Assessment of bacterial reduction after chemo-mechanical preparation (using 3% sodium hypochlorite) with or without intracanal dressing (calcium hydroxide paste (Ca(OH)2) or 2% chlorhexidine digluconate gel (CHX)) in necrotic pulps associated or not with apical lesion.
MethodsProspective clinical trial, in 69 adult patient's teeth with pulpal necrosis associated or not with apical periodontitis. Microbiological root-canal-sampling occurred before treatment (S1), after chemo-mechanical preparation (S2) and after 14 days intracanal dressing (S3). Colony Forming Units (CFU) were counted after growth in aerobic, anaerobic and microaerofilic cultures. Comparison of the median CFUs treatments and culture media was done with the Friedman test. Comparison of the intracanal dressing effect at S3 was done with the Wilcoxon and the Mann–Whitney tests. Because of the huge differences in bacterial counts variations were expressed as log10 to analyze differences among intracanal medication groups. S2 and S3 counts were expressed as percentage of CFU reduction regarding S1 counts.
ResultsSignificant differences were detected between S1, S2 and S3 (Friedman test; p<0.001), showing a significant decrease from S1 to S2 (Wilcoxon test; p<0.004), followed by a significant increase from S2 to S3 (p<0.001) for the CHX group, maintenance for the Ca(OH)2 group in aerobic/anaerobic (Wilcoxon test; p=0.777/0.227), and increase in the microaerofilic culture (Wilcoxon test; p=0.047). The two groups only differed significantly in S3 (Mann–Whitney test; p≤0.001), with a worse performance in the CHX group.
ConclusionsTreatment significantly reduced the number of bacteria but failed to render all root canals sterile. Ca(OH)2 performed better than CHX gel.
Avaliar a eficácia da preparação químico-mecânica com ou sem medicação intracanalar (pasta de hidróxido de cálcio [Ca(OH)2] ou gel de digluconato de clorhexidina a 2% [CHX]) no tratamento de dentes necrosados com ou sem lesão periapical.
MétodosEnsaio clínico prospetivo em 69 dentes monocanalares com necrose pulpar ou periodontite apical. A colheita microbiológica ocorreu antes do tratamento (S1), após preparação qui¿mico-mecânica (S2) e após 14 dias de medicação intracanalar (S3). Mediram-se as unidades formadoras de colónias (UFC) em aerobiose, anaerobiose e microaerofilia. As medianas de UFP de diferentes tratamentos e culturas foram comparadas com o teste de Friedman. A comparação do efeito da medicação intracanalar em S3 utilizou os testes Wilcoxon e t. Mann-Whitney. Dada a grande variabilidade de UFC nos distintos momentos de colheita, as diferenças entre S1, S2 e S3 foram traduzidas em logaritmos de 10. As contagens de S2 e S3 foram expressas como percentagem de redução de carga bacteriana relativamente a S1.
ResultadosEncontraram-se diferenças significativas entre S1, S2 e S3 (teste Friedman; p<0,001), com decréscimo significativo de S1 para S2 (teste Wilcoxon; p<0,004), e aumento significativo de S2 para S3 (p<0,001) no grupo de CHX, manutenção em aerobiose e anaerobiose (teste Wilcoxon; p=0,777/0,227) e aumento em microaerofilia (teste Wilcoxon; p=0,047) para o grupo experimental com Ca(OH)2. Os 2 grupos só diferiram significativamente em S3 (teste Mann–Whitney; p≤0,001), com pior desempenho da CHX.
ConclusõesO tratamento reduziu, de forma significativa, a carga bacteriana, mas não esterilizou os canais radiculares. O Ca(OH)2 apresentou melhor desempenho que a CHX.
