The COVID-19 pandemic has a significant impact on the physical and mental health of people around the world. Parkinson's patients need to visit hospitals regularly to evaluate and adjust the dosage of drugs. Studies have shown that anxiety, exacerbated by existing conditions ‒ including the challenges posed by the COVID-19 pandemic ‒ can aggravate the symptoms of Parkinson's disease. This study aims to assess the impact of the quarantine period on the severity of movement symptoms, quality of life, non-motor symptoms, and the relationship with sex, education level, and age in patients with Parkinson's disease.
MethodsThis descriptive cross-sectional study includes 200 patients with Parkinson's disease who were treated in Bu Ali Hospital from April 2019 to the end of 2021. Primary data was collected using the patients' files, which included information on age, gender, education level, medications and dosage, duration of Parkinson's disease, the severity of the disease was evaluated based on the Hohen and Yehr (H&Y) criteria, and Quality of Life (QOL) and the impact of the quarantine period on their illness and quality of life has been collected through the Parkinson's Disease Questionnaire (PDQ-39) questionnaire. The collected data were analyzed using SPSS software, descriptive statistics, t-test, and analysis of variance.
ResultsThe quarantine period has a significant effect on the severity of Parkinson's disease and quality of life. The mean severity of the disease increased from 2.85 before quarantine to 3.30 during quarantine (p < 0.05), indicating an increase in motor symptoms. Similarly, quality of life scores in all dimensions decreased from 62.8 before quarantine to 48.2 during quarantine (p < 0.05), indicating a decrease in quality of life. Pearson's correlation test was used to investigate the relationship between age and the change in patients’ quality of life and the change in disease severity (p < 0.05).
ConclusionThe quarantine period due to the COVID-19 epidemic has had a significant impact on the severity of Parkinson's disease and the quality of life of patients. The findings of this study indicate the need to develop strategies to provide better health care, social support, and physical activity for patients with Parkinson's disease during quarantine and the pandemic.
Parkinson's disease is the second most common age-related neurodegenerative disease, following Alzheimer's disease.1,2 The most common type of Parkinson's disease occurs without an apparent cause.3 This idiopathic type is not secondary to some known reasons, and it exhibits a sustained response to treatment with dopaminergic medications.4,5
Pathologically, a prominent feature of Parkinson's disease is the destruction of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies in the remaining cells of this region.6
The prevalence of COVID-19 has had a profound impact on people's lives worldwide, leading to unprecedented changes in various areas, including health. Many countries have been compelled to enact strict measures to halt or reduce the transmission chain of the virus.7 These measures include social distancing and the shutdown of all non-essential activities and businesses.8 Healthcare systems shifted their focus and priority to COVID-19 treatment, resulting in the abrupt interruption of many clinical services.9 These laws have had a significant effect on the lives of individuals with chronic diseases, including Parkinson's patients.10 Their daily routines, access to healthcare providers, medication availability, and activities such as physiotherapy and exercise were disrupted.11 This disruption may exacerbate Parkinson's symptoms and reduce the quality of life.12
Since elderly individuals bear the highest burden of two severe diseases, namely COVID-19 and neurodegenerative diseases, understanding the relationship between COVID-19 and its impact on conditions like Parkinson's is of paramount importance.1 Studies indicate that quarantine, by significantly disrupting access to healthcare facilities, has led to the exacerbation of symptoms in many patients. Among all the symptoms, the worsening of bradykinesia has been reported most frequently.13
Furthermore, studies have shown that anxiety resulting from COVID-19 infection and the subsequent quarantine exacerbates the symptoms of Parkinson's disease.14 Given the ongoing COVID-19 pandemic and continued quarantine and social distancing, the authors still lack extensive information about the impact of quarantine on the severity of Parkinson's disease.2 Based on conducted studies, there is no clear information regarding the impact of age and gender during quarantine on patients with Parkinson's.14
Translation: The hypothesis exists that individuals with Parkinson's disease may be at a higher risk of contracting COVID-19 due to indirect associations with age, coexisting medical conditions, and the frequency of medications used. Respiratory disorders are a direct risk factor for severe respiratory complications resulting from COVID-19 infection in this population. Furthermore, quarantine conditions may indirectly impact both the motor and non-motor symptoms of Parkinson's disease, potentially leading to an exacerbation of the disease's symptoms.15
The methods of caring for individuals with Parkinson's disease need improvement. Apart from clinics and organizations specifically for these patients, there are no specialized rehabilitation programs available, and advanced treatment options are still in development, which means their accessibility for patients is limited.16
This study was conducted to investigate the impact of the quarantine period on the severity of motor symptoms in Parkinson's patients, to assess the effect of the quarantine period on the quality of life or non-motor symptoms of Parkinson's patients, and to examine Parkinson's disease symptoms based on gender, level of education, age of patients, and duration of the disease.
