While Transcatheter Aortic Valve Replacement (TAVR) is increasingly performed in patients with severe aortic stenosis, the impact of Hematologic Malignancies (HM) on associated outcomes, remains unclear. The authors The authors used a contemporary national database to investigate whether HM is associated with adverse clinical and financial outcomes following elective TAVR.
Materials and methodsThe authors The authors identified all adult (≥ 18-years) hospitalizations for elective TAVR in the 2016–2021 Nationwide Readmissions Database. Multivariable models were constructed to evaluate the association of HM with in-hospital mortality, major complications, non-home discharge, Length of Stay (LOS), index hospitalization costs, and 30-/90-day non-elective readmissions.
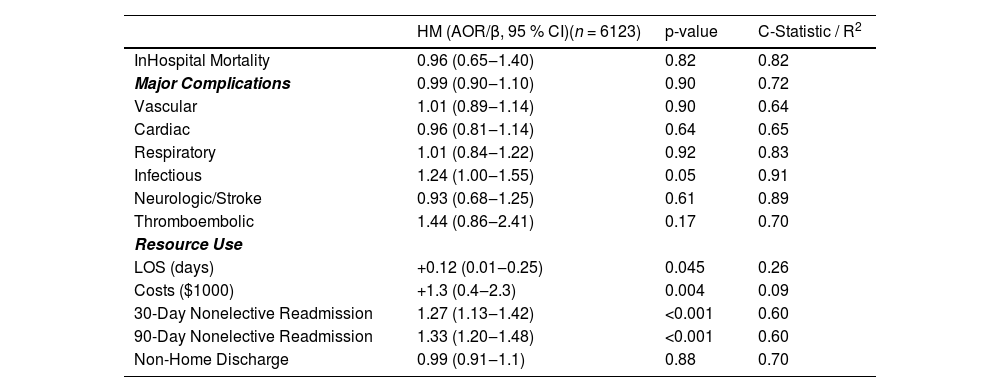
ResultsOf an estimated 336,998 TAVR procedures, 6123 (1.8 %) involved patients with HM. After risk adjustment, HM was not linked to in-hospital mortality (AOR = 0.96; 95 % CI 0.65–1.40; p = 0.82) or major complications, but conferred a modestly prolonged LOS (β+0.12 days; 95 % CI 0.01–0.25; p = 0.04) and increased costs (β+$1300; 95 % CI $400–$2300; p = 0.01). Moreover, HM was associated with higher odds of 30-day (AOR = 1.27; 95 % CI 1.13–1.42; p < 0.001) and 90-day (AOR = 1.33; 95 % CI 1.20–1.48; p < 0.001) non-elective readmissions. Notably, among 217 HM patients with a history of stem cell transplantation, in-hospital mortality risk was substantially elevated (AOR = 7.7; 95 % CI 1.53–38.8; p = 0.01).
ConclusionIn conclusion, although HM did not adversely impact in-hospital mortality or major complications following TAVR, it was linked to modestly increased resource utilization and readmission. Patients with prior stem cell transplantation represent a particularly vulnerable subgroup, underscoring the need for multidisciplinary pre-operative evaluation and specialized perioperative care pathways.
Initially introduced for patients deemed to be at prohibitive surgical risk, Transcatheter Aortic Valve Replacement (TAVR) has been increasingly adopted for severe Aortic Stenosis (AS).1,2 By 2019, TAVR procedures in the United States had surpassed surgical aortic valve replacement at a ratio of approximately 1.3:1.3 Although advances in TAVR technology and procedural safety have reduced complication rates, comorbid conditions such as renal, pulmonary, and hepatic dysfunction continue to increase morbidity and mortality among TAVR recipients.4–7
Driven by an aging and growing population, the 2014 AGES–Reykjavík study projected that the number of patients with severe AS would more than triple by 2060.8 Meanwhile, multiple studies have reported a steady rise in the incidence of Hematological Malignancies (HM) since 1990, with future projections indicating an even steeper increase.9,10 Notably, early TAVR trials largely excluded patients with HM, primarily because of the elevated risks associated with altered coagulation and immunosuppression.11,12 As a result, the concurrent rise in both AS and HM presents a growing concern for TAVR candidates with HM. This issue is further underscored by a retrospective analysis of 417 consecutive patients who underwent TAVR in Finland between 2008 and 2017, which found that individuals with HM had a 50 % mortality risk at four years, compared with 35 % among age-matched controls.13 Despite these important findings, large-scale contemporary investigations of TAVR outcomes in patients with HM remain scarce.
