Coronavirus disease (COVID-19) is an infectious illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It emerged in 2019 and quickly became a global pandemic, resulting in numerous deaths worldwide. Despite the devastating impact of SARS-CoV-2 on human life, it also spurred the development of advanced vaccine platforms. Within a remarkably short time frame, 11 vaccines have been approved for human use, marking a significant historical achievement. These include mRNA, whole inactivated, recombinant protein, and adenoviral vector platforms. Notably, these new-generation vaccine platforms represent a departure from previously utilized methods and form the backbone of SARS-CoV-2 preventive strategies. In order to enhance the efficacy of vaccines, it is crucial to have a comprehensive understanding of their underlying virological and immunological characteristics. The recent emergence of variant strains, particularly the Omicron variant, has raised doubts regarding the effectiveness of current vaccines and emphasized the need for a universal platform for future vaccinations.
This review focuses on discussing various vaccine platforms based on their molecular design, their ability to stimulate the immune system, safety concerns, potential efficacy against viral variants, and prospects for the future.
La enfermedad por coronavirus (COVID-19) es una enfermedad infecciosa causada por el síndrome respiratorio agudo severo-Coronavirus 2 (SARS-CoV-2), que creó una pandemia en 2019 y causó muchas muertes en todo el mundo. A pesar del impacto catastrófico de SARS-CoV-2 en la vida humana durante los últimos 2 años, el desarrollo de plataformas de vacunas de nueva generación fue un recuerdo de esta aparición viral. La construcción de 11 plataformas aprobadas para uso humano en un período tan corto es un logro histórico. Las plataformas de ARNm, inactivadas por completo, proteínas recombinantes y vectores adenovirales son plataformas de vacunas aprobadas. Sin embargo, las estrategias preventivas de SARS-CoV-2 se basan principalmente en las plataformas de vacunas de nueva generación que rara vez se habían utilizado antes. Comprender las bases de las vacunas, incluidas las características virológicas e inmunológicas, es esencial para mejorar el modo de acción. Además, la aparición de variantes preocupantes y recientemente la variante ómicron, ha planteado muchas preocupaciones sobre el potencial preventivo de las vacunas actuales y la necesidad de una plataforma universal para el futuro. En esta revisión, se han discutido los principios de las diferentes plataformas de vacunas basadas en el diseño molecular, la inducción del sistema inmunológico, los problemas de seguridad de las vacunas, el potencial contra las variantes virales y las perspectivas futuras.
The emergence of SARS-CoV-2 posed a significant health challenge to humanity.1 Vaccine development efforts began soon after the release of the virus genome sequences, recognizing the potential of vaccines in preventing viral diseases.2 However, developing effective respiratory virus vaccines has been challenging due to limited success in inducing long-lasting mucosal immunity.3 Various platforms have been proposed for creating a vaccine against SARS-CoV-2, ranging from traditional inactivated virus vaccines to advanced nucleic acid-based vaccines.4
This article focuses on the progress and molecular foundation of SARS-CoV-2 vaccines. It delves into the viral replication process, emphasizing structural proteins and crucial stages in the virus's life cycle. The Spike glycoprotein, chosen as the primary antigen for vaccination purposes due to its ability to trigger a strong immune response, is examined in detail. The article also discusses approved vaccines for combating COVID-19, including those with Emergency Use Listing (EUL) from the World Health Organization. These vaccines encompass various platforms such as whole virus vaccines (attenuated and inactivated), protein subunit vaccines, viral vector vaccines, and nucleic acid-based vaccines. The molecular basis for engineering the Spike protein, particularly its Receptor Binding Domain (RBD), is thoroughly explored. Additionally, the structure and function of the Spike protein, its role in viral entry and fusion, and its significance as a target for neutralizing antibodies are examined.
The objective of this review article is to provide a comprehensive examination of revolutionary approaches to SARS-CoV-2 vaccine development, focusing on molecular and virological aspects. It aims to explore the underlying principles behind various vaccine platforms and shed light on their potential in combating the COVID-19 pandemic.
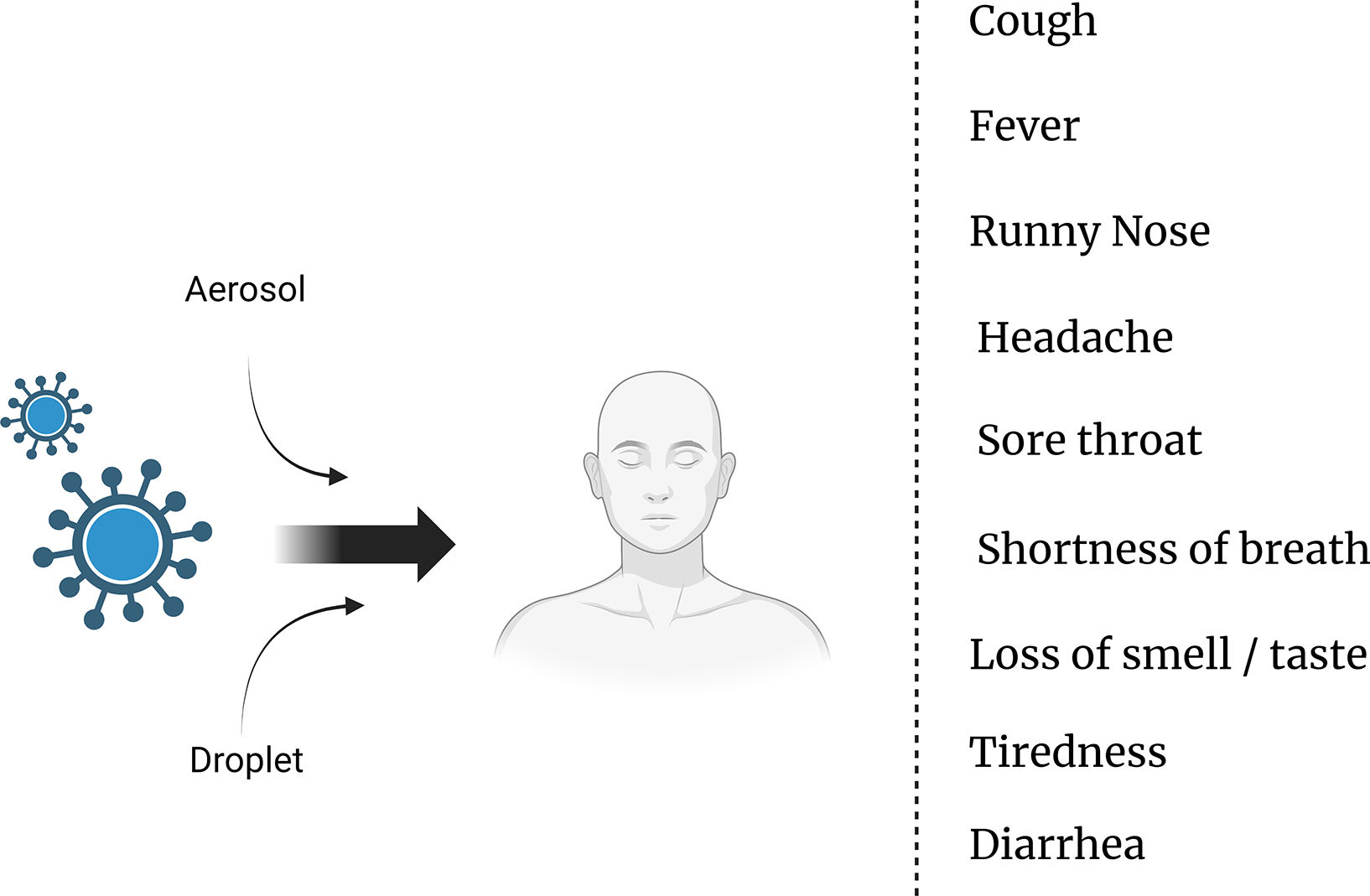
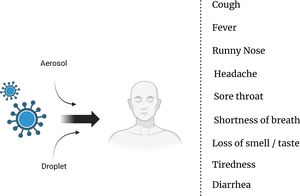
Modes of transmission and symptoms of COVID-19: Understanding the spread and identifying key indicatorsThe transmission of the SARS-CoV-2 virus, which causes COVID-19, primarily occurs through respiratory droplets when an infected person coughs, sneezes, talks, or breathes.5 These droplets can be inhaled by individuals in close proximity, typically within about 6 ft (2 m) of an infected person.6 Moreover, the virus can also spread by touching contaminated surfaces and then touching the face, particularly the eyes, nose, or mouth.7 It is important to note that the virus can be transmitted by individuals who are asymptomatic or presymptomatic, adding to the challenges in controlling its spread.8 As for the symptoms of COVID-19, they can vary from mild to severe and typically include fever, cough, shortness of breath, fatigue, muscle or body aches, loss of taste or smell, sore throat, congestion, runny nose, and headache.9 It is vital for individuals experiencing these symptoms to seek medical guidance and follow appropriate testing and isolation protocols to prevent further transmission10 (Fig. 1).