Apical periodontitis (AP) results from pulp space polymicrobial infection1–3 dominated by fastidious anaerobes, which, by symbiotic relationships, acquire specific sources of longer survival.4,5 Thus treatment must aim at all bacteria, microaerophilic, aerobic or anaerobic, strict or facultative, that is to say that the best scientific evidence-based documented procedure for the best outcome in endodontic treatment is based on the maximum disinfection of the root canal system.6
Regardless of the number of sessions, an effective bacteriological control is mandatory. The biologic concerns should always be a priority.7,8
Mechanical instrumentation coupled with sodium hypochlorite (NaOCl) irrigation dramatically reduces bacterial counts.9 However, since not all microorganisms are eliminated, intracanal dressing, primarily Calcium hydroxide paste (Ca(OH)2), has been advocated.10 Though, efficacy of this added step is controversial,11 both up to 97% bacterial reduction12–16 and increases in bacteria counts17,18 were reported. To overcome Ca(OH)2 limitations, 2% chlorhexidine digluconate gel (CHX) has emerged as an alternative.16,19,20 It has shown excellent antibacterial efficacy in vitro20 and a residual activity for up to 2 weeks or more.20 However, in vivo, liquid CHX resulted in bacterial load increase.16
Treatment outcome may also be influenced by interindividual variability. This is even more evident when individuals from different geographical locations are analysed.21–24
The present randomized clinical trial aimed at comparing the efficacy of chemomechanical cleaning alone and in combination with 2 intracanal dressings by 3 atmospheres of culture. To the best of our knowledge, this is the first report on the study of the microbiological microenvironment in root canals of Portuguese Patients.
Materials and methodsStudy adhered to Helsinki Declaration. Protocol was approved by the Ethics Committee of health Sciences Faculty of Fernando Pessoa University and an informed consent was obtained.
Using stringent criteria as described by others,25 sample included 69 subjects (35 women/34 men; mean 49.7 years) with 69 single-teeth (18 central and 16 lateral maxillary incisors, 10 mandibular and 10 maxilar premolars, 9 maxilar and 6 mandibular canines). From those, 26 were only necrotic and 43 had AP. All were chemomechanically prepared and randomly divided into two groups: calcium hydroxide paste (Ca(OH)2) or 2% chlorhexidine digluconate gel (CHX). The random order of assignment was previously generated, using Excel-generated random numbers, which were concealed in opaque, sealed, serially numbered envelopes and applied to participants as they entered the trial. Ca(OH)2 (Sigma–Aldrich, Sintra, Portugal) was manufactured with sterile saline solution (SSS) and CHX was prepared by a private Pharmacy and maintained at 4°C till usage. All the irrigants and medicaments were always freshly prepared.
Sample collection was performed as previously described.25 Briefly, after rubber dam application, its disinfection was performed with 3% hydrogen peroxide, until no further bubbling followed by a 3% NaOCl rinse for 1min as described.25 After access cavity, the operative field was disinfected as described above followed by inactivation of NaOCl with 5% sodium thiosulfate (Na2S2O3). For operative field disinfection control, a sterile cotton swab embedded in SSS was scrubbed on it and collected into tubes with 5mL Phosphate Buffer Saline (Sample Sx). For inclusion of the tooth in the study, Sx samples had to be uniformly negative. S1 sample was acquired by placing three successive size 25 sterile paper points in the canal for 1min each, approximately 1mm short of the root apex and used to soak up the fluid in the canal and transferred into vials containing 2mL sterile Reduced Transport Fluid (RTF). The working length (WL) was established with Root ZX apex locator (Morita, Kyoto, Japan) and confirmed by digital X-ray. Canals were enlarged with Protaper rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland). File sequence used was: Sx until resistance was encountered, S1, S2, F1, F2, and F3 at WL, according to each canal anatomy. Irrigation with 2mL of 3% NaOCl was applied between consecutive files using a 27G needle (Endoneedle, Elsodent, France) in an up-and-down motion to improve irrigant flow rate and a size 10K-file was used with WL+1mm (patency). In the cases of large canals, F4 and F5 files were used. After smear layer removal (5mL 10% Citric Acid, 5mL 3%NaOCl and 5mL 5%Na2S2O3), S2 sample was acquired. After drying, intracanal dressings were applied using lentulo spiral fillers with clockwise motion without water rinse and packed with a sterile cotton pellet at the canal entrance level. Another sterile cotton pellet input plug was placed and teeth were sealed with Coltosol (Coltène/Whaledent Inc., USA) for 14 days.
At the second appointment, after evaluation of the integrity of temporary restoration, restoration removal and similar field disinfection, another Sx was retrieved as described. Then, intracanal dressing was removed was with 5mL SSS for the Ca(OH)2-group and Lecitin, Tween 80 and Na2S2O3 solution for the CHX-group. Dressing removal was confirmed with a Carl Zeiss® Microscope, and S3 samples (to assess medication’ efficacy) were then collected as described for S1 and S2. After a final rinse, filling resorted to gutta-percha points and TopSeal (Dentsply, Maillefer, Ballaigues, Switzerland), the canal entrance was sealed with Synergy D6 Flow (Coltène/Whaledent, USA) and access cavity temporized.