In the present study, the authors the authors examined the association between hematologic malignancies and postprocedural outcomes in a contemporary, national cohort of patients undergoing elective TAVR. The authors The authors hypothesized that patients with HM would experience higher risks of adverse outcomes, including in-hospital mortality, major complications, non-home discharge, hospital length of stays, higher index hospitalization costs, and more frequent readmission at both 30- and 90-days.
Materials and methodsData source and study populationThe 2016‒2021 Nationwide Readmissions Database (NRD) was used to identify all elective adult (≥18-years) hospitalizations for TAVR, using previously reported International Classification of Diseases, Tenth Revision (ICD-10) codes (database was accessed December 14th, 2024).14 Using survey weighting methodology, the NRD provides accurate estimates for approximately 60 % of all hospitalizations in the US.15 As the NRD is deidentified and does not contain information that could identify individual participants during or after data collection, the study was exempt from full review by the Institutional Review Board at the University of California, Los Angeles.
Variable definitions and study outcomesPatient and hospital characteristics, including age, sex, insurance, income, and hospital teaching status, were defined according to the Healthcare Cost and Utilization Project data dictionary.16 The burden of chronic conditions was quantified using the validated CORE score, a machine-learning-derived index that closely estimates the risk of in-hospital mortality.17 Frailty was assessed according to the Hospital Frailty Risk Score (HFRS), a previously published and validated measure.18
The authors The authors used ICD-10 codes C81-C96 to identify Hematologic Malignancies (HM), and patients with these diagnoses comprised the HM cohort (Others: Non-HM).19 Among the HM cohort, a subgroup of patients with a history of Stem Cell Transplantation (SCT) was identified using ICD-10 codes Z94.81, Z94.84, T86.00, T86.01, T86.02, T86.03, T86.09, Z48.290, and T86.5 (Supplemental Table 1).20–22 Patients who lack key data (1.7 %), including age, sex, costs, in-hospital mortality, elective status, and income, were excluded from analysis.
The primary outcome of interest was mortality during index hospitalization. The development of major complications, Length of Stay (LOS), index hospitalization costs, non-home discharge, and 30- and 90-day non-elective readmissions were assessed as secondary outcomes. Major complications were categorized as neurologic/stroke (cerebral infarction, transient ischemic attack), cardiac (cardiac arrest, ventricular tachycardia, ventricular fibrillation, cardiac tamponade, myocardial infarction), vascular (injury to vessel, arterio-venous fistula, retroperitoneal bleed, accidental puncture, vessel repair), respiratory (pneumothorax, pneumonia, empyema, acute respiratory distress syndrome, acute respiratory failure, prolonged ventilation > 96-hours), thromboembolic (deep vein thrombosis, pulmonary embolism), and infectious (sepsis, systemic inflammatory response syndrome, mediastinitis, Clostridium difficile infection). Index hospitalization costs were calculated by applying hospital-specific cost-to-charge ratios to total episodic charges, followed by adjustment to the 2021 personal health index. Because the NRD only captures readmissions within the same calendar year as the index admission, patients discharged in December were excluded from the 30-day readmission analysis, and those discharged after September (i.e., in October, November, and December) were excluded from the 90-day readmission analysis. Individuals who did not survive their index hospitalization were similarly excluded from the readmission analysis.
Statistical analysisCategorical variables are reported as proportions ( %), while continuous variables are reported as medians with Interquartile Range (IQR). The Mann-Whitney U and Pearson’s Chi-squared tests were used to evaluate intergroup differences as appropriate. Multivariable regression models were then developed to assess the independent associations of HM with key outcomes of interest. The Least Absolute Shrinkage and Selection Operator (LASSO) was employed to select covariates for the regression models, including patient demographics (frailty, age, sex, income quartile, insurance status), comorbidity burden, and hospital characteristics (teaching status, bed size) (Supplemental Table 2).23 Model performance was evaluated using the c-statistic, calibration plots, and the coefficient of determination (R2), as appropriate. Regression results are presented as Adjusted Odds Ratios (AOR) or Beta coefficients (β) with 95 % Confidence Intervals (95 % CI). Freedom from readmission was assessed using Kaplan-Meier time-to-event analysis, while Cox proportional hazards models were employed to adjust for potential confounders. Readmission diagnoses were categorized by grouping similar Diagnosis-Related Group (DRG) codes for all patients with a linked second visit within 90-days of discharge from the index TAVR hospitalization. All statistical analyses were conducted using Stata version 16.0 (StataCorp LLC, College Station, TX), with statistical significance set at a p-value < 0.05.