Exploring the structure and life cycle of SARS-CoV-2: Insights into a beta-coronavirusSARS-CoV-2, a beta-coronavirus in the Coronaviridae family, shares its classification with other coronaviruses like SARS-CoV-1 and MERS-CoV.11 This family possesses a positive single-stranded genomic RNA enclosed by an envelope that features glycoproteins known as spikes.12 These spikes give the virus an appearance reminiscent of a “Sun Corona” when observed under an electron microscope.13
The genome of SARS-CoV-2 is approximately 30 kb in length and encodes 4 structural proteins: Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N). Additionally, it contains 16 predicted non-structural proteins (NSP) and at least 13 open-reading frames (ORF) responsible for RNA polymerization and the preparation of accessory proteins.14–17
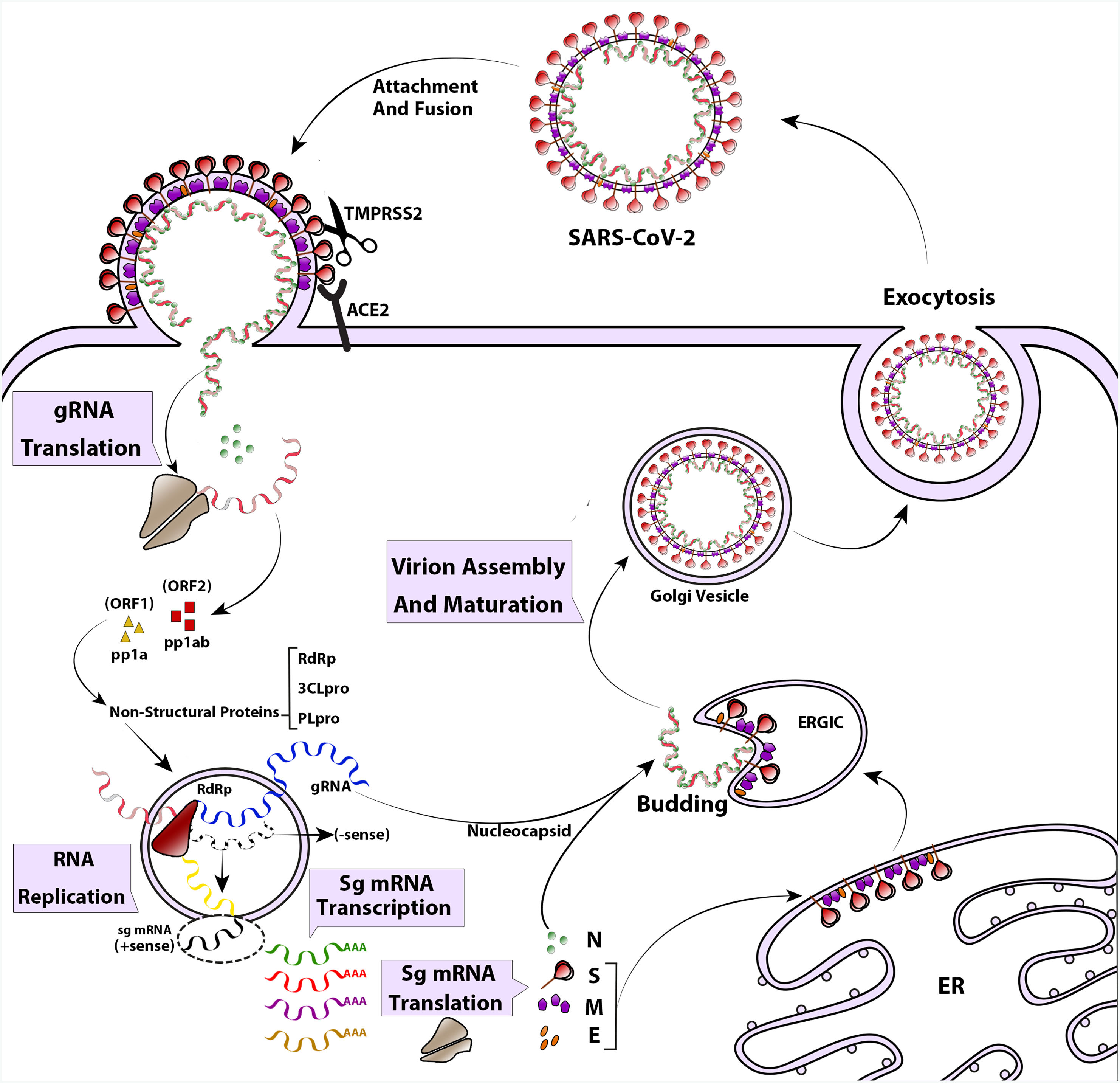
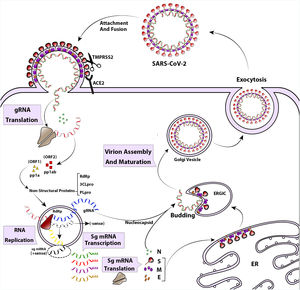
The life cycle of SARS-CoV-2 begins with the attachment of the pre-fusion spike protein to angiotensin-converting enzyme 2 (ACE2) receptors on the surface of airway epithelial ciliated cells. ACE2 receptors are found in the upper respiratory tract, as well as in other tissues such as the lungs, heart, kidney, intestine, and endothelium.18–20 The spike protein undergoes cleavage by a host enzyme called Transmembrane Protease Serine 2 (TMPRSS2) resulting in its rearrangement into the post-fusion shape.21 Once the virus enters the cell, its genome is uncoated and translated by the host's translation machinery. This translation generates viral proteins necessary for replication.12 Replication and transcription of the viral genome occur in a membranous compartment, where sub-viruses are formed with the help of several non-structural proteins encoded by ORF1a and ORF1ab.22 Proteins like RNA-dependent RNA polymerase (RdRp), Cap-providing enzyme, papain-like protease (PLpro), and main protease (3CLpro) play roles in viral genome replication during this process.23 Within these membranous complexes, the initial viral polyprotein is cleaved by viral proteinases (3CLpro and PLpro) into distinct protein units.24 Simultaneously, RdRp continues synthesizing the anti-genome strand in the replicase complex, providing templates for the production of viral genomic RNA.22 In the final stages of the replication cycle, structural proteins are produced, and virus assembly occurs in the ER–Golgi network.25 Spike proteins undergo glycosylation modifications in the ER inter-tubular environment, accompanied by M and E proteins. Ultimately, viral progenies are released through an exosome-mediated pathway26 (Fig. 2).
The life cycle and replication of SARS-CoV-2. The SARS-CoV-2 attaches to cell attachment factors and interacts with specific cellular receptors, like angiotensin-converting enzyme 2 (ACE2), along with other host factors such as the cell surface serine protease TMPRSS2. This interaction facilitates the virus entering and merging with the cellular or endosomal membrane. Once inside, the genomic RNA (gRNA) is released and undergoes immediate translation of 2 large open reading frames, ORF1a and ORF1b. These ORFs produce polyproteins pp1a and pp1ab, which are then processed into individual non-structural proteins (nsps). These nsps form the viral replication and transcription complex. As the nsps are expressed, viral replication organelles are created, which consist of specific vesicles and membranes that protect the viral gRNA during replication and transcription of subgenomic mRNAs (Sg mRNAs). The structural proteins are translated and move into the endoplasmic reticulum (ER) membranes, passing through the ER-to-Golgi intermediate compartment (ERGIC). Within the ERGIC, they interact with newly produced gRNA, enclosed in N (Nucleocapsid) proteins, leading to the budding of virions into secretory vesicular compartments. Finally, the infected cell secretes the virions by exocytosis.
The structural proteins of SARS-CoV-2 contain dominant B and T cell epitopes, making them potential candidates for immunization efforts.27 Comparisons between the structural protein sequences (S, M, E, and N) of SARS-CoV-2 and other coronaviruses reveal significant similarity in amino acid composition (approximately 75–90% coverage).28 As a result, the Spike glycoprotein, identified as the major antigen of the virus in previous studies, has been selected for vaccination purposes.29
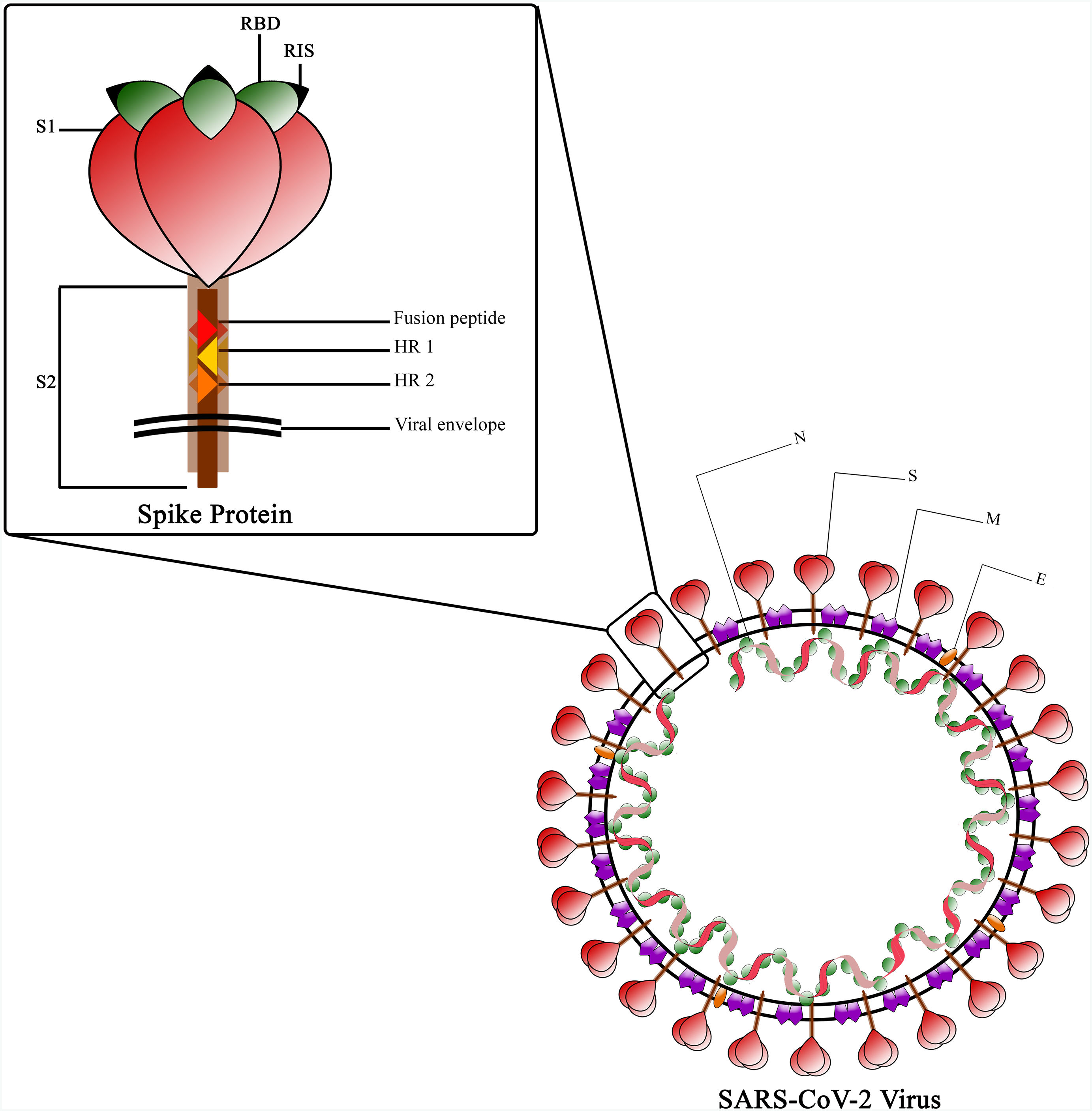
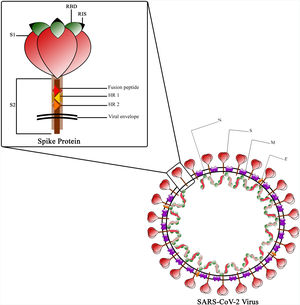