Samples in RTF vials were managed as described.25 Aliquots of 100μL of undiluted and highest dilution (10−3) were spread onto BHI (Liofilchem Diagnostic, Italy) and Anaerobe Basal (Oxoid, United Kingdom) agar plates both supplemented with 5% defibrinated horse blood. Plates were incubated within jars, at 37°C, anaerobically for 14 days and aerobically and microaerophilically (10% CO2) for 48h. Colony-Forming Units (CFUs) were then counted.
Statistical analysis was performed using IBM© SPSS© Statistics vs.20.0 (p<0.05).
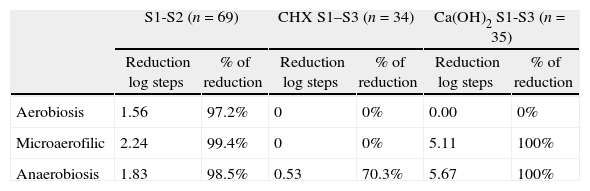
Differences among medication groups were calculated with log10 transformations. S2 and S3 counts were expressed as percentage of CFU reduction regarding S1 counts (Table 1).
Reduction log steps and percentage of bacterial reduction within the 3 atmospheres tested and the different intracanal dressings.
| S1-S2 (n=69) | CHX S1–S3 (n=34) | Ca(OH)2 S1-S3 (n=35) | ||||
| Reduction log steps | % of reduction | Reduction log steps | % of reduction | Reduction log steps | % of reduction | |
| Aerobiosis | 1.56 | 97.2% | 0 | 0% | 0.00 | 0% |
| Microaerofilic | 2.24 | 99.4% | 0 | 0% | 5.11 | 100% |
| Anaerobiosis | 1.83 | 98.5% | 0.53 | 70.3% | 5.67 | 100% |
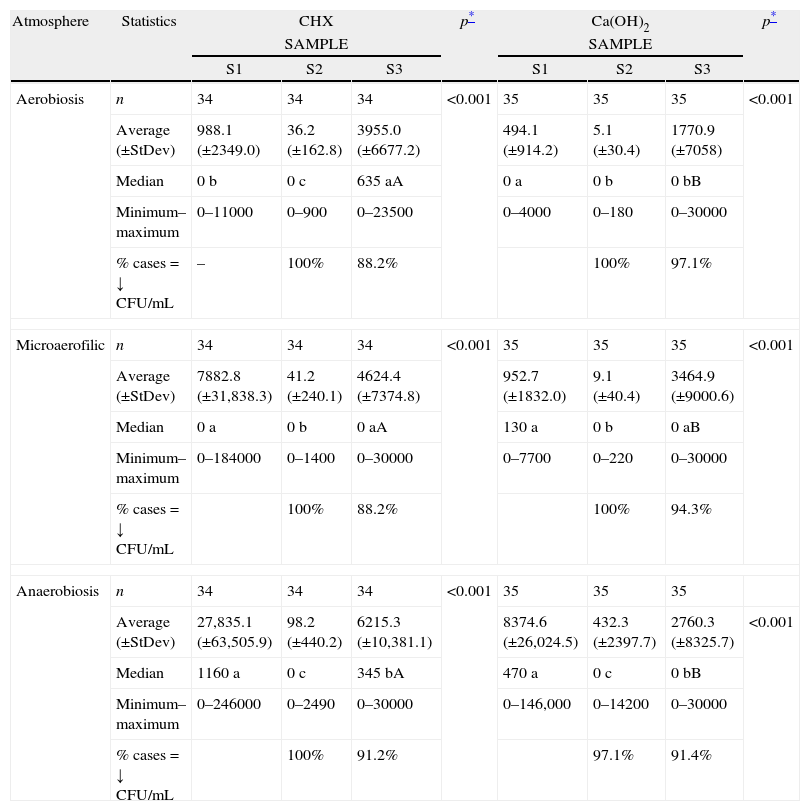
Differences in bacterial counts between S1, S2, and S3, among medication groups and initial diagnosis, were first analyzed with the Friedman test as non-normal distributions were always found (Shapiro–Wilk test). Upon detection of significant paired differences these were further investigated with the Wilcoxon test. Comparison of intracanal dressing effect at S3 was assessed using the Mann–Whitney test (Tables 2 and 3).