ResultsPatient demographics and clinical characteristicsOf an estimated total of 336,998 adult patients who underwent elective TAVR in the United States from 2016‒2021 included in the NRD, 6123 (1.8 %) were categorized as HM (Fig. 1). Leukemia was the most common HM subtype (45.8 %), followed by lymphoma (36.9 %) and myeloma (17.3 %). As shown in Table 1, the mean age at the time of TAVR was slightly lower in the HM group compared to the Non-HM group (78.5 vs. 78.9-years, p = 0.028). Compared to Non-HM patients, those with HM were less likely to be female (Non-HM: 44.2 % vs. HM: 36.1 %; p < 0.001). HM patients had a higher Comorbid Operative Risk than Non-HM patients as evaluated by a higher median CORE score (37 vs. 38; p < 0.001). However, HM patients had lower rates of obesity, diabetes, coronary artery disease, and hypertension. Additionally, HM patients were more frequently in the higher quartile of income (24.2 % vs. 29.5 %; p < 0.001) and were more frequently privately insured as compared to Non-HM patients (6.9 % vs. 8.2 %; p = 0.042) (Table 1).
Demographic, clinical, and hospital characteristics.
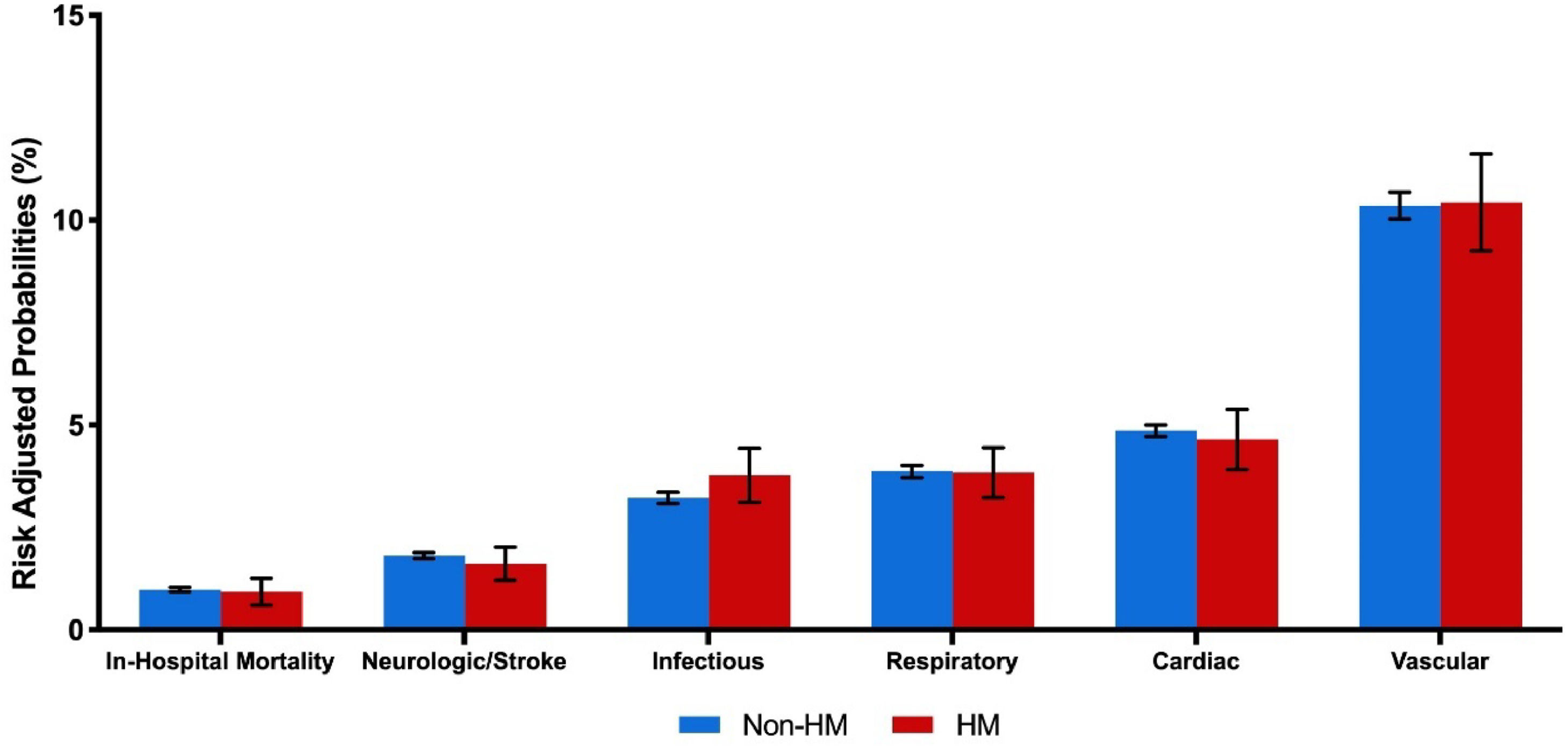
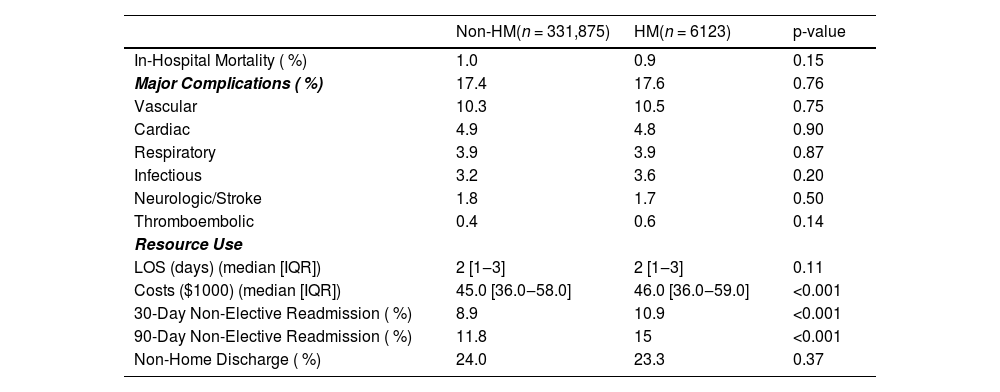
