNITI LONG- TERM EXTENSION (LTE) STUDY
1University of California San Diego, La Jolla, CA, USA. 2University of Birmingham, Birmingham, UK. 3Janssen Global Services, LLC, Malvern, PA, USA. 4Janssen Scientific Affairs, LLC, Horsham, PA, USA. 5Janssen Research & Development, LLC, Spring House, PA, USA. 6Robarts Clinical Trials, Western University, London, ON, Canada.
Introduction: In the IM-UNITI study, subcutaneous ustekinumab (UST) was safe & efficacious for maintenance in moderately to severely active CD patients who responded to UST induction therapy. In the IM-UNITI LTE, UST maintained clinically meaningful improvement in HRQoL through Week (W) 140. We present the final HRQoL results for patients receiving UST in LTE study through 5 years (W252).
Methods: Patients completing the W44 IM-UNITI study safety & e ficacy evaluations were eligible to continue in the LTE (UST 90 mg every 12W [Q12W] or UST 90 mg Q8W; placebo not evaluated). HRQoL was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ) & Medical Outcomes Study 36-Item Short Form (SF-36). Clinically meaningful improvement was defined as an IBDQ change ≥ 16 points or change in SF-36 mental component summary (MCS) or physical component summary (PCS) score ≥ 5 points. Data for all treated patients from randomized/nonrandomized populations were summarized through W252. For continuous endpoints, patients with treatment failure between W44-W252 had induction baseline (BL) values carried forward and patients with insu ficient data had last value carried forward. Binary endpoints were analyzed using a nonresponder imputation approach.
Results: Mean IBDQ & SF-36 scores (tables) at maintenance BL were comparable for both UST regimens. At W252, for both UST regimens, improvements in IBDQ & SF-36 scores that were achieved by maintenance BL were maintained; further improvement was observed in IBDQ bowel & emotional symptoms scores. From BL to W252, clinically meaningful improvement in IBDQ total score was achieved (Q12W: 40.8%; Q8W: 43.2%). Clinically meaningful improvement was also observed in SF- 36 PCS (Q12W: 37.5%; Q8W: 37.7%) and MCS scores (Q12W: 33.9%; Q8W: 31.0%).
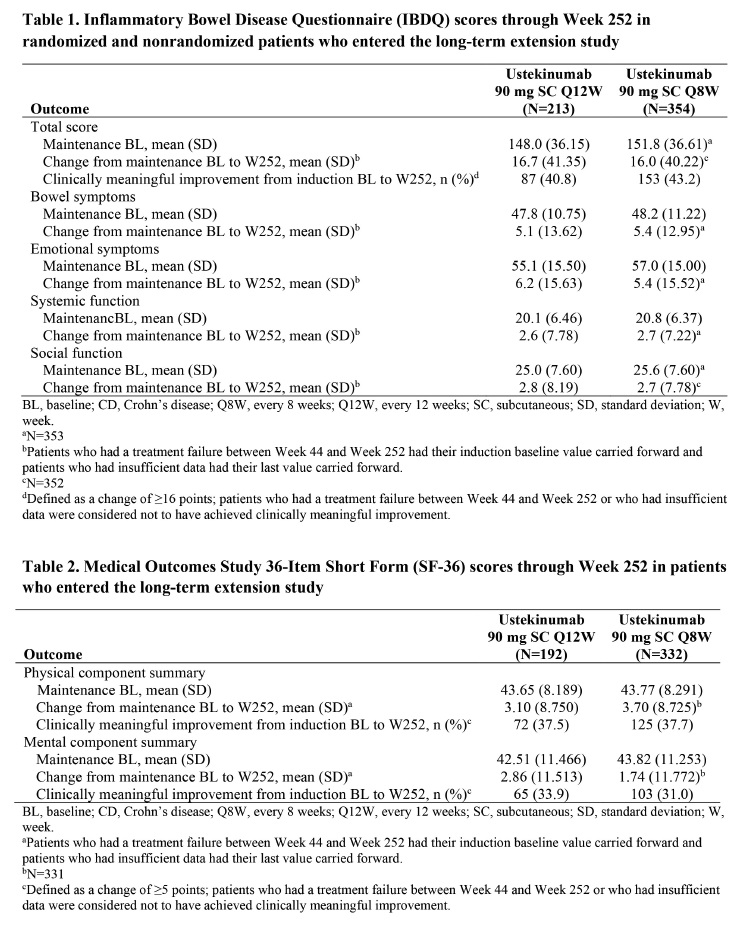
Conclusions: Long-term (5 years) treatment with UST 90 mg (Q12W/Q8W) was effective at maintaining improvements in HRQoL that were achieved during UST induction therapy in CD patients.