The Spike protein is a class I fusion protein that exists as a trimeric transmembrane protein and serves as the primary antigenic component of the viral particle.30 It undergoes cleavage by host cell furin-like protease during virus maturation, due to the presence of a cleavage site within the spike protein itself.31 The resulting cleaved fragments, known as subunit 1 and 2 (S1 and S2), possess the ability to infect new host cells and facilitate fusion with the host cell membrane, which is a prerequisite for infection.32 The Receptor Binding Domain (RBD), located in the globular head of the spike monomer within the trimer complex, plays a crucial role.33 The RBD is responsible for binding to ACE-2 and contains essential epitopes targeted by neutralizing antibodies.34,35 The spike stem region, known as S2, consists of the fusion peptide (FP) and 2 stabilizing heptad repeats (HR)1 and 2, which are essential for the penetration process.36 While the RBD undergoes glycosylation in post-translational modification facilitated by Golgi, some of its epitopes are covered by glycans, except for the receptor interaction site (RIS)37 (Fig. 3). The RBD region spans amino acids 319–541 and is located approximately in the middle of S1.38 Within the protein's extra-cytoplasmic domain (ectodomain), the RBD is the most exposed domain and has significant immunogenic potential.36 Immunization with the spike protein induces the production of neutralizing antibodies in animals, effectively protecting them against viral challenges.39
The RBD motif is particularly rich in epitopes and serves as a potent inducer of humoral immunity, while the extracellular domains of the Spike protein are suitable targets for both arms of the immune system.40 Expression of the RBD alone generates an acceptable level of neutralizing antibodies compared to the full Spike protein.41
Overview of the history of coronavirus vaccinationInfectious Bronchitis Virus (IBV)IBV is a coronavirus that mainly affects chickens.42 Live attenuated and killed vaccines for IBV have been developed and used in the poultry industry to protect chickens from this highly contagious and economically significant disease.43
Porcine Epidemic Diarrhea Virus (PEDV)PEDV is a coronavirus that affects pigs and can cause severe diarrhea, vomiting, and dehydration, especially in piglets.44 Vaccines have been developed and used in the pig farming industry to control and prevent outbreaks.45
SARS-CoV-1During the 2002–2003 SARS-CoV-1 outbreak, scientists worked to develop a vaccine to combat the virus.46 Some vaccine candidates showed promise in preclinical studies and animal models, but the outbreak eventually subsided before a fully approved vaccine could be widely distributed.47
After the SARS-CoV-1 outbreak, research into SARS-CoV-1 vaccines continued to some extent, but the focus shifted to other emerging infectious diseases.
MERS-CoVMERS-CoV was first identified in 2012 and has led to several outbreaks, primarily in the Middle East.48 Due to its high mortality rate (around 35%), there was an urgent need for a vaccine to control its spread.49
Vaccine development efforts for MERS-CoV have been ongoing, with various research groups and pharmaceutical companies working on potential vaccine candidates. Several approaches, such as viral vector vaccines and DNA vaccines, have been advanced to clinical trial.50
Some MERS-CoV vaccine candidates had shown promising results in animal studies and early-stage clinical trials, demonstrating their ability to induce an immune response.50 However, no MERS-CoV vaccine had received widespread approval for human use at that time.51
SARS-CoV-2The COVID-19 pandemic, caused by SARS-CoV-2, triggered an unprecedented global effort to develop vaccines in record time.52 By leveraging advancements in vaccine technology, several COVID-19 vaccines were developed, tested, and approved for emergency use in different parts of the world starting in late 2020.53
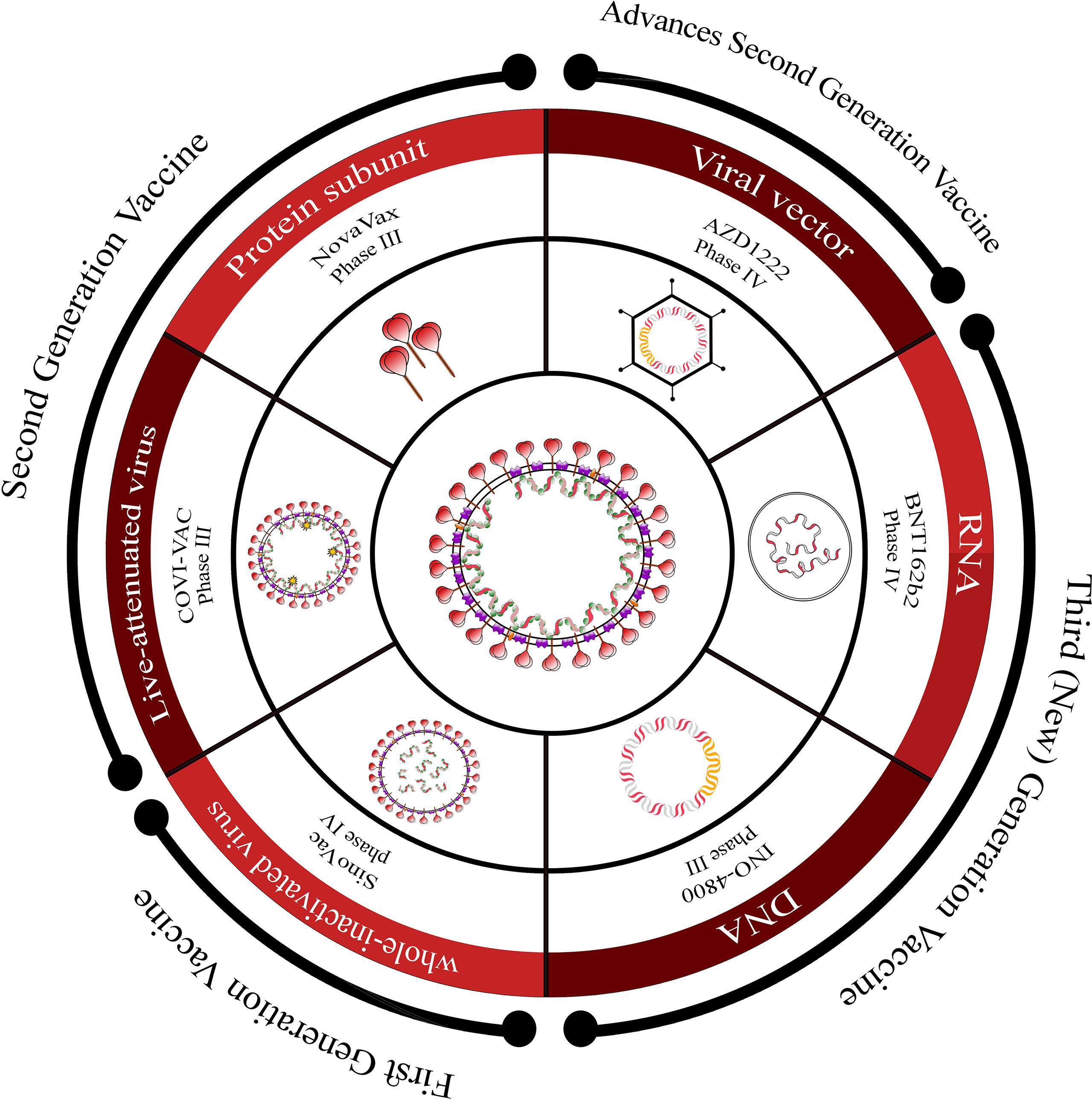
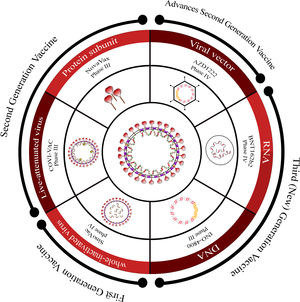
Overview of COVID-19 vaccine candidates: Progress and WHO emergency use listingCurrently, there are over 183 vaccine candidates undergoing clinical trials in various phases.54 These candidates encompass a range of platforms such as whole virus (attenuated or killed), viral vectors, protein subunit, and nucleic acid-based (RNA or DNA) vaccines, which are progressing through different stages of monitoring (Fig. 4).
Overview of vaccine platforms for SARS-CoV-2.
This figure provides an overview of the different vaccine platforms under development for SARS-CoV-2. It presents a schematic representation that includes first-generation, second-generation, advanced second-generation, and third (new)-generation platforms, along with information about the stage of development for each platform.