Materials and methodsStudy design and participantsIn this cross-sectional descriptive study, participants were selected through either random or convenience sampling methods. This included all patients previously diagnosed with Parkinson's disease according to specified criteria and attending Bu Ali Hospital's treatment center before the COVID quarantine. Initial data, including demographics, disease onset age, education level, medications, regular physician visits, symptoms, and disease severity. All participants were required to be diagnosed with idiopathic Parkinson's disease by a neurology specialist and be aged over 18.
The inclusion criteria were:
- •
Diagnosed with idiopathic Parkinson's disease by a neurology specialist.
- •
Aged over 18.
- •
History of COVID-19 infection was permissible provided the participants were asymptomatic and fully recovered at the time of data collection.
Exclusion criteria were:
- •
Patients with atypical Parkinsonism.
- •
Active COVID-19 infection during the time of the study.
- •
Severe medical conditions that could confound the study results.
Ultimately, 200 individuals meeting the inclusion criteria were selected for the study.
Data collection toolsPatients utilized the Persian version of the PDQ-39 questionnaire, which comprises 39 questions across eight domains. Notably, this version has been translated and validated in Iran, affirming its reliability and validity. It serves as a quality assessment tool for Parkinson's disease patients in the country.17
Disease severity and clinical stage were assessed by a neurologist using the Hoehn and Yahr criteria, which has 5 stages (1‒5). Patients were categorized according to disease severity as follows: a) Mild disease (HY: I‒II); b) Moderate disease (HY: III); c) Severe disease (HY: IV‒V).15
Participants in this study, upon consent, engaged via interviews (in-person or by telephone) or completed questionnaires through messaging apps. Patients visiting the clinic completed the questionnaire themselves when feasible. For those unable to visit, phone interviews were conducted, or questions were sent electronically.
The sampling included all Parkinson's disease patients diagnosed by a neurologist pre-quarantine, per Hoehn and Yahr criteria, who visited or were admitted to Bu Ali Hospital's Neurology Clinic during the 2020‒2021 pandemic period.
Sample size calculationThe sample size was calculated using the following formula: n:z(1−α/2)2×p(1−p)d2; n: Sample size, z: Researcher's confidence level, p: Proportion of the attribute in the population, d: Acceptable margin of error for the researcher.
Based on the standard deviation of the movement section in patients visiting the clinic in the Parkinson's study, which was 62.31, and considering the study precision of 5.4, and using the formula 0.05^2 = a, the sample size was calculated to be 189. Eventually, 200 individuals were enrolled in the study.17
Post-hoc analysis: impact of COVID-19 infection on PD severity and quality of lifeTo address the potential impact of previous COVID-19 infection on Parkinson's disease severity and quality of life, a post-hoc analysis was conducted. This analysis compared disease severity (using the Hoehn and Yahr criteria) and PDQ-39 scores between participants with and without a history of COVID-19 infection.
The analysis revealed that participants with a history of COVID-19 infection demonstrated a more pronounced worsening of PD symptoms and a greater decline in quality of life compared to those who had not been infected. No significant correlations were found between COVID-19 infection history and other demographic factors, suggesting that the observed differences were likely attributable to the infection itself.
Data analysisData analysis was conducted using Microsoft Excel spreadsheets and SPSS version 23.0. The responses from the PDQ-39 questionnaire encompassed 39 questions across eight dimensions: Movement (1-question), Activities of daily living (6-questions), Feeling of well-being (6-questions), Stigma (4-questions), Social support (3-questions), Cognition (4-questions), Communication (3-questions), and Physical discomfort (3-questions). Each question implemented a five-option Likert scale, where one option was selected. The scale ranged from the best state (score 0) to the worst state (score 4).
The scoring range for each dimension ranges from 0 to 100, where a score of zero denotes no issues, and a score of 100 reflects the poorest health condition. To calculate the score for each dimension, the sum of raw scores for the questions within that dimension is divided by the sum of the maximum possible raw scores for those questions. The resulting fraction is then multiplied by 100. These average scores across dimensions collectively form a unified index known as the PDSI (Parkinson's Disease Summary Index), which also ranges from zero to one hundred.