Effect of endodontic procedures on bacteria (CFU/mL) for both intracanalar dressings (CHX and Ca(OH)2), sampling moments (S1, S2 and S3) and the three atmospheres.
| Atmosphere | Statistics | CHX | p* | Ca(OH)2 | p* | ||||
| SAMPLE | SAMPLE | ||||||||
| S1 | S2 | S3 | S1 | S2 | S3 | ||||
| Aerobiosis | n | 34 | 34 | 34 | <0.001 | 35 | 35 | 35 | <0.001 |
| Average (±StDev) | 988.1 (±2349.0) | 36.2 (±162.8) | 3955.0 (±6677.2) | 494.1 (±914.2) | 5.1 (±30.4) | 1770.9 (±7058) | |||
| Median | 0 b | 0 c | 635 aA | 0 a | 0 b | 0 bB | |||
| Minimum–maximum | 0–11000 | 0–900 | 0–23500 | 0–4000 | 0–180 | 0–30000 | |||
| % cases=↓ CFU/mL | – | 100% | 88.2% | 100% | 97.1% | ||||
| Microaerofilic | n | 34 | 34 | 34 | <0.001 | 35 | 35 | 35 | <0.001 |
| Average (±StDev) | 7882.8 (±31,838.3) | 41.2 (±240.1) | 4624.4 (±7374.8) | 952.7 (±1832.0) | 9.1 (±40.4) | 3464.9 (±9000.6) | |||
| Median | 0 a | 0 b | 0 aA | 130 a | 0 b | 0 aB | |||
| Minimum–maximum | 0–184000 | 0–1400 | 0–30000 | 0–7700 | 0–220 | 0–30000 | |||
| % cases=↓ CFU/mL | 100% | 88.2% | 100% | 94.3% | |||||
| Anaerobiosis | n | 34 | 34 | 34 | <0.001 | 35 | 35 | 35 | |
| Average (±StDev) | 27,835.1 (±63,505.9) | 98.2 (±440.2) | 6215.3 (±10,381.1) | 8374.6 (±26,024.5) | 432.3 (±2397.7) | 2760.3 (±8325.7) | <0.001 | ||
| Median | 1160 a | 0 c | 345 bA | 470 a | 0 c | 0 bB | |||
| Minimum–maximum | 0–246000 | 0–2490 | 0–30000 | 0–146,000 | 0–14200 | 0–30000 | |||
| % cases=↓ CFU/mL | 100% | 91.2% | 97.1% | 91.4% | |||||
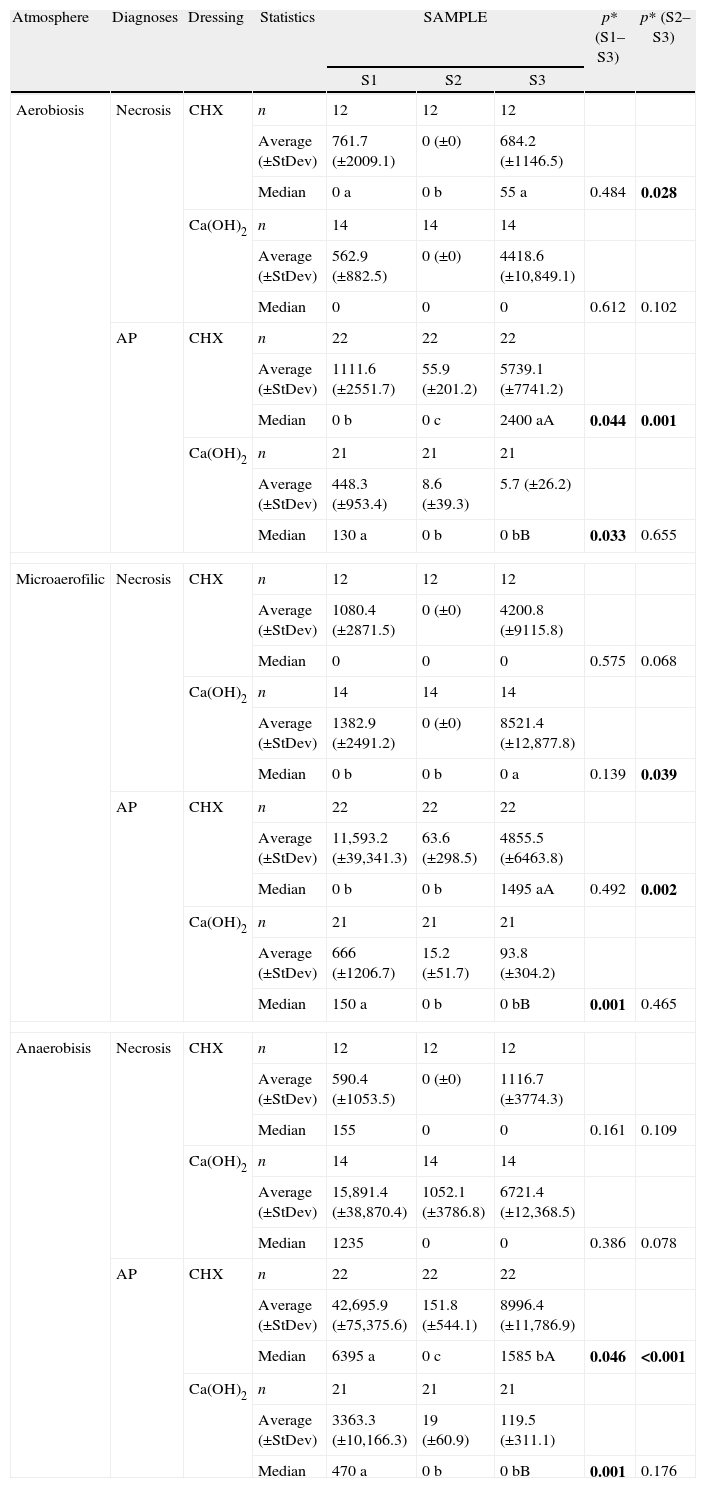
Effect of endodontic procedures on bacteria (CFU/mL) per initial diagnosis, for both intracanalar dressings (CHX, Ca(OH)2), sampling moments (S1, S2 and S3) and three atmospheres.
| Atmosphere | Diagnoses | Dressing | Statistics | SAMPLE | p* (S1–S3) | p* (S2–S3) | ||
| S1 | S2 | S3 | ||||||
| Aerobiosis | Necrosis | CHX | n | 12 | 12 | 12 | ||
| Average (±StDev) | 761.7 (±2009.1) | 0 (±0) | 684.2 (±1146.5) | |||||
| Median | 0 a | 0 b | 55 a | 0.484 | 0.028 | |||
| Ca(OH)2 | n | 14 | 14 | 14 | ||||
| Average (±StDev) | 562.9 (±882.5) | 0 (±0) | 4418.6 (±10,849.1) | |||||
| Median | 0 | 0 | 0 | 0.612 | 0.102 | |||