On unadjusted analysis, in-hospital mortality rates (Non-HM: 1.0 % vs. HM: 0.9 %; p = 0.15) and any perioperative complication (17.4 % vs. 17.6 %; p = 0.76) were similar between groups. Specifically, there was no significant difference in vascular (10.3 % vs. 10.5 %; p = 0.75), neurologic/stroke (1.8 % vs. 1.7 %; p = 0.50), or infectious-related complications (3.2 % vs. 3.6 %; p = 0.2). Median length of stay was also similar (2 days [IQR 1‒3] vs. 2 days [IQR 1‒3]; p = 0.11). However, HM patients incurred higher hospitalization costs ($45,000 [IQR 36,000‒58,000] vs. $46,000 [IQR 36,000‒59,000]; p < 0.001) and demonstrated increased rates of both 30-day (8.9 % vs. 10.9 %; p < 0.001) and 90-day (11.8 % vs. 15.0 %; p < 0.001) non-elective readmissions (Table 2). Following unadjusted survival analysis, HM patients experienced more frequent all-cause readmissions within 90 days of index discharge (Fig. 2).
Unadjusted outcomes, stratified by the presence of hematological malignancies.
After comprehensive risk adjustment, HM status was not significantly associated with an increased likelihood of in-hospital mortality (AOR = 0.96; 95 % CI 0.65–1.40; p = 0.82; C-statistic = 0.82). Furthermore, it was not significantly correlated with increased risks of vascular (AOR = 1.01; 95 % CI 0.89–1.14), neurologic/stroke (AOR = 0.93; 95 % CI 0.68–1.25), or infectious complications (AOR = 1.24; 95 % CI 1.00–1.55), as shown in Fig. 3 and Table 3.
Adjusted outcomes, stratified by the presence of hematological malignancies.