The data was initially assessed using the Kolmogorov-Smirnov test to confirm the normality of the distribution (p > 0.05). For comparing changes in the quality of life and disease severity before and during the COVID-19 pandemic quarantine, a paired t-test was used, revealing significant differences across these periods.
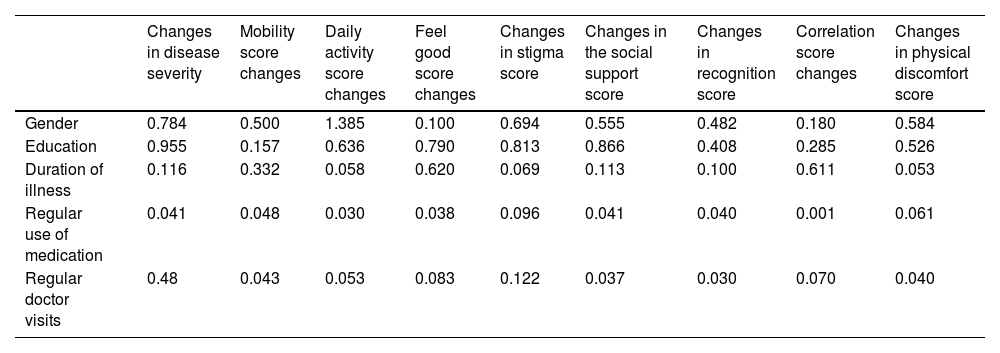
To further investigate the relationships between demographic and clinical variables (such as age, gender, education, disease duration, regular medication use, and physician visits) and the observed changes in quality of life and disease severity, Pearson's correlation coefficient was employed. Pearson's correlation was particularly used to examine the association between age and changes in quality of life and disease severity, as the data was normally distributed (p > 0.05). The analysis revealed a significant positive correlation between age increased disease severity and decreased quality of life during the quarantine period (p < 0.05).
Additionally, a one-way analysis of variance (ANOVA) was conducted to compare the mean differences between groups categorized by gender, education, disease duration, regular medication use, and regular physician visits in relation to changes in quality of life and disease severity. The ANOVA test helped determine whether these categorical variables had a significant impact on the changes observed. The results showed no significant association between gender, education level, or disease duration and changes in quality of life or disease severity. However, patients who adhered to regular medication use and physician visits exhibited significantly less decline in quality of life and smaller increases in disease severity (p < 0.05).
The study was approved by the Ethics Committee of the Islamic Azad University, Tehran Medical Branch (IR.IAU.TMU.REC.1401.066).
This work was in line with the STROBE criteria.
ResultsIn this study, 200 patients with Parkinson's disease were enrolled in a descriptive-analytical cross-sectional survey. Using the Parkinson's Disease Questionnaire (PDQ39) and the Jahan and Yaher criteria, the quality of life of the patients and the severity of their disease were assessed and compared before and during the quarantine resulting from the COVID-19 pandemic (Table 1).
Descriptive characteristics.
On average, participants were assessed approximately 6-months after the onset of the COVID-19 pandemic-related quarantine measures, which began in early 2020. However, it is important to note that the exact duration of quarantine for each participant varied, as precise documentation of individual quarantine periods was not uniformly available. Therefore, while the authors provide an approximate average of 6-months, this estimate reflects the general period during which most participants were under quarantine restrictions rather than a precise measure for each individual.
The patients were initially examined for demographic variables. The average age of the participants was 65.83±3.85 years. The youngest patient was 56-years-old, and the oldest was 81-years-old.
It was also observed that most of the examined patients (63.5 %) were men. Of the 200 participants, 34 individuals (17 %) had a history of COVID-19 infection before the study, though none were actively infected during the study period. Patients were also questioned regarding their level of education.
Patients were also questioned regarding their level of education. It was observed that 81 individuals (40.5 %) had completed secondary school, 75 individuals (37.5 %) were either illiterate or had primary education, and 44 individuals (22.0 %) had university education.
Additionally, it became evident that in 108 patients (54.0 %), <5-years had passed since the diagnosis of the disease, while in 92 patients (46.0 %), >5-years had passed since the diagnosis.
The examined patients were also assessed for regular medication use and regular visits to the treating physician during the quarantine due to COVID-19. It was evident that most of these patients (55.5 % and 60.0 %) did not have regular medication use or regular visits to the treating physician.
