Bronchial Asthma, characterized by chronic airway inflammation and remodeling, poses significant clinical challenges. Long-term use of conventional therapies often leads to metabolic complications and drug resistance. The bioactive alkaloid Tetramethylpyrazine, derived from Ligusticum chuanxiong Hort. (Apiaceae), demonstrates multi-target therapeutic potential through pathway modulation. TMP restores Th1/Th2 and Th17/Treg immune balance via Gαs/cAMP/PKA and AMPK/NF-κB signaling while suppressing ORMDL3-mediated collagen deposition. Clinical evidence demonstrates effective symptom alleviation with minimal adverse effects, establishing TMP as a promising complementary strategy for asthma management.
BA is a chronic airway disorder driven by Th2-mediated inflammation, clinically manifesting as bronchial hyperresponsiveness and variable airflow obstruction.1 Its prevalence is notably elevated in pediatric populations,2 with global incidence rising due to environmental pollution, lifestyle shifts, and aging demographics. Current projections estimate approximately 400 million cases worldwide by 2025,3,4 posing substantial health burdens and socioeconomic challenges. The Global Initiative for Asthma recommends ICS-LABA combination therapy as first-line treatment. Although effective against acute inflammation and bronchoconstriction,5 long-term use causes adverse effects ranging from oral candidiasis to osteoporosis.6 Safety concerns, particularly β2-adrenoceptor-mediated cardiotoxicity, have intensified demand for novel therapeutics. Recent studies reveal TMP's unique ability to concurrently target the inflammation-immunity-remodeling axis.7 This review synthesizes TMP's anti-asthma mechanisms, deciphers its multi-target networks, and proposes integrative strategies to overcome current therapeutic limitations.
TMP: Source and pharmacodynamicsLigusticum chuanxiong Hort. (Apiaceae), a canonical herb in Traditional Chinese Medicine, was first documented in Shennong Bencao Jing (circa 200 BCE) for promoting blood circulation and relieving pain. Modern studies confirm its circulatory benefits derive from enhancing microcirculation and inhibiting platelet aggregation.8 The rhizome harbors diverse bioactive compounds: phthalides, phenolic acids, polysaccharides, and alkaloids.9 Among these, TMP stands out as the principal alkaloid,10 exhibiting multi-target effects including anti-inflammatory, antitumor, neuroprotective, and antioxidant activities11–15 (Fig. 1). Although clinically used for cardiovascular and neurodegenerative diseases,16–18 TMP shows exceptional potential in respiratory disorders by simultaneously suppressing airway inflammation and remodeling in asthma.19,20
Regulation of inflammatory cytokines and immune balanceRestoring Th1/Th2 balanceFunctioning as pivotal regulators of adaptive immunity, CD4+T-lymphocytes undergo differentiation into distinct Th (Th1, Th2, Th17) cells and Treg cells.21 In asthma, Th1/Th2 and Th17/Treg imbalance drives immunopathology (Fig. 2). IL-12 and IFN-γ activate STAT4/T-bet signaling to induce Th1 differentiation,22 enhancing macrophage phagocytosis for antimicrobial defense.23 IFN-γ further amplifies Th1 responses via STAT1-mediated T-bet upregulation, which suppresses IL-4 activity and establishes early protective immunity.24,25 Conversely, IL-4 activates STAT6/GATA-3 to polarize Th2 cells, triggering excessive IL-5/IL-13 secretion that fuels allergic responses.26 This cascade elevates IgE levels and recruits eosinophils through CCL11/CCL24, perpetuating type 2 inflammation.27 Asthma pathogenesis thus hinges on Th1/Th2 imbalance sustained by type 2 cytokines.28 TMP complements glucocorticoids by restoring immune equilibrium.7 In OVA-sensitized murine models, Xiong et al.29 demonstrated TMP's dual immunomodulatory effects. Administration of 80 mg/kg TMP significantly suppressed eosinophil infiltration and downregulated Th2-associated markers IL-4 and GATA-3 (p < 0.05). Concurrently, this treatment upregulated Th1-related factors IFN-γ and T-bet (p < 0.05) through coordinated T-bet/GATA-3 ratio modulation. Yan et al.30 conducted a clinical trial evaluating TMP as adjunctive therapy in pediatric asthma. Patients receiving 2.5‒5 mg/kg/d TMP for 10 days exhibited significant immunological shifts: serum IL-4 levels decreased from 56.30 ± 14.32 pg/mL to 38.24 ± 12.10 pg/mL (p < 0.05). Simultaneously, IFN-γ concentrations increased from 16.58 ± 6.25 ng/mL to 28.42 ± 10.37 ng/mL (p < 0.05). This bidirectional cytokine modulation effectively reversed Th1-Th2 clonal drift.