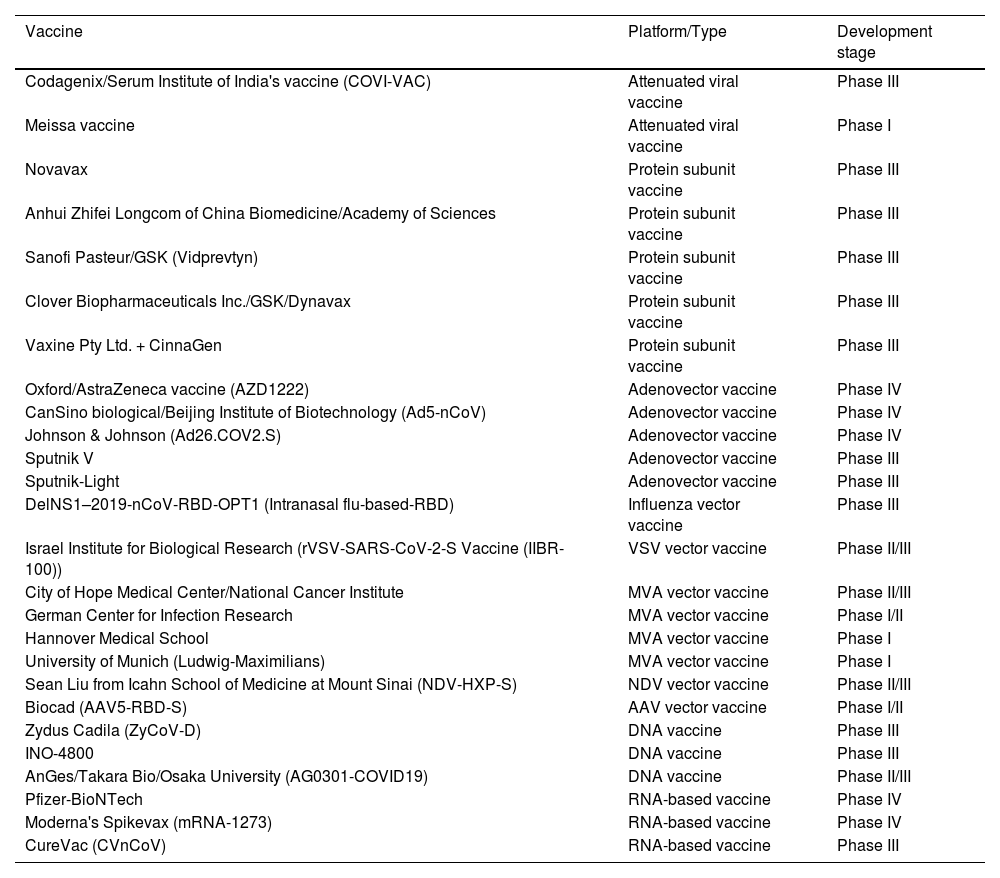
The World Health Organization (WHO) has granted Emergency Use Listing (EUL) to the following 11 COVID-19 vaccines: Pfizer-BioNTech (BNT162b2), Oxford/AstraZeneca (AZD1222), Janssen (Johnson & Johnson), Moderna (Spikevax or mRNA-1273), Novavax, Sinopharm (Beijing), Sinovac (CoronaVac), Bharat Biotech, CanSino, Serum Institute of India (COVOVAX), and Serum Institute of India (Covishield). Table 1 provides studied vaccines (excluding inactivated vaccines) divided by platform/ type and development stage.
Studied vaccines (excluding inactivated vaccines) divided by platform/type and development stage.
| Vaccine | Platform/Type | Development stage |
|---|---|---|
| Codagenix/Serum Institute of India's vaccine (COVI-VAC) | Attenuated viral vaccine | Phase III |
| Meissa vaccine | Attenuated viral vaccine | Phase I |
| Novavax | Protein subunit vaccine | Phase III |
| Anhui Zhifei Longcom of China Biomedicine/Academy of Sciences | Protein subunit vaccine | Phase III |
| Sanofi Pasteur/GSK (Vidprevtyn) | Protein subunit vaccine | Phase III |
| Clover Biopharmaceuticals Inc./GSK/Dynavax | Protein subunit vaccine | Phase III |
| Vaxine Pty Ltd. + CinnaGen | Protein subunit vaccine | Phase III |
| Oxford/AstraZeneca vaccine (AZD1222) | Adenovector vaccine | Phase IV |
| CanSino biological/Beijing Institute of Biotechnology (Ad5-nCoV) | Adenovector vaccine | Phase IV |
| Johnson & Johnson (Ad26.COV2.S) | Adenovector vaccine | Phase IV |
| Sputnik V | Adenovector vaccine | Phase III |
| Sputnik-Light | Adenovector vaccine | Phase III |
| DelNS1–2019-nCoV-RBD-OPT1 (Intranasal flu-based-RBD) | Influenza vector vaccine | Phase III |
| Israel Institute for Biological Research (rVSV-SARS-CoV-2-S Vaccine (IIBR-100)) | VSV vector vaccine | Phase II/III |
| City of Hope Medical Center/National Cancer Institute | MVA vector vaccine | Phase II/III |
| German Center for Infection Research | MVA vector vaccine | Phase I/II |
| Hannover Medical School | MVA vector vaccine | Phase I |
| University of Munich (Ludwig-Maximilians) | MVA vector vaccine | Phase I |
| Sean Liu from Icahn School of Medicine at Mount Sinai (NDV-HXP-S) | NDV vector vaccine | Phase II/III |
| Biocad (AAV5-RBD-S) | AAV vector vaccine | Phase I/II |
| Zydus Cadila (ZyCoV-D) | DNA vaccine | Phase III |
| INO-4800 | DNA vaccine | Phase III |
| AnGes/Takara Bio/Osaka University (AG0301-COVID19) | DNA vaccine | Phase II/III |
| Pfizer-BioNTech | RNA-based vaccine | Phase IV |
| Moderna's Spikevax (mRNA-1273) | RNA-based vaccine | Phase IV |
| CureVac (CVnCoV) | RNA-based vaccine | Phase III |
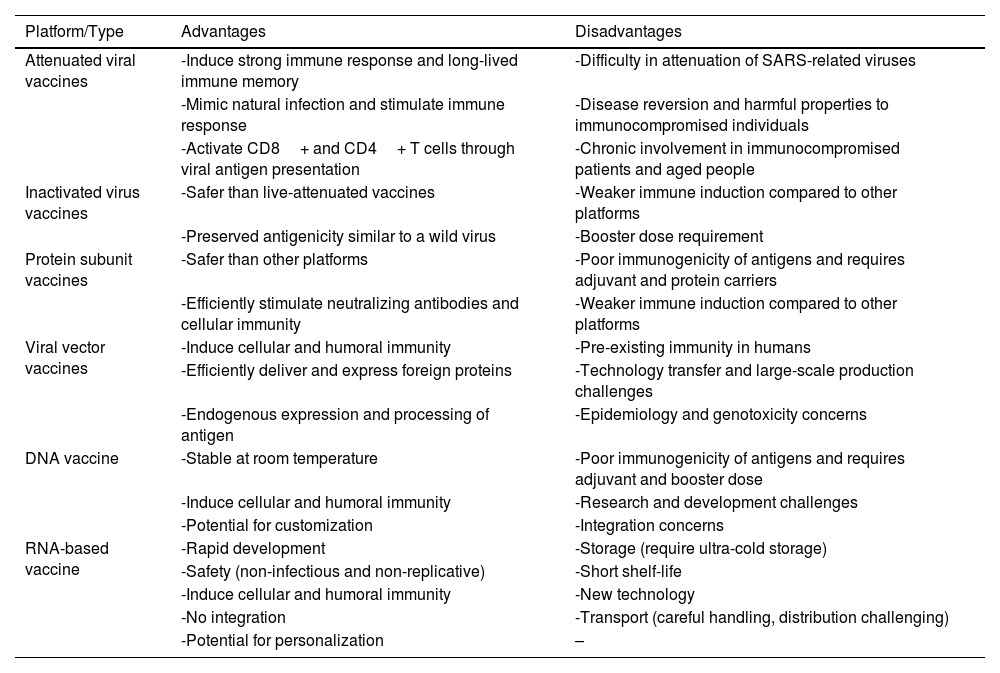
Whole viral vaccines, such as live-attenuated or inactivated vaccines, are extensively utilized compared to other vaccination approaches.55 These vaccines possess the significant advantage of stimulating innate immunity, which serves as an adjuvant for triggering adaptive immunity.56Table 2 provides an overview of different vaccine platforms along with their advantages and disadvantages.
Advantages and disadvantages of different vaccine platforms.