The severity of Parkinson's disease in patients was assessed using the Hoehn Yahr scale. According to this scale, patients with unilateral involvement of limbs are considered Grade 1, bilateral involvement of limbs without balance impairment is Grade 2, bilateral involvement of limbs with balance disorder is Grade 3, patients with severe disability are Grade 4, and those requiring a wheelchair for mobility are Grade 5. In both periods, most patients were in Grade 3 (27.5 and 27.5 %) or Grade 4 (24.5 and 22.0 %).
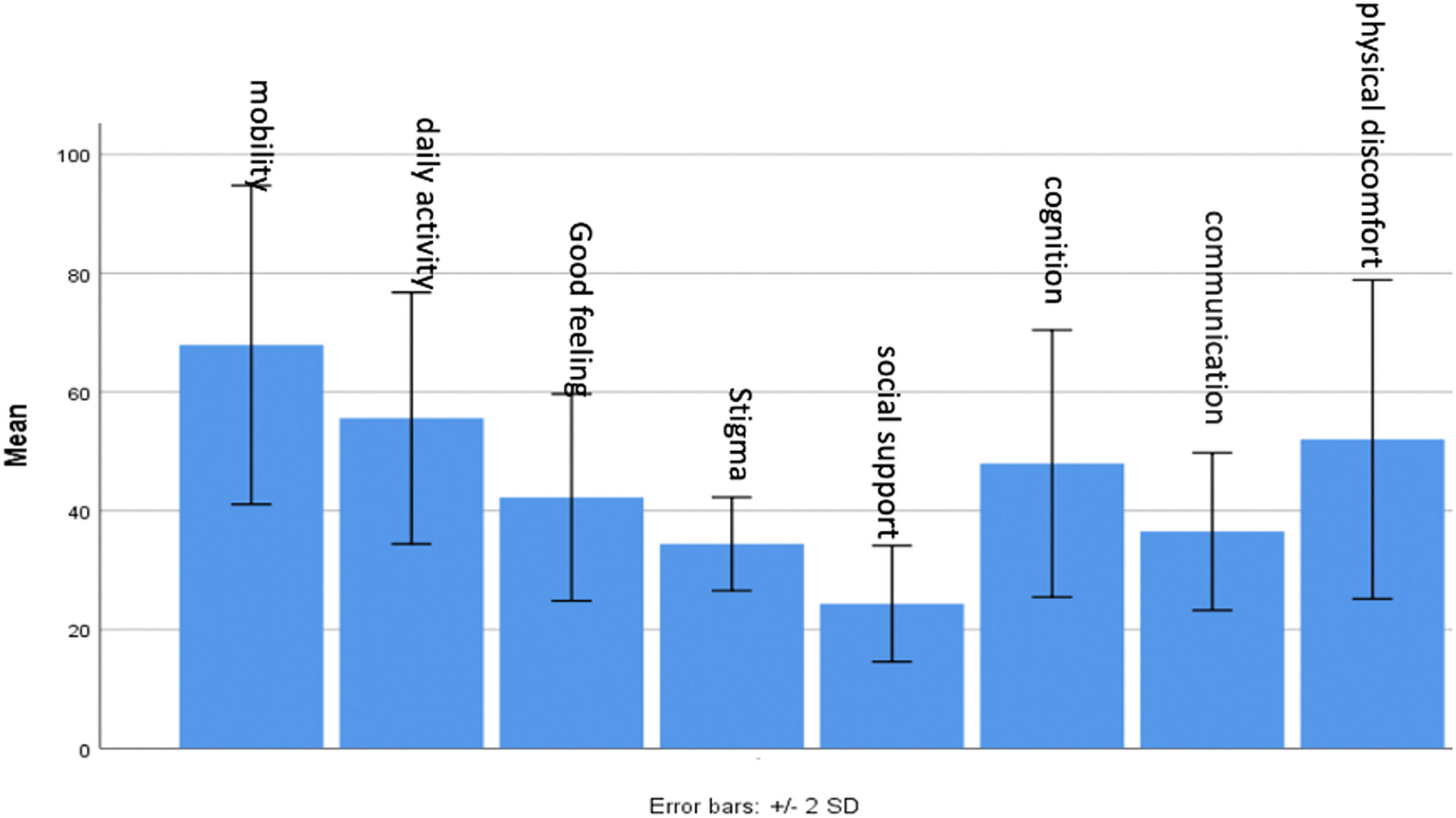
The PDQ39 questionnaire was utilized to evaluate the quality of life among patients, encompassing various aspects such as mobility, daily activities, well-being, stigma, social support, cognition, communication, and bodily discomfort. Each question is scored from 0 to 100, with 0 indicating the best condition and 100 representing the worst state (Fig. 1).