| AP | CHX | n | 22 | 22 | 22 | |||
| Average (±StDev) | 1111.6 (±2551.7) | 55.9 (±201.2) | 5739.1 (±7741.2) | |||||
| Median | 0 b | 0 c | 2400 aA | 0.044 | 0.001 | |||
| Ca(OH)2 | n | 21 | 21 | 21 | ||||
| Average (±StDev) | 448.3 (±953.4) | 8.6 (±39.3) | 5.7 (±26.2) | |||||
| Median | 130 a | 0 b | 0 bB | 0.033 | 0.655 | |||
| Microaerofilic | Necrosis | CHX | n | 12 | 12 | 12 | ||
| Average (±StDev) | 1080.4 (±2871.5) | 0 (±0) | 4200.8 (±9115.8) | |||||
| Median | 0 | 0 | 0 | 0.575 | 0.068 | |||
| Ca(OH)2 | n | 14 | 14 | 14 | ||||
| Average (±StDev) | 1382.9 (±2491.2) | 0 (±0) | 8521.4 (±12,877.8) | |||||
| Median | 0 b | 0 b | 0 a | 0.139 | 0.039 | |||
| AP | CHX | n | 22 | 22 | 22 | |||
| Average (±StDev) | 11,593.2 (±39,341.3) | 63.6 (±298.5) | 4855.5 (±6463.8) | |||||
| Median | 0 b | 0 b | 1495 aA | 0.492 | 0.002 | |||
| Ca(OH)2 | n | 21 | 21 | 21 | ||||
| Average (±StDev) | 666 (±1206.7) | 15.2 (±51.7) | 93.8 (±304.2) | |||||
| Median | 150 a | 0 b | 0 bB | 0.001 | 0.465 | |||
| Anaerobisis | Necrosis | CHX | n | 12 | 12 | 12 | ||
| Average (±StDev) | 590.4 (±1053.5) | 0 (±0) | 1116.7 (±3774.3) | |||||
| Median | 155 | 0 | 0 | 0.161 | 0.109 | |||
| Ca(OH)2 | n | 14 | 14 | 14 | ||||
| Average (±StDev) | 15,891.4 (±38,870.4) | 1052.1 (±3786.8) | 6721.4 (±12,368.5) | |||||
| Median | 1235 | 0 | 0 | 0.386 | 0.078 | |||
| AP | CHX | n | 22 | 22 | 22 | |||
| Average (±StDev) | 42,695.9 (±75,375.6) | 151.8 (±544.1) | 8996.4 (±11,786.9) | |||||
| Median | 6395 a | 0 c | 1585 bA | 0.046 | <0.001 | |||
| Ca(OH)2 | n | 21 | 21 | 21 | ||||
| Average (±StDev) | 3363.3 (±10,166.3) | 19 (±60.9) | 119.5 (±311.1) | |||||
| Median | 470 a | 0 b | 0 bB | 0.001 | 0.176 | |||
a, b, c – different letters stand for significant differences (median) between collection sampling moments, according to the *Wilcoxon test; A, B – different letters stand for significant differences (median) between intracanalar dressings (at S3), according to the Mann–Whitney test. Bold indicates p<0.05.
Randomization of intracanal dressing proved to be independent of diagnosis (Chi-square test, p=0.687), tooth position (p=0.436) and “master apical file” (p=0.233).