Mechanisms of Th1/Th2 and Th17/Treg Balance in BA and the Intervention of TMP. FOXP3, Forkhead Box Protein P3; Gata, GATA binding protein; IFN-γ, Interferon-γ; IL, Interleukin; RORγt, Retinoic acid Receptor-related Orphan Receptor γt; STAT, Signal Transducer and Activator of Transcription; T-bet, T-box expressed in T-cells; TGF-β, Transforming Growth Factor-Beta; Th, T-Helper; TMP, Tetramethylpyrazine; TNF-α, Tumor Necrosis Factor-α; Treg, Regulatory-T.
The transmembrane glycoprotein CD44 regulates cellular adhesion, migration, and signaling through interactions with ECM components such as hyaluronic acid.31 In asthma, CD44 overexpression facilitates eosinophil and neutrophil adhesion, driving airway infiltration and Th2-polarized inflammation.32 Kumar et al.33 found that CD44-knockout mice exposed to house dust mites and cigarette smoke showed significantly reduced inflammatory cell counts (p < 0.05) and suppressed T2 cytokine production. Compared to wild-type controls, these mice also exhibited fewer peribronchial eosinophils and mucus cells, confirming CD44 inhibition as an effective anti-inflammatory strategy. Li et al.34 revealed TMP's dose-dependent suppression of CD44 expression in asthma models. High-dose TMP (80 mg/kg) demonstrated superior efficacy to both lower-dose TMP (40 mg/kg) and dexamethasone (0.5 mg/kg) in mitigating pathological features.
Restoring Th17/Treg balanceThe Th17/Treg balance is a pivotal axis in asthma immunopathology, paralleling the importance of Th1/Th2 equilibrium. Th17 differentiation is driven by STAT3/RORγt signaling, contrasting with Treg development via STAT5-dependent FOXP3 activation.35 Th17 cells exacerbate inflammation by secreting IL-17A/IL-22/TNF-α, which recruit neutrophils and induce bronchoconstriction.36 Conversely, Tregs sustain immune tolerance through IL-10/IL-35 secretion, suppressing effector T-cell responses.37 Li et al.38 identified elevated serum IL-17 levels in acute asthma patients versus stable patients and healthy controls (p < 0.05). OVA-sensitized models mirrored this trend, showing increased IL-17 production (p < 0.05) with Th17/Treg imbalance driving disease progression. Moreover, Ji et al.39 revealed a critical limitation of dexamethasone: while effectively suppressing Th2 cytokines (IL-4/IL-5), it fails to inhibit Th17-derived IL-17. In contrast, TMP demonstrated dual immunomodulatory capacity by simultaneously blocking both Th2 and Th17 cytokine production while enhancing IL-10 secretion, albeit without altering FOXP3 expression patterns. This unique immunomodulatory profile reduces eosinophil/neutrophil infiltration and corrects Th17/Treg imbalance in allergic airway inflammation. Supporting evidence from Pan's team40 observed that TMP administration (1 mL/kg) upregulated both FOXP3 gene and protein expression in cerebral ischemia models. This cross-model evidence further supports TMP's regulatory capacity over the FOXP3/RORγt axis.
Modulating the Gαs/cAMP/PKA pathwayThe Gαs/cAMP/PKA axis is a critical signaling pathway disrupted in asthma. As a GPCRs component, Gαs dysfunction promotes airway inflammation (Fig. 3). Upon activation, Gαs dissociates from Gβγ to stimulate AC, converting ATP to Camp.41 This second messenger (cAMP) activates PKA, inducing phosphorylation of CREB/CREM transcription factors to maintain IL-2 production, T-cell proliferation, and anti-inflammatory mediator expression.42 In asthma, this pathway is suppressed, marked by reduced cAMP, impaired PKA activity, and inflammatory cell influx.43 Wang et al.44 demonstrated that daily 40 mg/kg TMP administration in OVA-induced asthmatic rats not only upregulated pulmonary Gαs expression (p < 0.01) but also restored cAMP levels and enhanced PKA activity (p < 0.01), collectively reactivating the impaired Gαs/cAMP/PKA pathway. This pathway reactivation significantly suppressed key Th2 cytokines ‒ TNF-α, IL-4, and IL-5 ‒ while improving immune balance (p < 0.01). PDE is a class of enzymes that hydrolyze cAMP and terminate its biochemical actions. Experimental validation shows that 40 mg/kg TMP administration suppresses PDE expression at both mRNA and protein levels. This dual inhibition of cAMP hydrolysis effectively attenuates airway hyperresponsiveness, as evidenced in preclinical models.45 TMP demonstrates partial β2-AR agonism, activating the cAMP/PKA pathway to induce bronchodilation comparable to salbutamol. Crucially, this effect occurs without the tachycardia or other adverse effects linked to conventional β-agonists.46
Mechanisms of the Gαs/cAMP/PKA Pathway in BA and the Intervention of TMP. AC, Adenylate Cyclase; ATP, Adenosine Triphosphate; Camp, cyclic Adenosine Monophosphate; CREB, cAMP-Response Element Binding; CREM, cAMP-Response Element Modulating; GPCRs, G Protein-Coupled Receptors; IL, Interleukin; PKA, Protein Kinase A; TMP, Tetramethylpyrazine.