| Platform/Type | Advantages | Disadvantages |
|---|---|---|
| Attenuated viral vaccines | -Induce strong immune response and long-lived immune memory | -Difficulty in attenuation of SARS-related viruses |
| -Mimic natural infection and stimulate immune response | -Disease reversion and harmful properties to immunocompromised individuals | |
| -Activate CD8+ and CD4+ T cells through viral antigen presentation | -Chronic involvement in immunocompromised patients and aged people | |
| Inactivated virus vaccines | -Safer than live-attenuated vaccines | -Weaker immune induction compared to other platforms |
| -Preserved antigenicity similar to a wild virus | -Booster dose requirement | |
| Protein subunit vaccines | -Safer than other platforms | -Poor immunogenicity of antigens and requires adjuvant and protein carriers |
| -Efficiently stimulate neutralizing antibodies and cellular immunity | -Weaker immune induction compared to other platforms | |
| Viral vector vaccines | -Induce cellular and humoral immunity | -Pre-existing immunity in humans |
| -Efficiently deliver and express foreign proteins | -Technology transfer and large-scale production challenges | |
| -Endogenous expression and processing of antigen | -Epidemiology and genotoxicity concerns | |
| DNA vaccine | -Stable at room temperature | -Poor immunogenicity of antigens and requires adjuvant and booster dose |
| -Induce cellular and humoral immunity | -Research and development challenges | |
| -Potential for customization | -Integration concerns | |
| RNA-based vaccine | -Rapid development | -Storage (require ultra-cold storage) |
| -Safety (non-infectious and non-replicative) | -Short shelf-life | |
| -Induce cellular and humoral immunity | -New technology | |
| -No integration | -Transport (careful handling, distribution challenging) | |
| -Potential for personalization | – |
The live vaccine platform is a potent method for inducing immune response and generating long-lived immune memory.57 It mimics natural infection and stimulates the immune response similar to a wild pathogen, making it highly effective against intracellular pathogens.58 This platform also activates CD8+ and CD4+ T cells through intracellular replication of the virus, resulting in endogenous expression and processing of viral antigens presented on MHC-I or II classes.39
However, attenuating SARS-related viruses is more challenging compared to other viruses like measles, poliovirus, and rubella, due to their large genome, undiscovered gene properties, and complex ORFs-containing region. Previous attenuated vaccines have shown promise in eradicating certain diseases, but there are concerns about disease reversion and potential harm to immunocompromised individuals, highlighting the predictable difficulties that should be considered.59 Additionally, live vaccines have limitations such as the requirement for sterilization and drying before release, as well as the need for cold-chain maintenance during transport.60 Another disadvantage is the chronic involvement observed in immunocompromised patients and the elderly population.61
The Codagenix/Serum Institute of India's vaccine (COVI-VAC) is currently in phase III of clinical trials. Codagenix utilizes a technology called “codon deoptimization” through a special algorithm to develop an attenuated virus.62
The Meissa vaccine presents a valuable option for COVID-19 immunization through inhalation injection.63 It demonstrates exceptional efficacy with just 1 dose during phase I clinical trials.54 The vaccine's development entails modifying the RSV virus by incorporating mutations to weaken its pathogenicity, followed by the insertion of the SARS-CoV-2 spike protein.64
Inactivated virus vaccinesA killed or inactivated virus vaccine refers to a vaccine that contains virus particles that have been rendered non-infectious through chemical and physical methods, resulting in the destruction of the viral genome and structural integrity.65 Unlike live-attenuated vaccines, the drastic changes in killed vaccines prevent virus replication, eliminating the risk of back mutation and virulence in immunocompromised patients.66 Despite their inability to propagate within a host, the antigenicity of inactivated vaccines is preserved, mimicking that of wild viruses, and exposing similar epitopes after antigen processing. Chemical methods such as formalin, β-propiolactone, psoralen, or physical methods like UV-irradiation can be used to maintain the antigenic structure of the virus intact, resembling a native virus.67 Influenza, Polio, Rabies, and Hepatitis A infections have seen successful immunization outcomes with the use of inactivated vaccines.68
The primary objective of developing inactivated vaccines is to induce the production of IgG and IgA neutralizing antibodies without triggering immunopathogenesis.69 Upon injection of an inactivated vaccine into a cell, such as a dendritic cell, the whole virus is processed into fragments, and toll-like receptors detect the single-stranded RNA of the virus.70,71
Inactivated vaccines, like other platforms, face certain challenges including weaker immune induction compared to other platforms, the need for booster doses, and the requirement for adjuvants to enhance innate stimulation.72,73
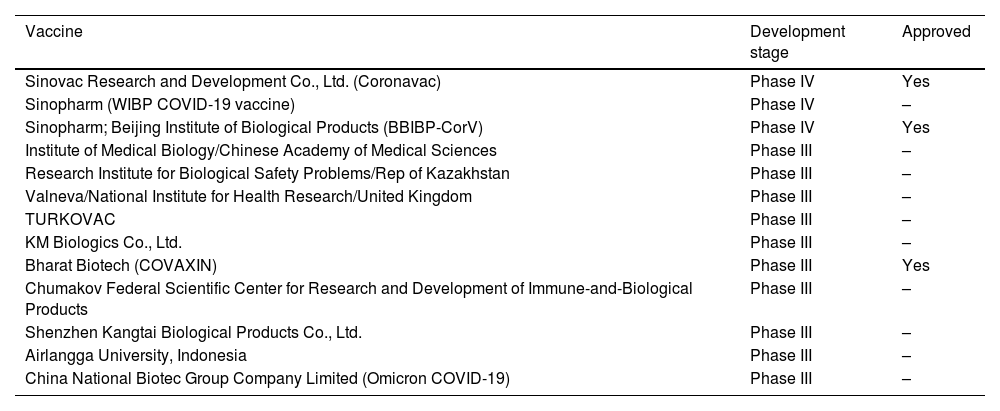
Due to the relative ease and shorter development time compared to other platforms, there are several inactivated vaccines available for SARS-CoV-2, some of which have received approval. Please refer to Table 3 for a list of these vaccines.
Inactivated vaccines divided by platform/type and development stage.
| Vaccine | Development stage | Approved |
|---|---|---|
| Sinovac Research and Development Co., Ltd. (Coronavac) | Phase IV | Yes |
| Sinopharm (WIBP COVID-19 vaccine) | Phase IV | – |
| Sinopharm; Beijing Institute of Biological Products (BBIBP-CorV) | Phase IV | Yes |
| Institute of Medical Biology/Chinese Academy of Medical Sciences | Phase III | – |
| Research Institute for Biological Safety Problems/Rep of Kazakhstan | Phase III | – |
| Valneva/National Institute for Health Research/United Kingdom | Phase III | – |
| TURKOVAC | Phase III | – |
| KM Biologics Co., Ltd. | Phase III | – |
| Bharat Biotech (COVAXIN) | Phase III | Yes |
| Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products | Phase III | – |
| Shenzhen Kangtai Biological Products Co., Ltd. | Phase III | – |
| Airlangga University, Indonesia | Phase III | – |
| China National Biotec Group Company Limited (Omicron COVID-19) | Phase III | – |
The second-generation vaccine utilizes protein-based vaccines, such as subunit vaccines.55 Subunit vaccines are created by extracting, recombinantly engineering, isolating, or synthesizing a single antigen or its truncated version and formulating it with immune adjuvants.74,75 Examples of successful subunit vaccines used in humans include the Hepatitis B virus and Papillomavirus vaccines.76 These vaccines are considered safer than other platforms because they only contain the external antigen of the virus and avoid other viral agents.77
Recombinant protein vaccines can effectively stimulate IgG and IgA neutralizing antibodies as well as the cellular arms of immunity (Th1 and Th2) if the critical antigens and relevant adjuvants are precisely selected and formulated.74,78 While structural virus proteins are suitable options for subunit vaccine design, they are weak immune stimulators when used as a single component formula due to the absence of other viral ingredients. To overcome the poor immunogenicity of these antigens, adjuvants and protein carriers are added to induce effective immunity.79,80
Several subunit vaccines, including those containing full-length or truncated forms of structural proteins, have been evaluated for preventing coronaviruses.74,79,81 Antigen candidates for protein vaccine design include different parts of the Spike protein, particularly its ectodomain and the RBD domain. In animal models of SARS-CoV-1 and MERS-CoV infection, trimeric S protein has demonstrated superior neutralizing antibody production compared to the monomeric form.75,82
For SARS-CoV-2 infection, various companies and institutions are optimizing their subunit vaccine formulas, focusing on spike-derived components, especially RBD.83 Numerous similar subunit vaccines have undergone clinical trials.
One example is the Novavax Company, which has developed a Spike protein production method using the Baculovirus expression system. Their vaccine is a recombinant nanocomplex form of S ectodomain multimer that resembles a natural particle. It is currently undergoing phase III clinical trials and has shown an efficiency of approximately 89%. The vaccine is administered in 2 doses via muscle injection, and the protein vials can be stored in a refrigerator.54,84
Another candidate is the recombinant RBD-Dimer, an alum adjuvant compound tested by Anhui Zhifei Longcom of China Biomedicine/Academy of Sciences. It is currently in phase III clinical trials and utilizes CHO cells for RBD expression. Recombinant proteins are formulated to elicit both humoral and cellular immunity.54,85
Sanofi Pasteur/GSK companies have developed the Vidprevtyn vaccine, which is based on a multimer spikes protein similar to the natural type. During the production process using the baculovirus system, further glycosylation modification is applied. Phase III clinical trials began in May 2021.54
Clover Biopharmaceuticals Inc./GSK/Dynavax have collaborated on a project involving a trimeric form of the SARS-CoV-2 S-protein. This engineered protein is prepared using Trimer-Tag® technology and is currently in phase III clinical trials.54
Vaxine Pty Ltd., in collaboration with the Iranian company CinnaGen, has produced a recombinant spike protein vaccine with an adjuvant. Phase II trials have been conducted in Iran, and registration for phase III trials began in August 2021.64
Viral vector vaccinesAdvanced second-generation vaccines are based on non-pathogenic viral vectors.55 These engineered recombinant vectors serve as carriers for delivering foreign proteins for gene therapy and vaccination purposes.86 The technology involves placing an antigen inside the vector's genetic chamber using appropriate promoters.87 Both replicating and non-replicating vectors can be used,88 with non-replicating vectors having essential genes deleted to prevent replication. Viral vector vaccines effectively infect antigen-presenting cells and stimulate an innate immune response, enhancing the duration of the immune response.89 This platform induces cellular and humoral immunity by mimicking a natural infection90,91 (Fig. 5). An advantage of this platform is that it can be developed without complete viral genome sequence information, relying solely on the antigen sequence.92 Viral vectors are adaptable and fast in responding to emerging viruses like SARS-CoV-2, which is why they are utilized in the pioneering COVID-19 vaccine. However, understanding the epidemiology and genotoxicity of the viral vector is crucial.93 Technology transfer and large-scale production/purification present challenges in the development of this vaccine platform.94 Various viral vectors, including adenovirus, vaccinia virus, influenza, Newcastle disease virus (NDV), lentivirus, vesicular stomatitis virus (VSV), measles, and Sendai virus, have been evaluated as candidates for vaccines against SARS-CoV-1 and MERS-CoV.95,96 Among the viral vectors tested for COVID-19, adenoviral-based vaccines are particularly significant.