First appointment protocol succeeded in decreasing bacteria counts in all cases (p<0.001), and in eliminating bacteria bellow detection level in 59 of 69 cases (85.5%). Results show a very efficient chemomechanical preparation demonstrated by the strong CFU reduction from S1 to S2 (Table 1): 97.2% (aerobiosis), 99.4% (microaerofilic atmosphere) and 98.5% (anaerobiosis), corresponding to 1.56, 2.24 and 1.83 log steps reduction, respectively (Wilcoxon test, p<0.001 for all atmospheres).
A further reduction in positive samples from S2 to S3 was not always observed. After dressing with Ca(OH)2, 70.6% of the canals (24 in 34) were negative for bacteria growth, whilst, in the CHX-group, only 28.6% (10 in 35 cases) proved negative. For CHX-group this resulted in a 70.3% CFU reduction for anaerobiosis (0.53 log step reduction), while Ca(OH)2 showed over 5 log reduction, accounting for 100% reduction (Table 1).
A consistent significant CFU decrease between S1 and S2 (Wilcoxon test; p<0.001) was found for both experimental groups (Table 2).
The same was not true for S2–S3 comparison: CHX-group showed a significant (p<0.05) increase in all atmospheres; Ca(OH)2-group showed similar counts in aerobiosis, and significantly (p<0.05) increased counts in microaerofilic and anaerobic atmospheres (Table 2).
Comparison of CFU counts at similar sampling moments between the groups showed that significant differences were only observed in S3 (Mann–Whitney test; p<0.001), with a worst performance of CHX (Table 2).
Next, CFU counts were compared according to the initial diagnosis (Table 3): in teeth presenting only necrosis, neither intracanal dressings showed significant CFU reduction between S1 and S3 or S2 and S3. However, those presenting AP showed a significant reduction between S1 and S3 when treated with Ca(OH)2 (p≤0.033 for all atmospheres) and a maintenance of low CFU counts between S2 and S3 (p>0.05), while CHX shows a significant increase between S2 and S3 (p≤0.002 for all atmospheres).
Thus, comparison at S3 shows significantly lower values in Ca(OH)2 than in CHX-group (Mann–Whitney test; p≤0.002 for all atmospheres), a fact even clearer with AP diagnosis.