The transcription factor NF-κB drives chronic inflammation in asthma when abnormally activated (Fig. 4). At rest, NF-κB remains inactive by binding to IκB. External triggers like bacteria or viruses activate TLRs, leading to IκB kinase-mediated IκBα phosphorylation and degradation. This exposes nuclear localization signals, allowing NF-κB to enter the nucleus and bind κB sites on the IL4 promoter, triggering excessive Th2 cytokine production.47 AMPK acts as a key regulator connecting energy metabolism and inflammation. It blocks NF-κB activity through two main methods. First, it phosphorylates IκB kinase to disable its function. Second, it increases SIRT1 production to remove acetyl groups from p65. Together, these actions lower inflammatory molecules and protect against asthma.48 In OVA-induced asthmatic mice, Xu et al.49 showed that intraperitoneal TMP (100‒200 mg/kg) boosted AMPK phosphorylation and increased p-AMPK/AMPK ratios in bronchial cells while suppressing NF-κB p65 protein. This combined action effectively controlled airway inflammation. Liu et al.19 additionally, confirmed TMP's suppression of NF-κB signaling, which lowers IL-1β and TNF-α levels to ease airway inflammation in neutrophilic asthma models. These combined results highlight TMP's promise for developing new asthma treatments.
Modulating the MAPK/ERK pathwayMAPK enzymes respond to various triggers (e.g., IL-1, IL-6) by activating serine/threonine kinases. In asthma, abnormal p38α MAPK activation drives disease progression, transmitting extracellular signals to the nucleus to alter gene expression and cellular responses.50 Liang et al.51 found OVA sensitization increased phosphorylated p38 MAPK and nuclear p65 levels in murine lungs while reducing oxidative stress markers MDA and GSH-Px (p < 0.05). The p38 MAPK pathway activates ERK1/2 through a three-tier signaling cascade (MAPKKK→MAPKK →MAPK), critically amplifying airway inflammation52 (Fig. 5). Mei et al.53 discovery that daily 80 mg/kg TMP treatment reduced p38 MAPK staining intensity in asthmatic mice from 0.217 ± 0.014 to 0.156 ± 0.003 (p < 0.05), effectively alleviating bronchial smooth muscle spasms, airway narrowing, and inflammatory cell infiltration. Wei's team54 confirmed the TMP suppresses p38 MAPK signaling to decrease neutrophils, lymphocytes, and eosinophils while lowering IL-4/IL-5 and related chemokines in asthma models. Beyond inflammation control, p38 MAPK contributes to airway remodeling. Inhibiting this pathway reduces fibroblast-derived IL-6/IL-8 and bronchial cell CTGF production, countering fibrosis and structural changes.55,56
In asthmatic inflammation, pro-inflammatory factors like IL-1β and TNF-α activate the MAPK/ERK pathway, rapidly inducing transcription of the proto-oncogene c-fos. The c-fos protein then combines with JNK to form the AP-1 complex, which drives expression of downstream inflammatory mediators (COX-2, MMPs) that amplify airway inflammation and hyperresponsiveness.57 Animal studies58 demonstrated that TMP treatment significantly reduced lung c-fos levels in asthmatic rats, with mRNA decreasing from 0.40 ± 0.06 to 0.19 ± 0.05 and protein from 0.56±0.07 to 0.31 ± 0.12 (p < 0.01). By suppressing AP-1 complex formation, TMP further lowered IL-8 and TNF-α release, confirming its therapeutic effect on airway inflammation. In simulated weightlessness models using tail suspension,59 TMP modulated ERK1/2 signaling to suppress excessive c-fos expression (0.14 ± 0.01 in TMP-treated vs. 0.16 ± 0.01 in tail-suspended controls; p < 0.05). This intervention reduced abnormal proliferation of vascular endothelial and smooth muscle cells; effectively mitigating lung tissue damage caused by simulated microgravity.
Additional mechanismsSCF exacerbates asthma by activating mast cells and modulating immune responses. It promotes mast cell survival, proliferation, and activation, driving IL-13 secretion that amplifies airway inflammation and hyperreactivity.60 Furthermore, SCF enhances mast cell migration to inflammatory sites via c-Kit receptor activation.61 Normal mice-maintained serum SCF levels at 48.6 ± 11.2 pmoL/L, which surged to 114.9 ± 27.3 pmoL/L (p < 0.01) after 1 % OVA challenge. TMP dose-dependently reversed this increase, reducing levels to 70.6 ± 7.9 pmoL/L with 40 mg/kg and near-baseline 51.4 ± 8.1 pmoL/L at 80 mg/kg.62 The observed SCF reduction implies TMP's regulatory effect on this pathway, warranting further investigation into its therapeutic potential for asthma treatment.
