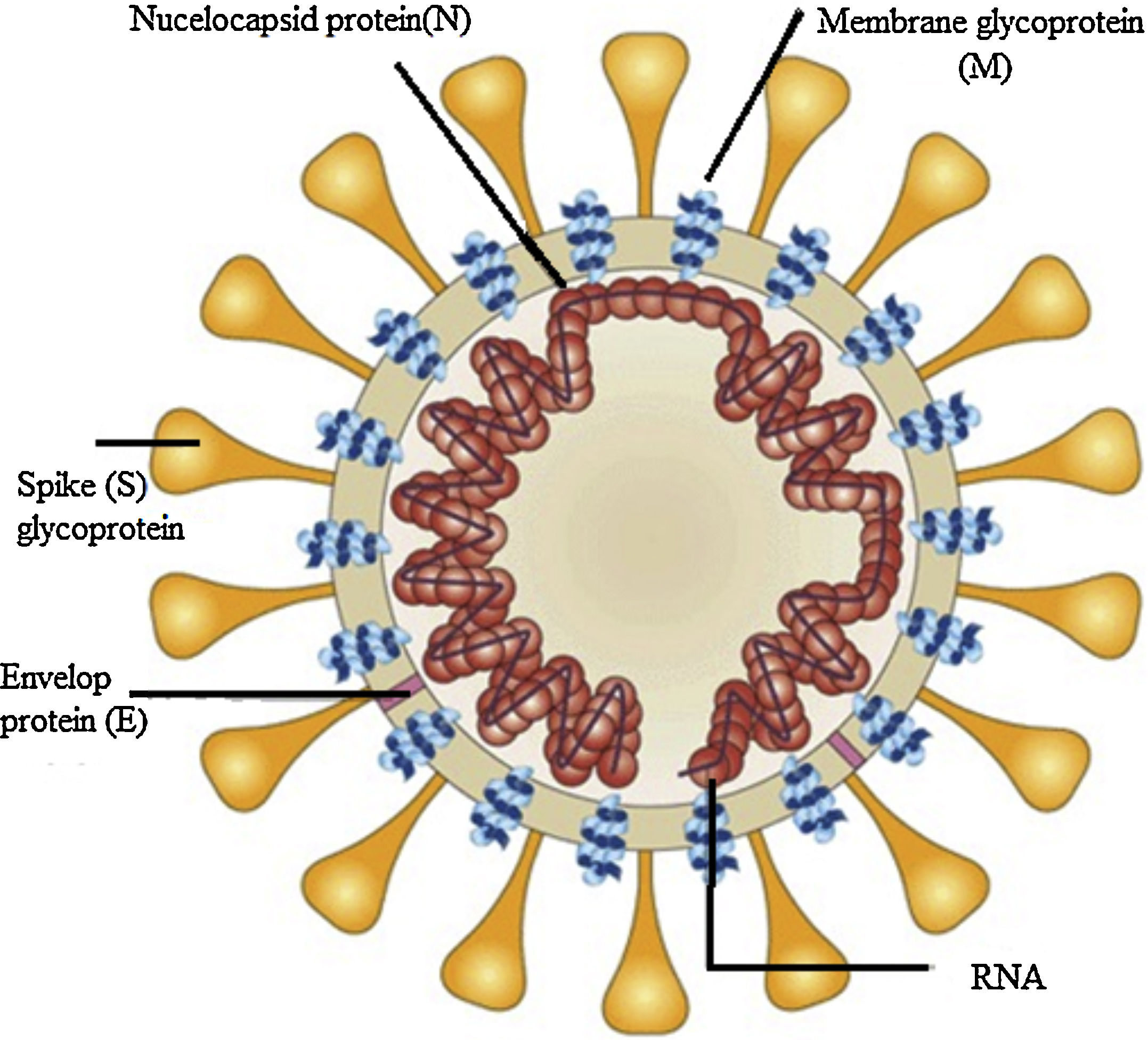
The emergence of the strain of coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) that causes corona virus disease 2019 (COVID-19) and its impact on in the world have made imperative progress to develop an effective and safe vaccine. Despite several measures undertaken, the spread of this virus is ongoing. So far, more than 1,560,000 cases and 1000,000 deaths occurred in the world. Efforts have been made to develop vaccines against human coronavirus (CoV) infections such as MERS and SARS. However, currently, no approved vaccine exists for these coronavirus strains.