DiscussionAP is caused by a habitat-adapted polymicrobial infection of the pulp space. The microbial flora typically consists of a restricted group of species, dominated by pigmented Gram-negative anaerobes with fastidious environmental and nutritional requirements.1,26
There is a current trend to include AP in the category of biofilm-induced diseases which is a major step forward to the understanding of root canal infection.27–29
Culture medium was used because of its ability to detect exclusively viable bacteria and the correlation shown by previous studies between negative cultures and a more favorable treatment outcome.18,26
In order for microorganisms to multiply in artificial media, they must have available the required nutrients and proper physicochemical conditions, including temperature, moisture, atmosphere, salt concentration and pH. Thus, given the poly-microbial nature of the endodontic micro flora and the fact that a synergistic balance is required for the survival of strict anaerobes in the presence of other aerobic and facultative anaerobes,6,30 it can be suggested that growth of bacteria in enriched broth media in different atmospheres may be more suitable because it may allow detection of even the less frequent bacteria species in the canal. These were the reasons for using a more laborious laboratory protocols (3 different atmospheres) than other reports in this field that only assessed anaerobic culture.13–16,25
The 14-days anaerobic atmosphere culture results were used, in contrast with the aerobic and microaerophilic atmospheres where 48h were sufficient to assess bacterial growth.
Clinicians may consider chemomechanical cleaning and shaping of the root canal system as total disruption of the endodontic microbial ecosystem.31 Our data are in total agreement with previous reports that have repeatedly demonstrated significant bacteria reductions with chemomechanical instrumentation32 and that waiting for treatment completion at a later date results in higher bacterial counts.30,33–35 Nevertheless, similar to other reports,36,37 the present data clearly showed that cleaning and shaping alone were insufficient to produce a sterile root canal.
Intracanal dressings also failed to render all root canals sterile. This was particularly true in the CHX-group. This could result from a number of reasons: (a) presence of bacteria in sampling inaccessible areas23; (b) a flaw during S2 sample collection38; (c) contamination during introduction of the intracanal drug39; (d) persisting bacteria either intrinsically resistant to drug or protected from the medicaments by biofilms40–42; (e) inactivation of medicament by compounds in pulp space.43
CHX activity is pH dependent,38 and is greatly reduced by dentin, inflammatory exudates, bacterial products, or the necrotic tissue.20,43 This could explain the reversal growth in 25 cases, which is in accordance with a previous report.16 Our results suggest that CHX has a limited effect as intracanal medication for 2 weeks in vivo.
The percentage of Ca(OH)2 related positive cultures observed in this study was within the range reported in previous studies.17,20 The reported variation of Ca(OH)2 effectiveness may be explained by a large variety of factors including: tooth-type, initial microbial loading, operator-associated variables, instrumentation technique, irrigants, elimination of smear layer, integrity of temporary restorations, and geographic differences of endodontic infectious bacteria.23
Immunological response to pulpal necrosis and AP is different, and this may explain the dissimilar performance of the Ca(OH)2 in these two clinical conditions. In fact, despite its well-known antimicrobial activity, Ca(OH)2 actions far exceed it. It is known to inactivate endotoxins, stimulate mineralization, dissolve organic material, and produce a chemical and physical barrier.39–44 Its immunoregulator properties were demonstrated in an in vitro study,45 where Ca(OH)2 either reduced cytokine basal expression or prevented increase of all cytokines tested during the experimental period. Another possibility is that persistent Ca(OH)2 in root canals can denature Interleukin-1α, Tumor Necrosis Factor-α, and calcitonin gene-related peptide,46,47 suggesting a dampening effect of Ca(OH)2 in periapical inflammation after root canal cleaning procedures. Thus differences observed in Ca(OH)2 performance between necrotic and AP teeth may also be related to differential activities of the drug in the presence of different basal immunological involvements.
ConclusionFrom a microbiological point of view, treatment of necrotic teeth with intracanal dressing to render root canals bacteria-free before filling seems to be questionable. Nevertheless, the present results indicate that if this strategy is used, Ca(OH)2 should be preferred to CHX. Results also indicate that if a two session protocol with intracanal dressings is chosen, removal of residual canal flora by chemomechanical cleaning at the second visit is mandatory.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to acknowledge the financial support of Abel Salazar Institute for the Biomedical Sciences (Oporto University, Portugal) which was attributed in the context of Ana Moura Teles PhD thesis at this Institution. The clinical and laboratory infrastructure and Human based support were provided by Fernando Pessoa University/Fernando Pessoa Foundation (Oporto, Portugal). Author M.C. Manso acknowledges Fundação para a Ciência e a Tecnologia through grant no. PEst-C/EQB/LA0006/2011.