P-selectin and E-selectin, as adhesion molecules, drive airway inflammation by mediating leukocyte-endothelial adhesion and migration. In patients experiencing acute asthma exacerbation, P-selectin levels are slightly elevated but return to baseline within 36 months.63 This sustained elevation critically exacerbates eosinophil infiltration.64 E-selectin, mainly endothelial-derived, promotes leukocyte rolling and firm adhesion, facilitating transendothelial migration. This process releases histamine and leukotrienes, causing bronchoconstriction, mucus hypersecretion, and chronic airway remodeling.65 Preclinical studies in OVA-sensitized rats revealed that 80 mg/kg TMP significantly reduced serum P-selectin levels from 72.39±3.78 ng/L to 24.17±1.98 ng/L (p < 0.05). This reduction outperformed both 40 mg/kg TMP alone and the combination of 40 mg/kg TMP with 0.25 mg/kg dexamethasone. Further analysis showed prolonged 80 mg/kg TMP administration achieved efficacy comparable to 0.5 mg/kg dexamethasone.66 Clinically, intravenous TMP (3‒5 mg/kg) significantly lowered serum P-selectin in pediatric asthma patients (p < 0.01), with 95 % therapeutic efficacy surpassing the 70 % observed with glucocorticoids (p < 0.05).67 Higher doses (40‒80 mg/day IV) also modulated soluble E-selectin and improved lung function.68 These findings collectively reveal TMP’s dual mechanism: disrupting P/E-selectin-mediated leukocyte adhesion/migration and rebalancing inflammatory networks to suppress airway pathology.
Asthma often involves platelet activation and aggregation. TMP counters this by lowering intracellular calcium levels in platelets, thereby reducing their activation, aggregation, and adhesion. This suppression decreases lymphocyte and eosinophil mobilization while significantly curtailing IL-5 and IL-13 production in asthmatic lungs, demonstrating potent immunomodulatory and anti-inflammatory effects.69
Suppressing airway remodelingAirway remodeling represents a core pathological feature of chronic asthma, characterized by irreversible structural alterations. Key changes encompass epithelial damage, thickened basement membranes, smooth muscle overgrowth, goblet cell metaplasia, excessive blood vessel formation, and abnormal ECM buildup.70 Chronic inflammation drives EMT, causing epithelial cells to lose polarity and transform into fibroblasts. This process promotes excessive type I/III collagen deposition beneath the basement membrane, leading to fibrosis and airway narrowing. Concurrently, TGF-β and VEGF activate fibroblasts/myofibroblasts to overproduce fibronectin while inhibiting MMP activity, disrupting ECM homeostasis.71 Aberrant activation of PDGF and EGF pathways drives ASMC proliferation