AOR, Adjusted Odds Ratio, CI, Confidence Interval; LOS, Length of Stay; R2, LOS and Costs; All others, C-Statistic; Ref, Non-HM.
With respect to resource utilization, HM status was associated with an increased length of stay (β + 0.12-days; 95 % CI 0.01‒0.25) and higher index hospitalization costs (β+$1300; 95 % CI, $400‒$2300). Moreover, HM was associated with greater odds of both 30-day (AOR = 1.27; 95 % CI 1.13‒1.42) and 90-day (AOR = 1.33; 95 % CI 1.20‒1.48) non-elective readmissions (Table 3). Cardiovascular problems were the most common cause for readmission, accounting for ∼40 % of events. Infectious, gastrointestinal, and respiratory causes each accounted for ∼10 % of readmissions, trailed by renal causes. Of these, only infection-related rehospitalizations differed significantly between groups, with a higher frequency in the HM cohort (Fig. 4).
History of stem cell transplant is associated with inferior TAVR outcomesSubgroup analysis was conducted among HM patients with a history of Stem Cell Transplantation (SCT). Of the 6123 HM patients in the analysis, 217 (3.5 %) had a history of SCT. Compared to HM patients without SCT, those with SCT had higher odds of in-hospital mortality (AOR = 7.7; 95 % CI 1.53‒38.8) and an increased risk of respiratory complications (AOR = 2.96; 95 % CI 1.31‒6.68) (Table 4). There was no statistically significant association between SCT and the risk of vascular, neurologic/stroke, or infectious-related complications.
Adjusted outcomes, stratified by the presence of history of stem cell transplant.
AOR, Adjusted Odds Ratio; CI, Confidence Interval; LOS, Length of Stay; R2, LOS and Costs; All others, C-Statistic; Ref, HM with No History of Stem Cell Transplant.
Hematological Malignancies (HM) have previously been shown to be associated with adverse clinical outcomes following a variety of surgical procedures. However, in this large-scale contemporary study, the presence of HM was not associated with worse perioperative outcomes among TAVR recipients. Notably, in-hospital mortality and major perioperative complications were not influenced by the presence of HM. Despite these reassuring findings, HM was associated with a slight elevation in length of stay, costs, as well as increased 30-day and 90-day non-elective readmissions. Furthermore, a subgroup analysis of HM patients with a history of stem cell transplantation faced a nearly eight-fold increase in the odds of mortality after TAVR. Several of these findings merit further discussion.
In the present analysis, HM was not associated with significant adverse outcomes after TAVR. These findings are consistent with previous findings that a history of cancer is not associated with inferior short- or long-term survival in patients undergoing TAVR.24 The authors The authors expand on these findings by noting no significant increase in the risk of bleeding, thrombotic events, neurologic/stroke, or infectious-related complications among TAVR recipients with HM. However, despite the small sample size warranting cautious interpretation, these findings reveal a significantly elevated mortality risk associated with a history of prior Stem Cell Transplantation (SCT). As highlighted in prior studies, this increased risk likely reflects a greater burden of comorbidities and heightened frailty within this subgroup, potentially due to the progression and treatment of their underlying hematologic condition.24–27 Notably, the authors also observed a higher incidence of respiratory complications among SCT recipients. This is consistent with existing literature and likely stems from the long-term effects of the transplant process.28 These patients often remain immunocompromised due to both their underlying malignancy and ongoing post-transplant immunosuppressive therapy, increasing their susceptibility to serious respiratory infections.29 Additionally, conditioning regimens such as chemotherapy or radiation can cause lasting pulmonary injury, and chronic graft-versus-host disease may further compromise lung function.29–31 Together, these factors likely contribute to their increased vulnerability to respiratory complications following TAVR. Importantly, specialized oncological care pathways and co-management with hematology experts have been shown to improve outcomes for these vulnerable patients and should be considered as part of TAVR due to the increased risk demonstrated in this analysis.32,33 Taken together, while HM itself should not be a deterrent to TAVR, the significant risks observed in SCT patients underscore the importance of tailoring care to this high-risk population.
