Robotic surgery has proven effective in certain surgical procedures. However, in liver and pancreatic surgery (HBP) its use is still rare. The initial experience in HBP robotic surgery of a specialized unit of a tertiary hospital is presented.
MethodThe results of patients undergoing robotic HBP surgery between April 2018 and October 2020 have been prospectively studied. The data analyzed correspond to demographic data, surgical techniques performed, associated morbidity and mortality.
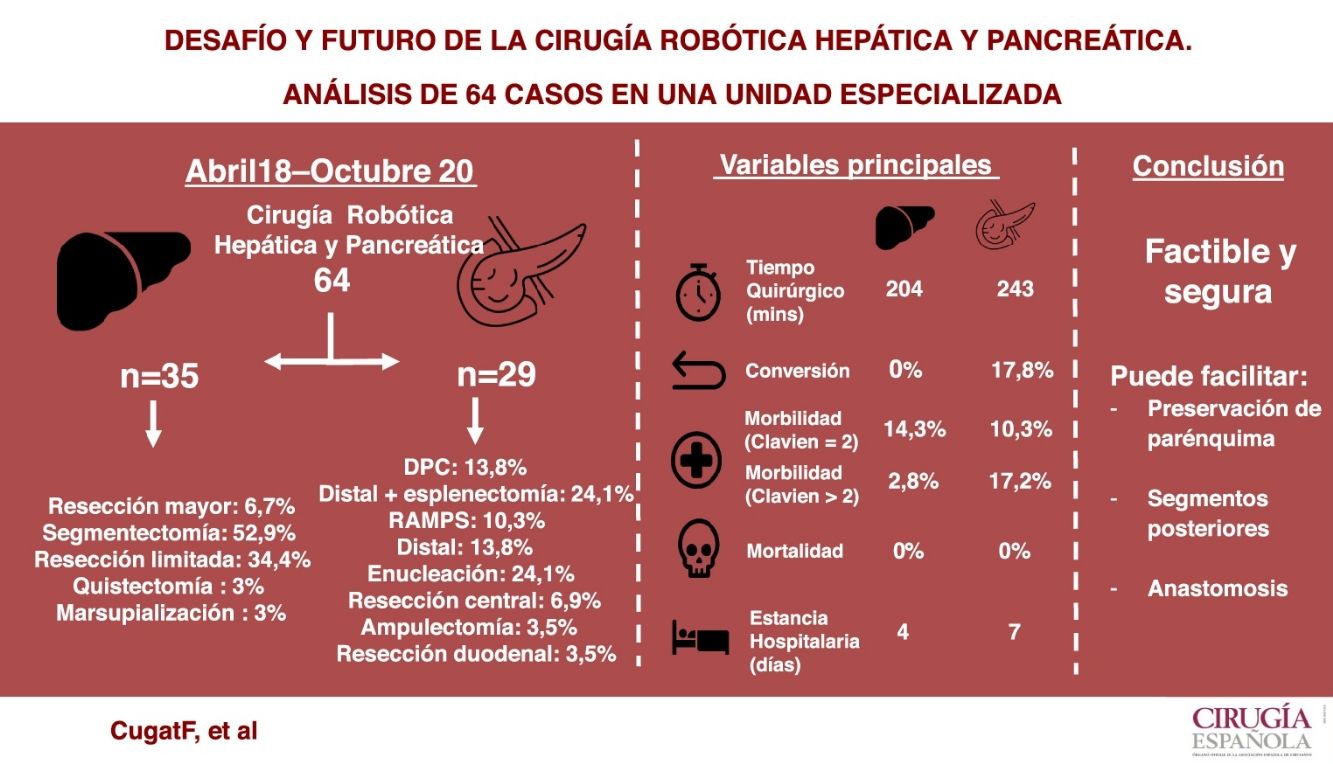
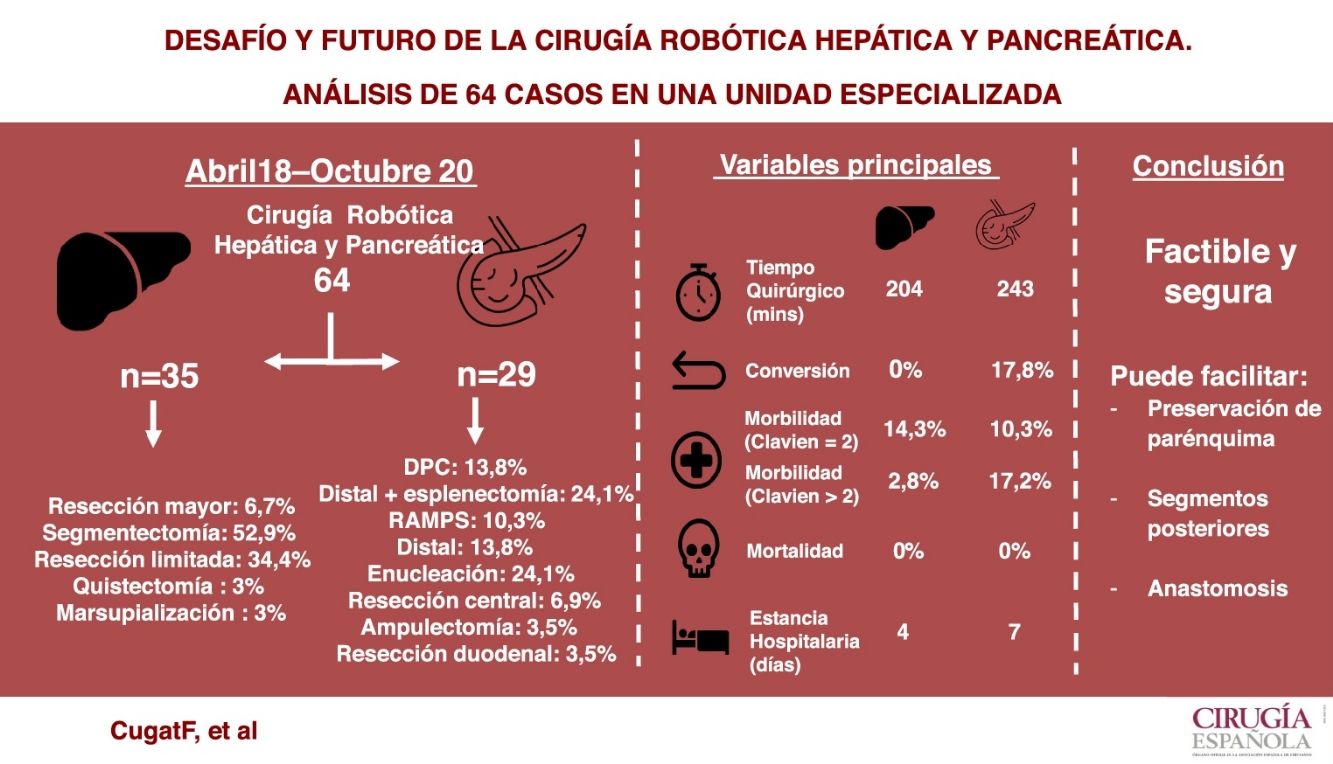
Results64 patients were operated, corresponding to 35 hepatectomies (major [6.7%], anatomic [52.9%], limited [34.4%], cystectomies [3%] and marsupialization [3%]), 29 pancreatectomies (distal [48.2%], central [6.9%], cephalic [13.8%], enucleations [24.1%], ampullectomies [3.5%] and duodenal resections [3.5%]).
In liver surgery the mean operative time was 204.4 min (100−265 min), the median postoperative complications according to the Clavien-Dindo scale was one (1–4), the mean blood losses 166.7 mL (100−300 mL), there was no conversion and the mean postoperative stay was four days (2–14 days).
In pancreatic surgery, the mean operative time was 243.8 min (125−460 min), the median of postoperative complications was two (1–4), blood loss of 202.3 mL (100−500 mL) associated to a conversion rate 17.8% and an average stay of seven days (3–23 days).
ConclusionsRobotic HBP surgery is safe and feasible. It is suggested that its use facilitates parenchymal sparing surgery, access to posterior liver segments and anastomosis in pancreatic reconstruction compared to laparoscopic surgery.