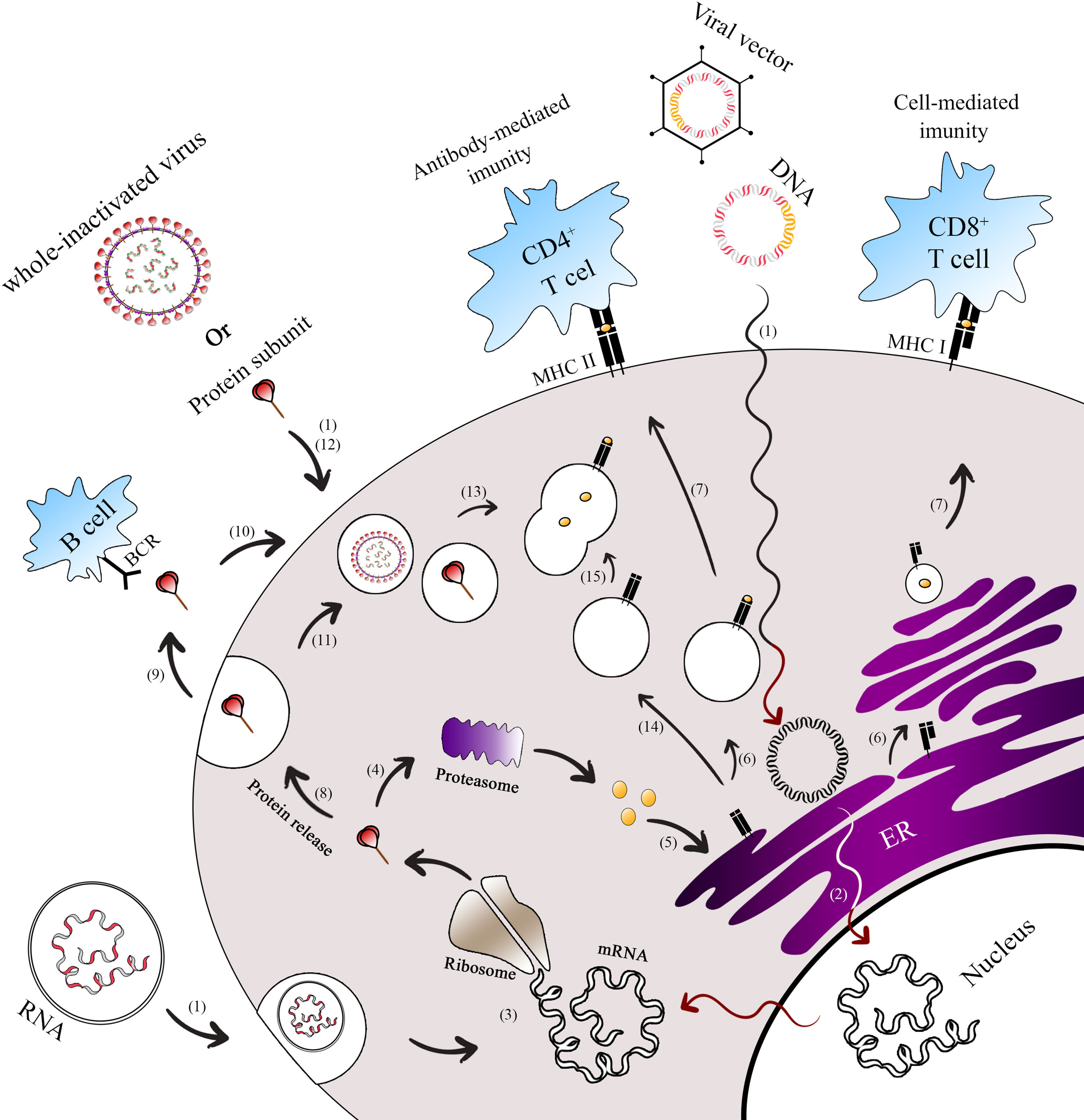
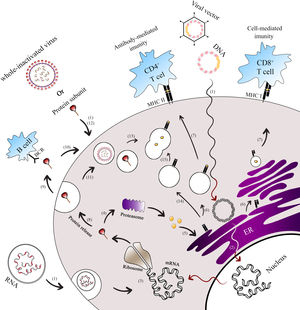
Antigen presentation paths of COVID-19 vaccine platforms. This figure illustrates the endogenous and exogenous expression paths for antigen presentation by Covid-19 vaccine platforms.1 Both next-generation vaccines (mRNA, DNA, viral vector-based) and classical vaccines (whole inactivated virus, protein subunit) enter the cell to initiate the immunization process.2 For DNA and viral vector vaccines, their genomes are released into the nucleus and transcribed into spike mRNA.3 This mRNA is then translated into spike proteins in the ribosome, forming the antigen (spike). Once the spike protein is formed, it can undergo different pathways.4 In the first path, the spike is broken down into peptides by the host proteasome.5 These peptides enter the endoplasmic reticulum (ER) and Golgi apparatus, where they undergo glycosylation.6 Subsequently, the peptides bind to major histocompatibility complex class I (MHC-I) and II (MHC-II).7 The MHC-I-peptide complex is presented on the cell membrane to CD8+ T cells, while the MHC-II-peptide complex is presented to CD4+ T cells.8 In the second path, the spike protein can be secreted9 and presented to B cells.10 Alternatively, it can be ingested by the endosome from outside the cell11 or enter the endosome without secretion.12 Moreover, subunit protein vaccines can be endocytosed in the form of spike protein or as a whole inactivated vaccine.13 In these cases, the spike protein is broken into pieces in the lysosome.14 The MHC-II in the vesicle from the ER then travels to the cell membrane.15 The vesicles containing peptide fragments of the vaccines fuse with vesicles containing MHC-II proteins, forming the MHC-II-peptide complex. This complex is recognized by CD4+ T cells, facilitating B cells to produce antigen-specific antibodies. The activation of CD8+ and CD4+ T cells occurs through the presentation of peptides on MHC-I and MHC-II, respectively.
* Exogenous expression paths for antigen presentation by vaccine platforms.
** Endogenous expression paths for antigen presentation by vaccine platforms.
Adenovectors have been extensively studied since the emergence of SARS-CoV-1 and MERS-CoV. In 2003, adenovectors expressing the S protein of SARS-CoV-1 were evaluated in the rhesus macaque model.96–98 Folegatti et al demonstrated that adenovectors expressing the complete spike protein also showed adequate immunogenicity in an animal model of MERS-CoV infection.91 Recently, a prime-boost regimen of ChAdOx1 was used in rhesus macaques to enhance MERS-CoV-targeted antibodies and eliminate viral particles from the respiratory tract. This study proved the protective ability of ChAdOx1 MERS-CoV against 6 circulating MERS-CoV strains.34
Adenovectors, whether replication-competent or replication-defective, are popular viral-derived carriers for heterologous antigens. By manipulating or replacing certain early genes, particularly the E1 region, the construction of adenovectors containing antigen sequences is feasible while preventing viral replication competence.99 Omitting other early genes like E3 and E4 can prevent destructive effects and increase vector capacity.100 Adenovectors have a high transduction rate, efficiently delivering gene cargo to the nucleus of both resting and dividing cells.101 They are considered safe in terms of genome stability and lack of integration, although they may experience initial dilution and deletion due to cell division.88 The existence of numerous serotypes of adenovirus in humans, birds, and mammals allows for the production of serotype-specific vaccines, minimizing neutralization of antibodies and enabling different patterns of tropism. However, pre-existing immunity to adenovirus poses challenges in vector targeting and the eradication of transduced cells through cell-mediated toxicity and trained CTLs.102 To address this issue, using rare human- or animal-derived serotypes such as Ad-26 and Ad-35 has proven to be a viable strategy, as seen in the design of vaccines like Oxford/AstraZeneca and Sputnik V. Scaling up adenovectors is relatively simpler compared to other viral vectors, although not as efficient as other platforms. Genetically engineered vectors are rescued using mammalian packaging cells expressing the E1 protein to produce recombinant vectors.103 Additionally, oral or nasal administration of adenoviral vector vaccines enhances mucosal immunity, which is particularly relevant for primary SARS-CoV-2 infection.104
Currently, several adenoviral vector vaccines targeting SARS-CoV-2 are under clinical evaluation in phases I-III. One notable example is the Oxford/AstraZeneca vaccine (AZD1222), which utilizes a chimpanzee-derived virus and entered its first clinical trial early on in the pandemic. This choice of animal serotype vector was motivated by the lack of pre-existing immunity in humans.105 Researchers from Oxford University and AstraZeneca published the first paper on a phase III clinical trial of this vaccine.106 Despite reports of side effects such as coagulopathy, which gained significant attention, these instances were rare, and the vaccine demonstrated high efficacy, making it an early candidate for COVID-19 control.107 AstraZeneca and Oxford subsequently developed a new version of the vaccine, AZD2816, which provides protection against beta, delta, and Omicron variants and can be administered as a nasal spray.108
In China, an adenovirus type 5 was selected as a carrier for a full spike encoding cassette in the vaccine known as Ad5-nCoV. CanSino Biological and the Beijing Institute of Biotechnology have been producing this vaccine since the outbreak, and it is currently approved in China.109
Johnson & Johnson, for their Ad26.COV2.S vaccine, chose a rare serotype of adenovirus (hAd26) to express the spike ectodomain for efficient immunization. In September 2020, Johnson & Johnson launched a Phase III trial using a single dose instead of a 2-dose regimen.110 Subsequently, on February 27, 2021, the U.S. Food and Drug Administration (FDA) issued an emergency use permit for Johnson & Johnson's vaccine in the United States, Canada, and other countries.111
Sputnik V, an adenoviral vector-based vaccine developed by the Gamaleya Research Institute in Russia, was the first globally distributed vaccine. It utilizes a prime-boost strategy, with a prime shot of AD-26 followed by boosting with AD-5 expressing the spike protein after a 21-day interval.112 The lyophilized form of the vaccine, known as “Gam-COVID-Vac Lyo,” has also shown positive results when administered intramuscularly to healthy volunteers. Clinical trials began in June, and the vaccine's efficacy rate of 91.6% has been published in The Lancet.113
Sputnik-Light is the initial component (rAd26) of Sputnik V, the world's first registered COVID-19 vaccine. It utilizes a well-studied human adenovirus vector platform, similar to Sputnik V. As a standalone 1-shot vaccine, Sputnik-Light has demonstrated higher efficacy against infection compared to most 2-shot vaccines.114,115
Besides the aforementioned adenovectors, several other non-replicative adenovectors are currently in Phase I clinical trials.54
Influenza vector vaccinesA vaccine that provides dual protection against both flu and SARS-CoV-2 has shown promise in terms of effectiveness. Various types of flu vaccines, such as inactivated, live attenuated, and recombinant, have been available on the market for a number of years.116 This suggests that it may be feasible to use each vaccine type to express antigens of SARS-CoV-2. In clinical and pre-clinical experiments, influenza vectors have been used to design vaccines that express the antigenic epitope of the SARS-CoV-2 spike protein.54 These vaccines can be generated through reverse genetic engineering of flu-vector or by attenuating the virus under non-permissive circumstances. One advantage of replicating flu-vectors is that they can be administered intranasally.117 By providing localized immune protection against SARS-CoV-2 variants, intranasal vaccines effectively reduce the transmission of circulating variants.118 Non-replicating influenza viral vectors expressing heterologous antigens are also suitable for SARS-CoV-2 immunization. However, the addition of adjuvants is necessary to enhance the immunization outcome.117