During the COVID-19 pandemic quarantine, the PDQ39 questionnaire was again employed to assess patients’ quality of life. Findings indicated that patients encountered difficulties across all evaluated criteria, resulting in a lower overall quality of life during the quarantine period (Fig. 2).
Analytical statisticsThe study assessed the difference in questionnaire scores between different sections, with data analyzed using the Kolmogorov-Smirnov test to confirm normal distribution (p > 0.05). Subsequently, a paired t-test was conducted, revealing a significant decline in patient quality of life across all sections during the COVID-19 pandemic quarantine (Table 2).
Evaluation of the difference in scores before and during the COVID-19 quarantine in the study participants.
Furthermore, the study evaluated changes in patient quality of life before and during the COVID-19 pandemic quarantine, initially verifying data normality using the Kolmogorov-Smirnov test (p > 0.05). A paired t-test was then employed, indicating a significant increase in disease severity among patients during the quarantine period (p < 0.05).
The study investigated the impact of age on changes in both quality of life and disease severity following the COVID-19 quarantine. Utilizing Pearson's correlation due to the normally distributed data (p > 0.05), it was found that higher age during the quarantine correlated with increased disease severity and lower quality of life (p < 0.05) (Table 3).
The study also explored how gender, education, disease duration, regular medication use, and physician visits influenced changes in patients’ quality of life and disease severity post-quarantine due to the COVID-19 pandemic. A one-way ANOVA test was employed to analyze the relationship between these variables and changes observed in both quality of life (as assessed by PDQ39 questionnaire scores) and disease severity (based on Hoehn and Yahr criteria) (Table 4).
Investigation of the effect of age on the quality of life in the research subjects.
No significant correlation was found between education, gender, or disease duration and changes in quality of life or disease severity during quarantine. However, patients who adhered to regular medication use or maintained regular visits to their physician showed less decline in quality of life and a smaller increase in disease severity based on PDQ39 questionnaire criteria (p < 0.05) (Table 5).
Examination of some background variables on the quality of life of the research subjects.
In 2019, the rapid spread of COVID-19 led the World Health Organization (WHO) to declare it a pandemic on March 11, 2020. This global crisis profoundly impacted various aspects of life, affecting physical and mental health worldwide. Vulnerable populations, such as the elderly and those with chronic conditions like Parkinson's Disease (PD), faced higher risks of contracting COVID-19 and experiencing severe outcomes.18
PD is a chronic neurological disorder affecting around 1 % of people over 60. During the pandemic, widespread implementation of long-term quarantine measures aimed to curb the virus's spread. However, reduced mobility, social distancing, and isolation measures could exacerbate psychological stress, potentially worsening PD symptoms and diminishing patients’ quality of life.19 Hence, recognizing the significance of Parkinson's disease and seeking to enhance the comprehension of how quarantine affects disease severity and patient quality of life, particularly amidst a dearth of similar studies, the authors conducted a cross-sectional analytical investigation on this matter.
In the 2021 study by Lopez et al., it was concluded that the pandemic's true effect on Parkinson's patients remains uncertain. However, prolonged quarantine is linked to a decline in their self-reported health status, potentially attributed to factors like heightened depression and anxiety, reduced physical activity, or social isolation. This finding aligns with the study.20 The present study supports this observation, indicating that quarantine not only exacerbates the physical symptoms of PD but also severely impacts mental well-being. These findings underscore the importance of addressing both physical and psychological health in PD management, particularly in situations of prolonged isolation. Practical measures could include ensuring regular physical activity, providing psychological support, and encouraging social engagement through virtual platforms to mitigate these adverse effects.
In Martinez et al.’s 2021 study, findings revealed a decrease in Mini-BESTest scores after two months of quarantine. Statistical analysis further revealed a worsening of disease severity (based on Hoehn and Yahr criteria) during quarantine, consistent with previous findings. Moreover, patients’ quality of life (assessed via the PDQ39 questionnaire) also significantly declined during this period (p < 0.05). However, unlike this study, changes in patients’ body mass index were not examined, highlighting a distinction between the two studies. The present study corroborates these results and suggests that healthcare providers should prioritize regular monitoring of PD patients during extended periods of isolation, potentially through telemedicine, to identify and address worsening symptoms early.