La cirugía robótica ha demostrado su eficacia en ciertos procedimientos quirúrgicos. Sin embargo, en cirugía hepática y pancreática (HBP) su uso es todavía poco frecuente. Se presenta la experiencia inicial en cirugía robótica HBP de una unidad especializada en un hospital de tercer nivel.
MétodoSe han estudiado en forma prospectiva los resultados de los pacientes intervenidos de cirugía HBP robótica entre abril de 2018 y octubre de 2020. Los datos analizados corresponden a datos demográficos, técnicas quirúrgicas realizadas y morbimortalidad asociada.
ResultadosSe intervinieron 64 pacientes, sometidos a 35 hepatectomías (mayores [6,7%], anatómicas [52,9%], limitadas [34,4%], quistectomías [3%] y marsupializaciones [3%]) y 29 pancreatectomías/resecciones duodenales (distales [48,2%], centrales [6,9%], cefálicas [13,8%], enucleaciones [24,1%], ampulectomías [3,5%] y resecciones duodenales [3,5%]).
En cirugía hepática el tiempo operatorio medio fue de 204,4 minutos (100−265 min), la mediana de complicaciones postoperatorias según la escala de Clavien-Dindo fue de uno (1–4), las pérdidas hemáticas medias de 166,7 mL (100−300 mL), no existió conversión y la estancia postoperatoria media de cuatro días (2–14 días).
En cirugía pancreática el tiempo operatorio medio fue de 243,8 minutos (125−460 min), la mediana de complicaciones postoperatorias de dos (1–4), las pérdidas hemáticas de 202,3 mL (100−500 mL) asociadas a una tasa de conversión del 17,8% y una estancia media de siete días (3–23 días).
ConclusionesLa cirugía robótica HBP es segura y factible. Se sugiere que su uso facilita la cirugía conservadora de parénquima, el acceso a segmentos posteriores hepáticos y la realización de anastomosis en la reconstrucción pancreática respecto a la cirugía laparoscópica.