There is currently a Phase III clinical trial underway for an influenza virus vector COVID-19 vaccine called DelNS1-2019-nCoV-RBD-OPT1 (intranasal flu-based-RBD). This vaccine is being developed by the University of Hong Kong, Xiamen University, and Beijing Wantai Biological Pharmacy (ChiCTR2100051391). It is the only replicating influenza vector vaccine currently in clinical trials, with the notable advantage of being administered through a nasal spray, which has demonstrated favorable safety and efficacy.119
VSV vector (replicating): rVSV-SARS-CoV-2-S vaccine (IIBR-100)Vesicular Stomatitis Virus (VSV) is an effective replicating vector used for vaccination against viral infections. One example is Ervebo®, an FDA-approved vaccine developed by Merck, which utilizes a recombinant VSV that expresses the glycoprotein of the Ebola virus (rVSVΔG-ZEBOV-GP).120 This particular virus demonstrates extensive tropism to antigen-presenting cells (APCs), induces potent cellular immunity, and has minimal evidence of pre-existing immunity in the human population, making it a suitable candidate for vaccination purposes.121
In the context of SARS-CoV-2, the Israel Institute for Biological Research is currently investigating a replicating VSV in clinical Phase II/III trials (NCT04990466).54
MVA vector (non-replicating)Edward Jenner pioneered eradication efforts by injecting the cowpox virus, which belongs to the same family as Vaccinia Virus (VV) and smallpox. VV was later utilized by scientists to eradicate smallpox due to its close relation and favorable properties. In 1982, VV served as a viral vector for expressing influenza genes, benefiting from its optimal characteristics.122,123 Modified Vaccinia Virus Ankara (MVA) vectors expressing heterologous antigens have been adopted for their stability, ample capacity, easy production and manipulation, cytoplasmic gene expression, and ability to induce long-lasting protective immunity. These features have been demonstrated in cancer immunotherapy applications.89 Clinical trials have been conducted on 4 MVA-SARS-2-Spike proteins developed by the University of Munich (Ludwig-Maximilians), City of Hope Medical Center/National Cancer Institute, German Center for Infection Research, and Hannover Medical School.54
NDV vector (replicating)The viral vector vaccine NDV-HXP-S, developed by Sean Liu from Icahn School of Medicine at Mount Sinai, is currently in Phase II/III clinical trials.54 Newcastle Disease Virus (NDV) possesses several advantageous features that make it a suitable candidate for vaccine production. These include the absence of pre-existing immunity, easy attenuation, and the existence of reverse genetics systems to rescue recombinant NDV. However, there are also limitations associated with this vector, such as persistent immunity against NDV, an increased risk of pathogenesis, low viral titer production, and potential carcinogenesis.124
AAV vector (non-replicating)AAV5-RBD-S is an adeno-associated virus vector-based COVID-19 vaccine currently undergoing Phase I/II clinical trials conducted by Biocad.54 This vaccine candidate boasts a notable advantage of remaining stable at ambient temperature for up to 1 month, as stated by the manufacturer.125 In previous studies involving Balb-C mice, an AAV vector-based vaccine candidate targeting SARS-CoV demonstrated effective mucosal immunity when administered via nasal spray.126
Nucleic acid-based vaccineThe third-generation vaccines for SARS-CoV-2 include nanoparticle and genetic vaccines. These vaccines contain DNA or RNA sequences that encode target antigens.55 They offer advantages such as producing antigen protein similar to an actual infection, activating both cellular and humoral immunity, and not requiring complex protein folding or production processes127,128 (Fig. 5). Nucleic acid-based vaccines, including DNA and mRNA vaccines, are easier to design and proceed into clinical trials compared to other platforms.129 Several biotech companies are utilizing nucleic acid-based vaccines to develop a vaccine against SARS-CoV-2.128
DNA vaccineOne platform of nucleic acid-based vaccines is the DNA vaccine. In this approach, the DNA sequence of the target antigen is inserted into a eukaryotic expressing plasmid and delivered into the host cell nucleus to express the antigen protein.130 DNA vaccines can be produced on a large-scale and provide long-term immunity. Unlike RNA or live attenuated vaccines, they do not require a cold chain for transmission.131 DNA vaccines offer additional advantages such as a robust cellular immune response, higher safety margin, simplified production process conforming to cGMP norms, absence of infectious agents, and suitability for large-scale production.132
Disadvantages of DNA vaccines include the potential integration into the host genome and the spread of drug-resistant bacteria in the environment.133 Poor immunogenicity and reactogenicity have been limitations preventing their approval as real vaccines, but strategies like prime-boost administration and adjuvant inclusion in the plasmid construct may optimize their efficiency.134 The route of delivery is another challenge for nucleic acid-based vaccines, with new methods like pyro-drive jet injectors being explored as practical devices.135 Several DNA-based vaccines against SARS-CoV-2 are currently undergoing clinical evaluation in different phases.
ZyCoV-D, developed by Zydus Cadila, is the first DNA vaccine to demonstrate effectiveness against SARS-CoV-2. It has received emergency use permit in India136 and is administered intradermally in three doses.54 The vaccine has an efficacy of approximately 66% with no serious side effects observed. However, its stability at room temperature is relatively low, lasting for about 3 months.136
INO-4800 is a DNA vaccine encoding the full Spike glycoprotein137 that is entering Phase III human testing.54 It can be stored at room temperature for about a year138 and has shown promising results in inducing neutralizing antibodies and T cell activity against B.1.351 variants.139
AG0301-COVID19, developed by AnGes/Takara Bio/Osaka University, is another DNA vaccine in Phase II/III clinical trial.54 It encodes the Spike glycoprotein and exhibits a long half-life, maintaining its properties for up to 1 year at room temperature.132,140
RNA-based vaccineRNA vaccines, a new generation technology, have proven to be effective in emergency situations. These vaccines utilize non-replicative mRNA molecules or self-replicating RNA constructs encoding antigens. They are a standalone platform that does not require virus isolation or characterization.141 The production of mRNA/self-amplifying RNA involves antigen sequence selection, sequence optimization, modified nucleotide screening, in-vitro artificial synthesis, delivery system optimization, and immunoassay.142 Upon injection, RNA vaccines initiate the expression of antigens in the cytoplasm without the need for access to the nucleus. Residing antigen-presenting cells (APCs) in the regional lymph nodes take up the mRNA and synthesize viral antigens.143 These antigens undergo endogenous processing and presentation to immune cells, mimicking the natural viral infection process. This results in the induction of cellular and humoral immunity144 (Fig. 5).