Such Previous research efforts to develop a coronavirus vaccine in the years following the 2003 pandemic have opened the door for the scientist to design a new vaccine for the COVID-19. Both SARS-CoV and SARS-CoV-2 has a high degree of genetic similarity and bind to the same host cell ACE2 receptor.
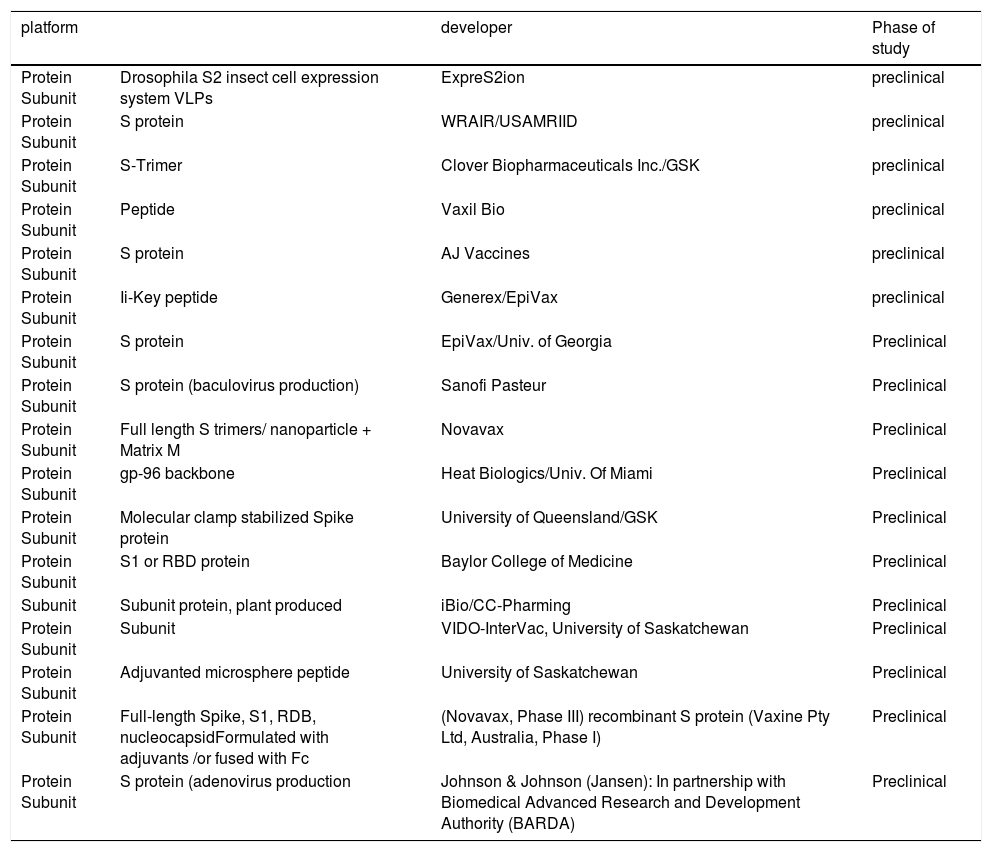
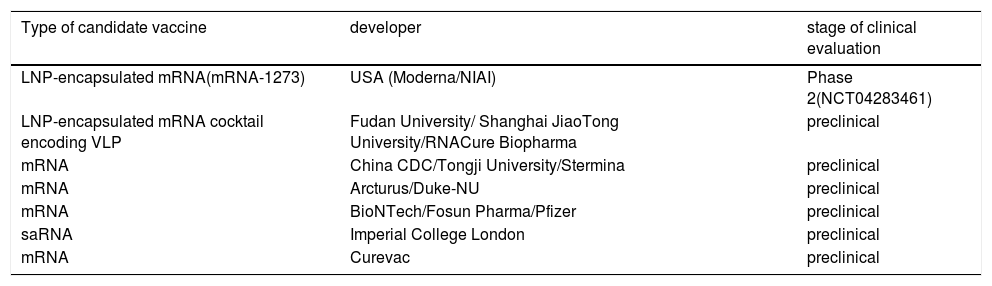
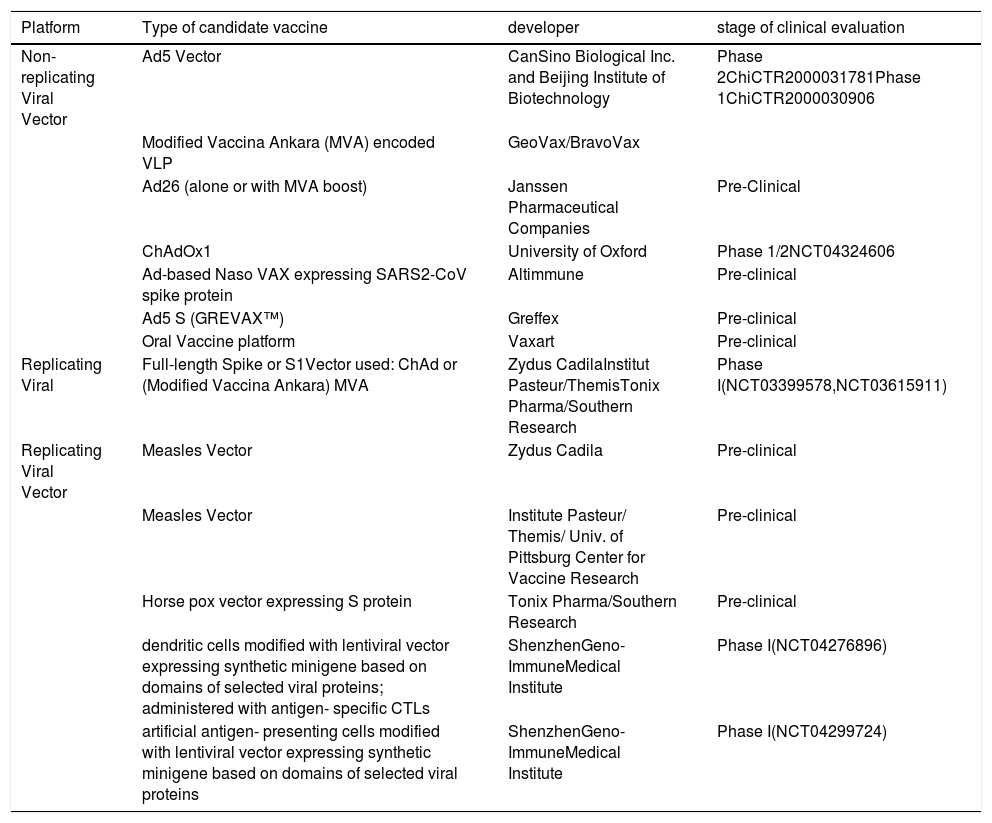
By using different vaccine development platforms including whole virus vaccines, recombinant protein subunit vaccines, and nucleic acid vaccines several candidates displayed efficacy in vitro studies but few progressed to clinical trials. This review provides a brief introduction of the general features of SARS-CoV-2 and discusses the current progress of ongoing advances in designing vaccine development efforts to counter COVID-19.
La aparición de la cepa de coronavirus SARS-CoV-2 (síndrome respiratorio agudo severo por coronavirus 2), que causa la enfermedad por coronavirus 2019 (COVID-19), y su impacto a nivel mundial, ha urgido el avance hacia el desarrollo de una vacuna efectiva y segura. A pesar de las diversas medidas adoptadas, la diseminación de este virus es continua. Hasta la fecha, se han producido más de 1.560.000 casos y 1.000.000 de muertes en todo el mundo. Se han realizado esfuerzos para desarrollar vacunas frente a las infecciones por coronavirus humano (CoV), tales como MERS y SARS. Sin embargo, actualmente no existe ninguna vacuna autorizada para estas cepas de coronavirus.
Los esfuerzos previos sobre investigación, para desarrollar una vacuna frente a coronavirus en los años posteriores a la pandemia de 2003, han abierto la puerta a los científicos para diseñar una nueva vacuna para el COVID-19. Tanto SARS-CoV como SARS-CoV-2 poseen un alto grado de similitud genética y capacidad de adherirse al mismo receptor ACE2 de la célula huésped.
Utilizando diferentes plataformas para el desarrollo de vacunas, incluyendo vacunas de virus completos, vacunas de subunidades de proteína recombinante y vacunas de ácido nucleico, algunas de estas han mostrado su eficacia en estudios in vitro, pero pocas de ellas han progresado hacia ensayos clínicos. Esta revisión aporta una breve introducción a las características generales del SARS-CoV-2, y trata el progreso actual de los avances en curso para diseñar el desarrollo de vacunas frente al COVID-19.