and hypertrophy, enhancing contractility and pro-fibrotic mediator secretion. This exacerbates airway hyperresponsiveness through structural and functional alterations.72 Concurrently, VEGF-mediated angiogenesis and NGF overexpression worsen airway edema and neuronal hypersensitivity, creating a self-perpetuating inflammation-remodeling cycle.73 These pathological cascades collectively result in irreversible airway narrowing, glucocorticoid resistance, and progressive lung function deterioration.
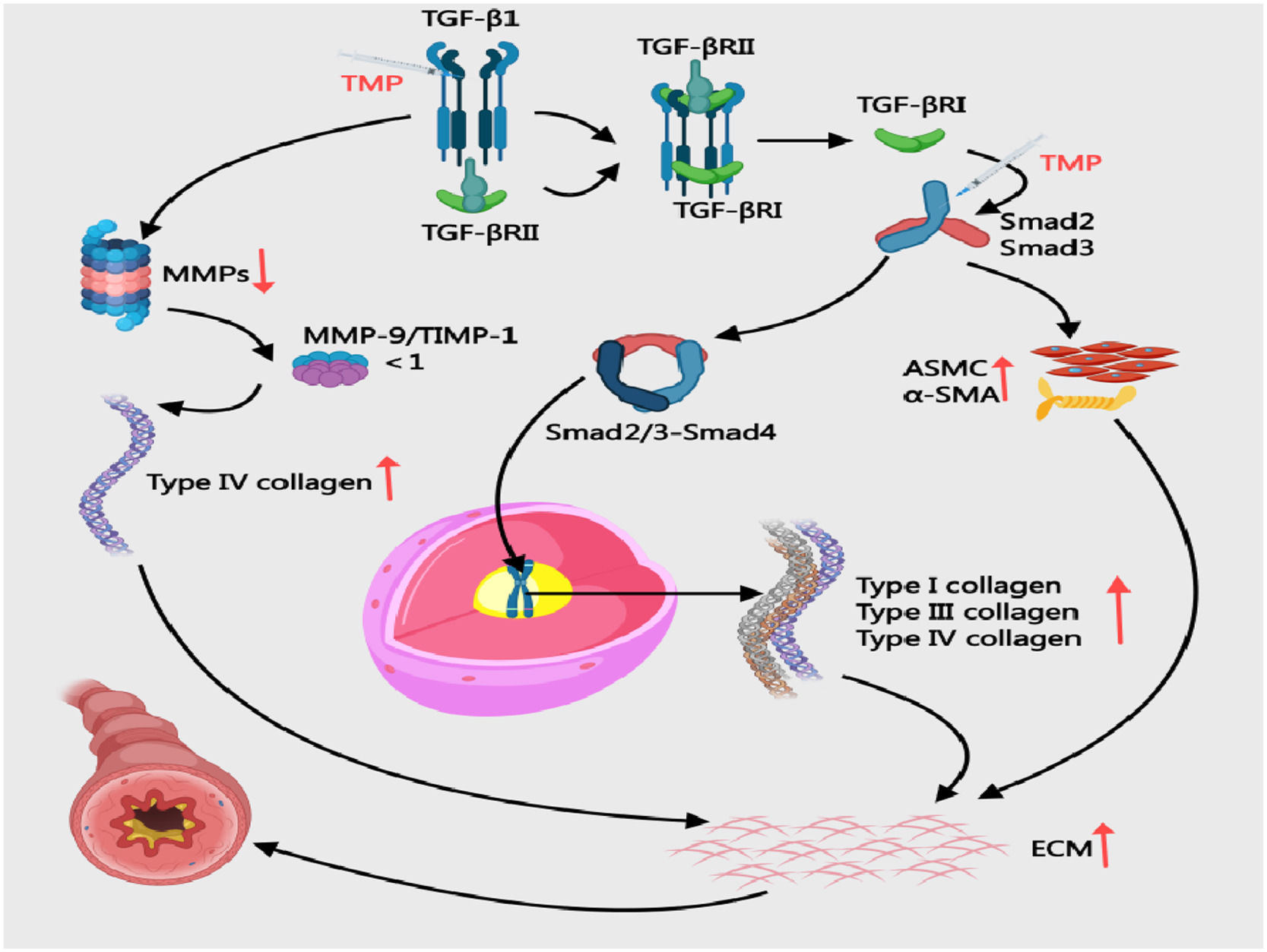
Modulating TGF-β/Smad signaling, and MMP-9/TIMP-1 balanceCollagens III/IV critically drive asthma airway remodeling by altering ECM structure/function, contributing to wall thickening and fibrosis.74 The TGF-β/Smad signaling pathway drives asthmatic airway remodeling through multifaceted mechanisms. Upon activation, TGF-β1 binds to TGF-βRⅡ and phosphorylates TGF-βRⅠ, initiating Smad 2/3 phosphorylation. These phosphorylated Smad proteins combine with Smad4 to form transcriptional complexes that migrate into the nucleus, directly upregulating collagen types I/III/IV and fibronectin expression. This molecular cascade promotes excessive ECM deposition and basement membrane thickening.75,76 Simultaneously, TGF-β1 suppresses MMPs production, impairing ECM degradation capacity.77 Additionally, the pathway induces EMT and activates fibroblasts, which stimulate ASMC proliferation and α-SMA overexpression. These cellular changes collectively exacerbate airway constriction and airway hyperresponsiveness78 (Fig. 6). In OVA/Al (OH)3-sensitized mice, 80 mg/kg TMP reduced TGF-β1 (0.456 ± 0.073 vs. 0.656 ± 0.049) and Smad2 (0.370 ± 0.042 vs. 0.591 ± 0.074) while increasing Smad7 (0.279 ± 0.056 vs. 0.137 ± 0.055; p < 0.05), effectively curbing collagen deposition.79 PKB and ERK proteins function as downstream effectors of the TGF-β/Smad pathway, indirectly modulated by this signaling cascade to drive airway remodeling.80 In CVA rats, 1.05 mg/kg TMP surpassed 32.5 mg/kg montelukast in suppressing TGF-β/PKB/ERK (p < 0.05), indicating pathway modulation.81 In OVA-sensitised asthmatic rats, intraperitoneal injection of 5 mg TMP resulted in lower expression of both MMP-9 and TIMP-1 compared to the asthma model group. This was accompanied by reduced airway smooth muscle thickness, decreased type IV collagen deposition, and attenuated progression of airway remodelling.82
Mechanisms of Collagen Deposition in BA and the Intervention of TMP. ASMC, Airway Smooth Muscle Cells; ECM, Extracellular Matrix; TGF-β1, Transforming Growth Factor-beta; TIMP, Tissue Inhibitor of Metalloproteinases; MMP-9, Matrix Metallopeptidase 9; TMP, Tetramethylpyrazine; α-SMA, α-Smooth Muscle Actin.