In Shalash et al.’s 2021, most PD patients reported a negative impact on mental health, physical activity, healthcare routines, and interest in virtual visits. Unlike this study, which compared quality of life and disease severity before and after quarantine, theirs compared these factors within a time frame and between control and patient groups.21
In Guo et al.’s 2020 study, patients who changed their regular medications due to quarantine experienced a greater decline in quality of life. Similarly, the present study found that patients who did not have regular physician visits and did not regularly take their medications showed a significant decline in quality of life and an increase in disease severity, consistent with Guo et al.’s findings.22
In a 2021 review study conducted in the UK, the findings suggest that Parkinson's Disease (PwPD) patients experienced exacerbated symptoms and declined mental health during the pandemic, likely due to changes in medical care, daily routines, social support, or physical activity. Although not compared directly with other chronic diseases, similar negative impacts on neurological symptoms and cognitive function were observed, as seen in studies on Alzheimer's disease. This highlights the broader effects of the pandemic on individuals with chronic conditions.22
A study in Israel involving 142 Parkinson's patients found that social distancing and COVID-19 quarantine negatively affected them. Nearly 60 % experienced a reduction in physiotherapy sessions, while almost 40 % reported increased negative emotions and fatigue, leading to a decline in well-being. Additionally, around 40 % reported health changes, including worsened symptoms and weight gain.23
In a German study involving 39 neurology specialists and one general practitioner, 75 % of the participants reported primarily treating Parkinson's patients, indicating a high level of specialization. Most respondents noted a worsening of Parkinson's symptoms during the pandemic, aligning with previous cohort studies. This deterioration was attributed to factors such as reduced physical activity, canceled appointments leading to inadequate medication management, and the cessation of group or individual activities.24
In a Polish study, 47 Parkinson's patients aged 43–90 were studied. Results show that the psychological effects of quarantine have a greater impact on Parkinson's patients’ quality of life than their activity level or symptoms.25
In a study in Portugal, clinical outcomes before and after the pandemic were compared among Parkinson's disease patients to assess differences in behavior and exercise habits. After two months of quarantine, changes in exercise behavior, daily life aspects, activities of daily living, quality of life, sleep, falls, and overall clinical impact were evaluated. 27 participants aged 57–92 were involved, with 10 (37 %) completely stopping physical activity, while 17 (63 %) maintained some level of active lifestyle.26
A study in Italy involving 12 Parkinson's patients found that those who had received prior training on quarantine conditions and were regularly monitored showed minimal changes after 2-months of quarantine, with only an increased risk of falls and weight gain observed. The study highlights the importance of comprehensive patient education and technology assistance in managing symptoms during quarantine.27,28
ConclusionAt the end of the study, it was observed that quarantine and isolation result in a decline in the quality of life and an increase in disease severity among patients with Parkinson's disease, which is consistent with the findings of most conducted studies. Additionally, it was noted that this decline in quality of life or increase in disease severity is more evident in older patients and those who did not regularly use medication or visit their treating physician periodically.
LimitationsThis study has several limitations. Firstly, the cross-sectional design limits the ability to infer causality between quarantine measures and the observed declines in quality of life and disease severity. Secondly, the reliance on self-reported data, particularly in the PDQ-39, may introduce bias due to participants’ subjective interpretation of their health status. Thirdly, the sample size, while sufficient for detecting significant differences, may not fully represent the broader PD population, particularly those with more severe forms of the disease. Lastly, the study did not account for potential confounding variables such as pre-existing mental health conditions, which may have influenced the outcomes. Future studies should aim to include larger, more diverse samples and utilize longitudinal designs to better understand the long-term impact of quarantine on PD patients.
Human and animal rightsNo animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (Islamic Azad University, Tehran Medical Branch (IR.IAU.TMU.REC.1401.066), and with the Helsinki Declaration of 1975, as revised in 2013. This study was approved by the Research Ethics Board of Islamic Azad University.
Consent for publicationInformed consent was obtained from each participant.
Data availability: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributionsConceptualization: FR, RHN. Methodology: FR, RHN. Software: MSF, DMM, KA. Validation: EE, ZA. Formal analysis: MSA. Investigation: HA. Resources: RHN, EE. Data Curation: KA. Writing-Original Draft: MSA, DMM. Writing-Review & Editing: MSA. Visualization: HA, ZA. Supervision: FR, RHN, EE. Project administration: ZA, FR. Funding acquisition: RHN, EE.
The authors deny any conflict of interest in any terms or by any means during the study.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.