Among TAVR patients with HM, resource utilization proved only marginally higher, with an additional $1300 in index hospitalization costs and an additional 0.12 days in the hospital length of stay. These findings represent a marked reduction from prior studies, with earlier studies reporting that cancer patients incur an average of $13,000 more in index hospitalization costs and 1.1 additional days of hospitalization compared to those without malignancy.34 This lower resource utilization aligns with a recent study noting an annual reduction of $1854 in TAVR-related costs, likely driven by rising procedural volumes, enhanced clinician expertise, and the implementation of fast-track discharge protocols.35–37 Continued initiatives to curtail resource utilization in this population remain critical to minimizing both financial impact on patients and strain on healthcare systems.
Despite the decline in costs and length of stay, the present analysis indicated HM patients face significantly higher risks for 30-day and 90-day non-elective readmissions compared to their Non-HM counterparts. Although many readmissions were driven by cardiac issues, HM patients exhibited a particular vulnerability to infectious rehospitalizations, consistent with findings by Sommer et al., who documented an increased infection risk among HM patients undergoing cardiac surgery.38 Given the unique yet wide spectrum of immunosuppression among patients with HM, this finding requires introspection into periprocedural protocols. Both the American Society of Clinical Oncology and the Infectious Diseases Society of America advocate prophylactic antimicrobial therapy for patients with cancer-related immunosuppression, while a systematic review by Chai et al. supports the use of prophylactic immunoglobulins and vaccinations to mitigate infection risk.39,40 Moreover, a prior study emphasized the importance of personalized discharge planning and timely outpatient follow-up for reducing readmission rates among HM patients.41 Adopting these strategies, alongside vigilant infection monitoring, could not only lower readmission rates but also enhance patient well-being and alleviate burdens on healthcare resources.
This study has several important limitations. As a retrospective analysis using administrative data, it is subject to inherent limitations in causal inference and residual confounding, despite robust multivariable adjustment. Key clinical details ‒ such as HM subtype, cancer stage, remission status, and treatment modalities (e.g., chemotherapy, immunotherapy) ‒ were not available and may influence both baseline risk and outcomes. The use of ICD-10 coding introduces the potential for misclassification bias, and differences in coding practices across institutions may further impact data accuracy. The focus on in-hospital and 90-day readmission outcomes, dictated by the structure of the NRD, precludes assessment of long-term survival, valve durability, and HM progression, all of which are critical for informing treatment decisions. The authors also excluded emergent TAVR procedures, potentially underestimating the risk faced by more acutely ill HM patients. Cost estimates were derived from inpatient charges and do not account for outpatient expenses or long-term financial burden. Additionally, the relatively small sample of patients with prior stem cell transplantation limits statistical power and generalizability of those findings. Finally, while the authors observed increased infection-related readmissions among HM patients, the absence of data on prophylactic strategies ‒ such as antimicrobial use, vaccinations, or immunoglobulin therapy ‒ limits actionable interpretation. Despite these limitations, these findings provide meaningful insight into the early outcomes of TAVR in patients with HM and underscore opportunities for improved risk stratification and multidisciplinary care. In conclusion, while hematologic malignancies were not independently associated with increased in-hospital mortality or perioperative complications following TAVR, they remain clinically significant due to their association with higher rates of infection-related readmissions and increased healthcare costs. Notably, patients with a history of stem cell transplantation experienced substantially elevated mortality, highlighting the need for tailored perioperative planning and multidisciplinary management. As TAVR continues to expand among medically complex populations, these findings may help refine patient selection and guide resource allocation to optimize outcomes in this vulnerable cohort. Future research should prioritize linkage to more granular datasets to evaluate long-term survival, quality of life, and cancer-specific factors such as stage, remission status, treatment history, and biomarkers. Additionally, prospective studies are warranted to assess the role of prophylactic strategies ‒ such as antimicrobial use and vaccination protocols ‒ in reducing infection risk among TAVR recipients with hematologic malignancies.
Ethical approval statementThis manuscript uses deidentified data from a publicly available administrative database and was thus deemed exempt from full review by the University of California, Los Angeles Institutional Review Board.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statementZeyu Liu: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. Esteban Aguayo: Writing – review & editing. Giselle Porter: Writing – review & editing. Konmal Ali: Writing – review & editing. Radoslav Zinoviev: Supervision, Writing – review & editing. Peyman Benharash: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
P. Benharash received fees from AtriCure as a surgical proctor. This manuscript does not discuss any AtriCure products or services. Other authors report no conflicts.
None.