The airway epithelium forms a continuous protective barrier, with intercellular junctions maintaining structural integrity.83 Post-injury, adhesion junction remodeling mediated by VEGF, TGF-β1, and FGF facilitates epithelial rearrangement and repair.84 Normally, myofibroblasts derived from EMT undergo apoptosis post-repair, leaving residual collagen for physiological ECM remodeling.85 In asthma, pathological EMT transforms polarized epithelial cells into migratory mesenchymal cells, driving airway remodeling and chronic inflammation86 (Fig. 7). Beyond the TGF-β1/Smad signaling pathway, additional pathways, including JAK/STAT, PI3K/PKB, and Notch signaling, are implicated in driving epithelial-mesenchymal transition during airway remodeling.87 IL-6 and IFN-γ activate the JAK/STAT pathway through receptor binding. This activation triggers STAT phosphorylation, leading to nuclear translocation of dimerized transcription factors. These factors reprogram gene networks that regulate cellular proliferation, apoptosis, and inflammatory responses ‒ all critical drivers of airway remodeling.88 Experimental evidence demonstrates that Ligusticum chuanxiong-containing Taohong Siwu Decoction attenuates pulmonary fibrosis by reducing TGF-β1, TNF-α, and α-SMA expression (p < 0.01), effectively mitigating alveolar EMT.89 However, current evidence gaps persist regarding TMP's capacity to modulate PI3K/PKB or Notch signaling in human asthmatic EMT processes.
The Role of EMT in BA and the Potential Effects of TMP. ECM, Extracellular Matrix; EMT, Epithelial-mesenchymal Transition; JAK, Janus Kinase; STAT, Signal Transducer and Activator of Transcription; TGF-β, Transforming Growth Factor-beta; MMPs, Matrix Metallopeptidases; TMP, Tetramethylpyrazine.
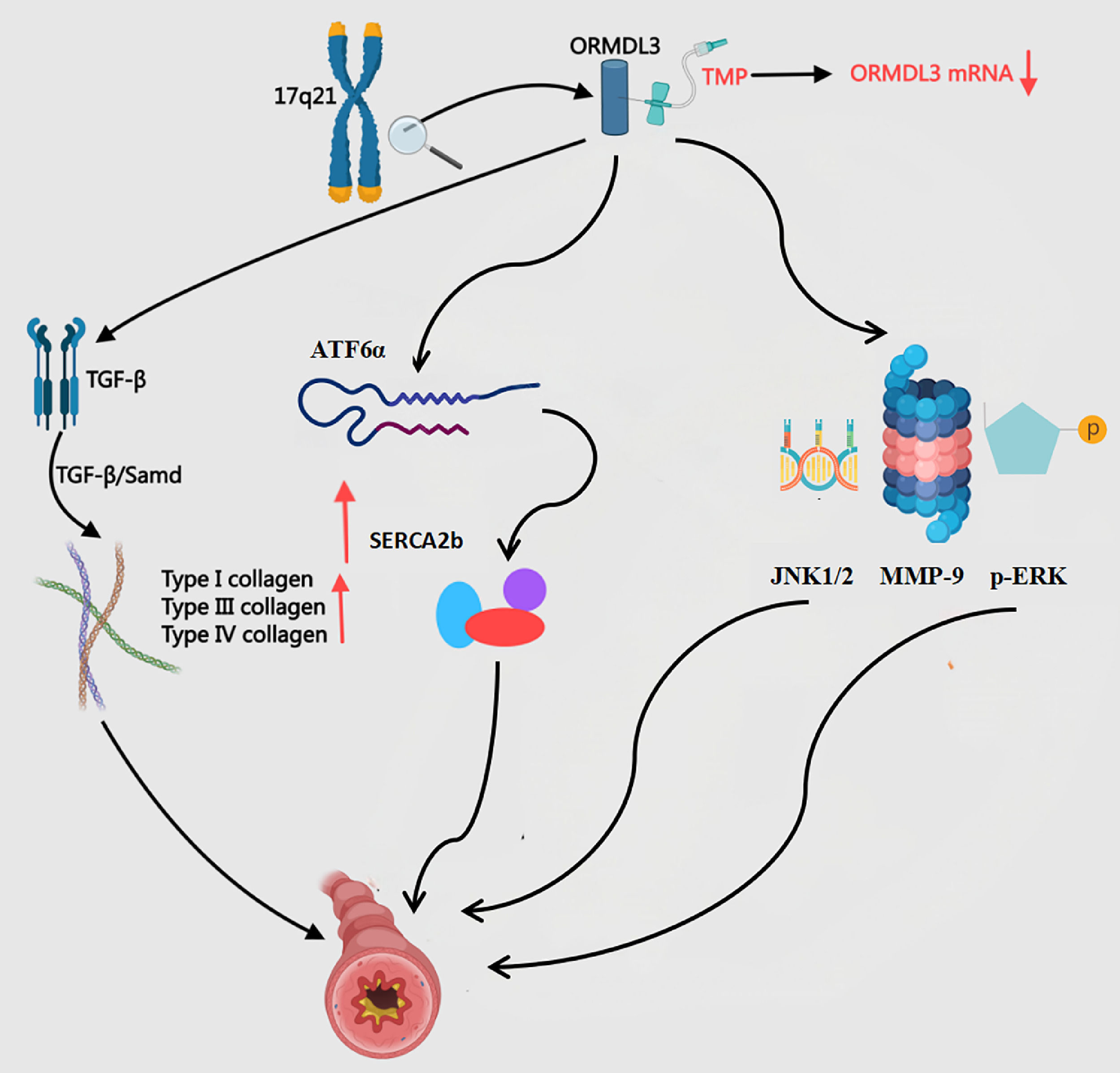
The endoplasmic reticulum transmembrane protein ORMDL3, encoded by the chromosomal locus 17q21, functions as a genetic susceptibility factor for bronchial asthma and drives airway remodeling through multi-mechanistic pathways90 (Fig. 8). This protein activates the UPR, inducing TGF-β release from airway epithelial cells to promote fibroblast activation and collagen deposition, thereby accelerating airway fibrosis.91 ORMDL3 activates the ATF6α pathway within the unfolded protein response (UPR), upregulates SERCA2b expression, and subsequently induces the production of multiple pro-inflammatory and pro-remodelling factors in human bronchial epithelial cells, thereby directly linking endoplasmic reticulum stress to the process of airway remodelling in asthma.92 ORMDL3 promotes thickening