One of the advantages of mRNA vaccines is their ability to induce chemokines such as CXCR3-ligands CXCL9, CXCL10, and CXCL11, which recruit macrophages and dendritic cells to the injection site.145 mRNA vaccines offer high potential, short production cycles, low production costs, and safe administration, making them a suitable alternative to conventional vaccines.146
Unlike DNA vaccines, mRNA vaccines do not integrate into the host genome and do not require extra sequences containing antibiotic resistance or immune stimulators. This makes them safer.147 mRNA vaccines also have advantages over protein or inactivated vaccines, as they eliminate the risks of protein contamination, high post-processing costs, activation of injected viruses, development of antibody-dependent enhancement (ADE), and dominant humoral immunity.148 Additionally, purification and isolation of proteins are hassle-free in mRNA vaccines.141
However, there are some restrictive factors for mRNA vaccine synthesis, including termini modifications such as 5´-Cap and poly-A tail, which improve mRNA stability against degradation by RNase. Storage and shipment of mRNA vaccines require a special cold chain.148
Prior to the COVID-19 pandemic, there were no approved RNA vaccines. However, in August 2021, the first fully approved RNA vaccine against COVID-19, the BNT162b2 vaccine developed by Pfizer-BioNTech, received full approval.149 Currently, there are at least 18 mRNA-based vaccines expressing the full Spike or S1 protein against SARS-CoV-2 undergoing clinical trials.54
The Pfizer-BioNTech vaccine utilizes nucleoside-modified mRNA that encodes the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein.150 The RBD antigen is modified with a foldon trimerization domain to enhance its immunogenicity.151 The vaccine is formulated in lipid nanoparticles for efficient delivery after intramuscular injection.152 It has shown high efficacy of 92%.153 Due to virus mutations and the emergence of the Delta variant, booster doses have been suggested.154
Another authorized RNA vaccine is Moderna's Spikevax or mRNA-1273. It can be stored for 30 days with refrigeration and 6 months at −4 °F (−20 °C). The formulation involves a lipid nanoparticle-encapsulated mRNA construct that expresses the full-length, perfused SARS-CoV-2 spike protein in the cytoplasm.155 Initially given in 2 doses 4 weeks apart, the FDA authorized 3 doses for immunocompromised individuals.156 Spikevax received full FDA approval on January 31, 2022.157
CureVac developed the CVnCoV vaccine, an mRNA-based SARS-CoV-2 vaccine encoding the spike protein. It is designed for 2 doses and can be stored easily for at least 3 months at 36–46 °F (2–8 °C).158 However, in Phase III trials, CVnCoV showed an efficacy of only 48%.159
Variants of concern and vaccine efficacyThe SARS-CoV-2 virus has undergone mutations, leading to the emergence of various variants. WHO classifies these variants into 2 groups: variants of concern (Alpha, Beta, Gamma, Delta, and Omicron) and variants of interest (Lambda and Mu).160 The prevalence of the Omicron variant in vaccinated populations raised concerns about vaccine effectiveness. Studies have indicated that 2 doses of the vaccine offer limited immunity, while a booster dose enhances immunity, although its protection diminishes over time.161 Another study on mRNA vaccines demonstrated that a fourth dose of Pfizer-BioNTech or Moderna vaccines provided significant protection.162
A study by Lau et al estimated that approximately 45% (41–48%) of the local population had been infected with the Omicron variant. After 7 days of vaccination, 3 and 4 doses of Pfizer-BioNTech or CoronaVac showed effectiveness against Omicron infection. The vaccine efficacy (VE) was estimated to be 48% (95% credible interval: 34–64%) and 69% (46–98%) for 3 and 4 doses of Pfizer-BioNTech, respectively. Furthermore, the VE was estimated to be 30% (1–66%) and 56% (6–97%) for 3 and 4 doses of CoronaVac, respectively. However, after 100 days of immunization, the VE declined to 26% (7–41%) and 35% (10–71%) for 3 and 4 doses of Pfizer-BioNTech, and to 6% (0–29%) and 11% (0–54%) for 3 and 4 doses of CoronaVac.163
According to a study, individuals with diabetes may experience lower vaccine effectiveness in terms of infection, symptomatic disease, and hospitalization compared to those without diabetes or the general study population.164
Given the available information, it is crucial to administer updated bivalent mRNA vaccines, such as COMINATY® Original/Omicron BA.4–5 COVID-19 and SPIKEVAX® Original/Omicron BA.4–5 COVID-19 bivalent vaccine, to effectively combat the spread of newly emerged variants.165
Disadvantages and reverse events of COVID-19 vaccinationWhile vaccines are currently recognized as the most effective method to ensure public safety and reduce mortality rates, the urgency of the situation has led to the granting of emergency use licenses for these vaccines, which has resulted in certain potential side effects being disregarded. Concurrently, there have been numerous accounts of adverse reactions following the administration of COVID-19 vaccines. Individuals with prior immune-related conditions or heightened susceptibility due to age and physiological factors have reported a higher frequency of side effects following COVID-19 vaccination.166
Neurological side effectsThe primary and most prevalent complications associated with specific vaccine brands include cerebral venous sinus thrombosis (more commonly associated with Oxford/AstraZeneca), transverse myelitis (more commonly associated with Pfizer-BioNTech, Moderna, Oxford/AstraZeneca, and Johnson & Johnson), Bell's palsy (more commonly associated with Pfizer-BioNTech, Moderna, Oxford/AstraZeneca), Guillain-Barré syndrome (more commonly associated with Pfizer-BioNTech, Oxford/AstraZeneca, and Johnson & Johnson), and the initial occurrence of multiple sclerosis (more commonly associated with Pfizer-BioNTech).166
Cardiovascular side effectsIn addition, there have been reports of significant concerns regarding cardiovascular-related side effects associated with the 4 widely used and well-known vaccines, specifically Oxford/AstraZeneca, Johnson & Johnson, Pfizer-BioNTech, and Moderna. These side effects include myocarditis, thrombosis, thrombotic thrombocytopenia, immune thrombocytopenia, cerebral sinus venous thrombosis, and acquired thrombotic thrombocytopenic purpura.167 Inactivated vaccines have a long-standing history of usage in preventing various infectious diseases, and as a result, they are generally regarded as safe.168 While uncommon, there is a possibility of allergic events linked to cardiovascular issues during vaccination. According to available literature, severe allergic reactions following the use of inactivated vaccines appear to be infrequent.169 However, healthcare professionals should be vigilant about the infrequent yet severe complication called type one Kounis syndrome, which can arise as a result of inactivated coronavirus vaccines.170
Acute eosinophilic pneumonia side effectsAcute eosinophilic pneumonia (AEP) is an uncommon condition that can occur either without a known cause or as a result of various agents.171 The specific T-helper immune response triggered by vaccination depends on the type of antigen used. For instance, previous studies have demonstrated that inactivated SARS-CoV-1 vaccines can induce pulmonary eosinophilia in animals after viral exposure, as well as eosinophil-related inflammatory reactions in monkeys during reinfection.172 Similar eosinophil-associated pulmonary diseases have been observed following RSV vaccination172 and there have been reported cases of AEP associated with influenza vaccination.173 Additionally, instances of AEP have been observed in individuals with COVID-19,174 either during their active infection or as a recurrence of respiratory symptoms after recovering from the disease.171 Given that SARS-CoV-1 and SARS-CoV-2 share a significant genetic similarity of over 80%,175 it would not be surprising if SARS-CoV-2 vaccines could potentially lead to a similar vaccine-related immunopathology.
Limitations of using current COVID-19 vaccinesThe current vaccines available for COVID-19 have certain limitations when it comes to age groups and specific clinical complications. Let's take a closer look at each vaccine:
Oxford/AstraZeneca Vaccine: Some countries have imposed restrictions on the use of the Oxford/AstraZeneca vaccine due to rare cases of blood clots. As a result, it is recommended that individuals under the age of 18 should not receive this vaccine until further studies are conducted. Moreover, people with a history of severe allergic reactions to any component of the vaccine should avoid taking it.176
Johnson & Johnson Vaccine: The U.S. FDA has limited the use of the Johnson & Johnson vaccine as a last resort for adults who are medically ineligible for another approved vaccine or have no access to an alternative option. This decision was made after reports of rare but potentially life-threatening blood clots, known as thrombosis with thrombocytopenia syndrome, in a small number of recipients. Individuals aged 18 and older may receive this vaccine under these specific circumstances.177
Pfizer-BioNTech Vaccine: While the Pfizer-BioNTech vaccine has been authorized for use in individuals aged 6 months and older, there are some considerations.178 People with a history of severe allergic reactions to any component of the vaccine should avoid taking it. Additionally, if an individual has a fever (body temperature over 38.5 °C), it is recommended to postpone vaccination until they are afebrile.179 The dosage of the vaccine may also vary based on age groups, with adjustments for those aged 6 months–4 years and 5–11 years. The WHO suggests using this vaccine in children aged 6 months–17 years only when high vaccine coverage has been achieved in priority-use groups.178
Moderna Vaccine: Several countries, including Finland, Sweden, Denmark, and Norway, have limited the use of Moderna's COVID-19 vaccine in young people under the age of 30 due to concerns about rare cardiovascular side effects.180 The safety and effectiveness of this vaccine have not been assessed in individuals younger than 18 years, so its Emergency Use Authorization does not include use in this age group.181 Moreover, for immunocompromised individuals, receiving a third primary series vaccine dose has shown only moderate effectiveness in increasing antibody levels. Therefore, it is essential for them to maintain physical precautions against COVID-19. Close contacts of immunocompromised persons should also consider getting vaccinated as appropriate for their health status.182
It is important for individuals to consult with healthcare professionals and follow the recommendations and guidelines provided by regulatory authorities and health organizations regarding COVID-19 vaccination, especially when it comes to specific age groups and clinical conditions.
Prospective and the experience we gained from the pandemicThe COVID-19 pandemic has presented a unique and unprecedented global health crisis that has spurred significant advancements in vaccine development. This experience has offered valuable insights and lessons that can shape the development of new generation vaccines.
Firstly, the urgent need for an effective COVID-19 vaccine highlighted the importance of expedited research and development processes. The unprecedented collaboration between scientists, researchers, pharmaceutical companies, and regulatory agencies led to an accelerated timeline for vaccine development. This experience demonstrated the significance of streamlined regulatory pathways, flexible clinical trial designs, and enhanced manufacturing capabilities to respond rapidly to emerging infectious diseases.
Secondly, the pandemic emphasized the significance of novel vaccine platforms and technologies. Traditional approaches such as inactivated or attenuated vaccines have been complemented by the emergence of new platforms, including mRNA and viral vector-based vaccines. These newer technologies demonstrated their efficacy and versatility during the development of COVID-19 vaccines. The success of mRNA vaccines, such as the Pfizer-BioNTech and Moderna vaccines, has paved the way for the application of mRNA technology in future vaccine development against various infectious diseases.
Furthermore, the scale and global impact of the pandemic have led to unprecedented levels of international collaboration. Scientists, researchers, and manufacturers across the globe have worked together to share knowledge, data, and resources in an effort to develop effective vaccines. This experience highlighted the importance of global cooperation, data sharing, and equitable access to vaccines. It also emphasized the need for robust and resilient vaccine supply chains to ensure the rapid and equitable distribution of vaccines worldwide.
Additionally, the pandemic has underscored the significance of vaccine confidence and public acceptance. Misinformation and vaccine hesitancy have posed challenges during the COVID-19 vaccination campaigns. As a result, there is a growing recognition of the importance of effective communication strategies, public engagement, and trust-building efforts to ensure the successful implementation of vaccination programs.
In conclusion, the COVID-19 pandemic has provided invaluable experience and knowledge in the development of new generation vaccines. The lessons learned from this global crisis can guide future research, development, and deployment of vaccines. The collaborative efforts, advancements in vaccine platforms, streamlined processes, global cooperation, and vaccine confidence gained from this experience will play a crucial role in preparing us to combat future pandemics and emerging infectious diseases effectively.
FundingThis work was financially supported by Shiraz University of Medical Science (Grant No. 23413).
Code availabilityNot applicable.
Authors' contributionsThe manuscript has been reviewed and approved by all authors. S.Y.H. and J.S. conceived the study concept. S.Y.H., A.L and F.E. gathered and evaluated the data. S.Y.H. and F.E. drafted the manuscript. A.M. and J.S. revised the manuscript. F.H.T., A.L designed the images and table. S.Y.H. and J.S. supervised the study.