and remodelling of the airway wall in asthma patients by activating the p-ERK/MMP-9 pathway and upregulating the expression of p-ERK and MMP-9.93 Furthermore, ORMDL3, which is highly expressed in asthma, exacerbates airway inflammation and remodelling by activating the JNK1/2–MMP-9 pathway, representing a potential novel therapeutic target for asthma treatment.94 Li et al.95 found that ORMDL3 overexpression in asthmatic mouse lungs boosts 16HBE-14° cell migration. This genetic alteration also activates NF-κB and MAPK/ERK phosphorylation while undermining dexamethasone's therapeutic efficacy. Such molecular resistance clarifies why glucocorticoids ‒ despite being first-line asthma therapies ‒ fail to prevent disease relapse. TMP administered at 400 μg/L significantly reduces ORMDL3 mRNA and protein levels in human bronchial epithelial cells (p < 0.01). This suppression effectively slows airway remodeling progression.96 Compared to glucocorticoids, TMP demonstrates longer-lasting therapeutic benefits.
The Mechanism of ORMDL3 in BA and the Intervention of TMP. ATF6α, Activating Transcription Factor 6 alpha; p-ERK, p-Extracellular Regulated Protein Kinases; JNK, c-Jun N-terminal Kinase; SERCA2b, Sarco/Endoplasmic Reticulum Ca2+-ATPase 2b; TGF-β, Transforming Growth Factor-Beta; MMP-9, Matrix Metallopeptidase-9; ORMDL3, Orosomucoid-Like-3; TMP, Tetramethylpyrazine.
VEGF plays a central role in airway remodeling (Fig. 9). Its overexpression stimulates vascular endothelial cell proliferation, increases microvascular density, and exacerbates airway wall edema, inflammatory infiltration, and hyperresponsiveness.97 Elevated VEGF correlates with key remodeling markers such as smooth muscle thickening and collagen deposition. Through activating downstream pathways like KDR, VEGF promotes ASMC proliferation and accumulation of matrix components (collagens I/III), leading to airway wall thickening and luminal narrowing.98 Additionally, VEGF synergizes with Th2 cytokines (IL-4/IL-5) to amplify eosinophilic infiltration, worsening airway inflammation and fibrosis.99 Nasser et al.100 reported significantly higher serum VEGF-A levels in asthmatic children compared to healthy controls (p < 0.05) via ELISA. In OVA-sensitized rats, Yan et al.101 observed that 2 mg TMP injections reduced airway VEGF (TMP: 13.27 ± 5.72 vs. BA: 18.59 ± 6.32 pg/min) and iNOS (TMP: 15.79 ± 6.61 vs. BA: 22.23 ± 7.91 pg/min), effectively suppressing remodeling. Both VEGF and iNOS levels positively correlated with WA/Pi and SMC-A/Pi ratios, confirming their roles in structural changes.
Inhibiting HUVECs functionHUVECs derived from neonatal umbilical veins play dual roles in vascular remodeling associated with asthma. During asthmatic pathophysiology, IL-8-mediated pro-angiogenic activity enhances HUVECs proliferation, migration, and permeability. The abnormal vascular growth and structural remodeling driven by these changes strongly correlate with persistent Th2/Th17 inflammatory responses.102 Microenvironmental homeostasis critically regulates HUVECs phenotypes. Studies by Faiz et al.103 demonstrate that removing inflammatory stimuli restores physiological ECM function in asthmatic conditions. This normalized ECM maintains HUVECs quiescence and vasodilatory capacity without inducing pathological angiogenesis. MSCs modulate HUVECs through dual mechanisms. First, Paracrine release of VEGF and HGF stimulates HUVEC proliferation and vascular regeneration. Second, Secretion of IL-10 and TGF-β suppresses Th2/Th17 polarization, blocking IL-5/IL-17A-induced endothelial activation to preserve vascular homeostasis.104 Thus, HUVECs operate within a microenvironment-dependent “dual regulatory network” ‒ exacerbating vascular remodeling under inflammation while promoting repair via MSC interactions during homeostasis. Although TMP lacks direct inhibitory effects on HUVECs, its broad anti-inflammatory and immunomodulatory properties may indirectly attenuate pathological HUVECs activation by mimicking MSC-mediated protective mechanisms. However, this hypothesis remains under investigation, requiring further experimental validation and clinical evidence to confirm its scientific validity and generalizability.
SummaryAs an alkaloid possessing potent anti-inflammatory and immunomodulatory properties, TMP demonstrates multifaceted therapeutic potential for asthma management. Research reveals TMP's ability to intervene in asthmatic pathology through multi-target mechanisms. It precisely restores immune balance by normalizing Th1/Th2 and Th17/Treg equilibrium, thereby correcting inflammation caused by immune dysregulation. TMP concurrently inhibits activation of critical signaling pathways ‒ including Gαs/cAMP/PKA, AMPK/NF-κB, and MAPK/ERK cascades ‒ to block inflammatory signal transduction and suppress inflammatory cell activation. Furthermore, TMP significantly reduces IL-4 and IL-17 expression levels, diminishing inflammatory cell adhesion and infiltration in airways, which effectively alleviates airway inflammation and remodeling. This multi-targeted mechanism reduces inflammatory cell infiltration and cytokine overproduction while improving airway hyperresponsiveness. Additionally, it mitigates bronchospasm and decreases fibrotic changes, ultimately enhancing respiratory function in asthma patients. Crucially, TMP exhibits minimal adverse effects alongside its therapeutic efficacy, highlighting its potential as a safe complementary or alternative therapy. TMP's unique mechanistic profile and favorable safety data establish a robust foundation for novel asthma therapeutics. These advancements offer prospects for safer, more effective clinical options to improve patient outcomes.
Despite these insights, current research faces significant limitations. Preclinical studies predominantly rely on animal models, whose physiological and immunological differences from humans create translational uncertainties. Clinical evidence remains scarce, with most trials being small-scale and preliminary. The lack of large multicenter randomized controlled trials prevents definitive conclusions about TMP's efficacy and safety in human asthma management. While TMP shows preclinical promise and early clinical signals, it remains investigational. Rigorous human studies must now address three priorities: mechanistic validation in human pathways, optimized dosing regimens, and long-term safety assessments across diverse populations. Until such evidence emerges, cautious interpretation of TMP's clinical potential is essential. Future research should bridge this translational gap through coordinated preclinical-clinical efforts to fully realize TMP's therapeutic value in asthma.
MethodologyThe authors conducted systematic searches across PubMed, Web of Science, CNKI, Wanfang Data, and VIP databases from inception through May 2025. Search strategies combined keywords including “Tetramethylpyrazine”, “Asthma”, and “Pharmacological Mechanisms” using Boolean operators (AND/OR). Inclusion criteria prioritized peer-reviewed English/Chinese publications investigating TMP's effects on airway inflammation and remodeling in human asthma patients, animal models, and cellular studies. Following deduplication, eligible literature was categorized into inflammation regulation and remodeling modulation domains. These were further stratified by TMP's specific mechanistic pathways, forming the foundation for this comprehensive review.
AbbreviationsThis review addresses intricate molecular mechanisms requiring concise presentation. To enhance clarity and readability, the authors employ standardized abbreviations for recurrent specialized terms and complex nomenclature. The alphabetized abbreviations list below provides full definitions, which will be used directly in subsequent sections without repetitive elaboration (Table 1).
Abbreviation table.
The article processing fee is funded by the Shandong Provincial Health Commission. Fei Yu received financial support from the Shandong Provincial Health Commission for the “Shandong Province Traditional Chinese Medicine Science and Technology Project” (Project Name: Research on the Mechanism of Ligustrazine and Its Derivatives in the Treatment of Chronic Refractory Asthma through the ORMDL3/ERK1/2/MMP-9/VEGFSignaling Pathway Based on the Blood-Activating and Stasis-Removing Method, n° M-20240301). The funding agency had no role in the review process, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statementZichao Han: Conceptualization, Methodology, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Fei Yu: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition. Zhen Ding: Data curation, Writing – review & editing, Supervision. Qinghua Liu: Writing – review & editing, Project administration. Xiangyi Zheng: Writing – review & editing. Lijuan Zhang: Writing – review & editing. Dong Li: Writing – review & editing. Chao Wang: Writing – review & editing.
The authors declare no conflicts of interest.
Special thanks go to Dr. Yu Fei and DeepSeek for helping with the English writing.