Robotic surgery has already demonstrated its effectiveness in certain surgical procedures, such as radical prostatectomy1,2. The increased vision and precision of the movements of robotic platforms make it possible to perform complex laparoscopic techniques in a small space. In this area, the use of surgical robots has significantly simplified and improved surgical techniques3.
In the case of hepatobiliary and pancreatic surgery (HBP), minimally invasive surgery has been implemented mainly laparoscopically, and the use of surgical robots is rare.
Laparoscopic liver surgery is complex surgery with proven efficacy. Nevertheless, it is only reproducible by a small number of surgeons with advanced laparoscopic skills4. The position of the organ, with posterior areas hidden from anterior view, makes it difficult to access certain lesions. To circumvent these circumstances, surgeons have used caudal approach techniques and changes in patient position or placement of intercostal trocars. A priori, robotic surgery, with its vision and ability to articulate instruments, would make it possible to obviate these problems and access areas that are ‘hidden’ from laparoscopic view5.
Laparoscopic pancreatic surgery has been shown to be effective for resections of the body and tail, while resection of the head of the pancreas is more controversial6–8. In this case, robotic surgery could have a role in pancreatic parenchyma-sparing procedures, given its microscopic vision and precision. In addition, it could be useful in the reconstructive phase of the digestive tract after pancreaticoduodenectomy (PD), simplifying the procedure thanks to the ease of its movements.
If so, the incorporation of robotic surgery in the field of hepatopancreatic surgery could facilitate the minimally invasive approach compared to laparoscopic surgery, which is highly demanding and has a long (and not always adequate) learning curve.
However, these presumed benefits have yet to be demonstrated in HBP surgery9.
In this present study, we present the initial experience in hepatic and pancreatic robotic surgery in selected cases of a unit specialized in HBP surgery at a tertiary university hospital in order to establish the advantages and disadvantages of this approach.
MethodsBetween April 2018 and October 2020, 64 patients underwent robotic HBP surgery with the DaVinci Xi® Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Thirty-five hepatectomies (major, anatomical, and limited), 28 pancreatectomies (distal, central, head, and enucleation) and one duodenal resection were performed. The surgical techniques for both liver and pancreatic surgery were developed simultaneously.
All patients were managed under the hospital surgical protocol10, with preoperative multimodal prehabilitation and an enhanced recovery after surgery (ERAS) protocol11.
Technical descriptionPatients were positioned in the supine position on a Pink Pad® positioning system, in the French position, with legs open, the right arm closed, and the table was placed in 15° anti-Trendelenburg position. Next, pneumoperitoneum (12−14 mmHg) was created with a Veress needle at Palmer's point. The positioning of the trocars was along a straight line below the umbilicus, starting with the periumbilical trocar for the 30° robotic stereo camera and the determination of the objective (pointing target). Two other 8-mm robotic trocars and a third 11-mm trocar were used for the robotic endostapler, adding an accessory trocar for the assistant as needed. The approach of the robotic arm was done on the patient’s right for hepatic and duodenal-pancreatic resections (with variable left decubitus), while it was performed on the left for resections of the body and tail of the pancreas and spleen (right decubitus). Docking was performed, from right to left, with bipolar forceps (T1), 30° robotic camera (T2), monopolar scissors/vessel sealer (T3) and tip up grasper (T4).
Liver surgeryThe liver was mobilized to provide adequate access to the transection area. Parenchymal transection was performed with extracorporeal Pringle hilar clamping using a systematic crush-clamp, bipolar forceps and monopolar scissors. Robotic Hem-o-locks were used to divide second-order portal veins, while suprahepatic veins or first-order portal veins were transected with a robotic endostapler, with a 45-mm white cartridge for the former and a blue 45-mm cartridge for the latter. Specimens were extracted in XL endobags through an accessory Pfannenstiel incision. Lastly, the aponeurosis of the 11-mm trocar and the accessory incision were closed.
Pancreatic surgeryFor parenchyma-sparing pancreatic resections (enucleations), after accessing the omental bursa, the stomach was pulled to the zenith with two Rummel tourniquets, inserted with a Reverdin needle, to obtain correct exposure of the pancreas. The lesion was then identified with perioperative ultrasound. The dissection and transection of the gland was performed with fine movements using fenestrated Maryland bipolar forceps and monopolar scissors.
For the PD, after transection of the gastric antrum with a 60-mm robotic endostapler, the omental bursa was accessed. The hepatic hilum was dissected to continue with an extensive Kocher maneuver. The retropancreatic portal vein was dissected, and the gland was divided with monopolar scissors. After rejecting the greater omentum cranially, the first jejunal loop was identified, which was divided with a 60 mm endostapler to proceed with the duodenal uncrossing. Finally, portal dissection and mesopancreas transection were performed with the robotic sealer. For reconstruction, enteric bypass with pancreaticojejunal anastomosis was performed with discontinuous 4/0 PDS suture, and for the duct-to-mucosa anastomosis a continuous double layer with 3/0 barbed suture was used. In the case of a pancreaticogastric anastomosis, a double crown of 3/0 barbed suture was applied on the posterior gastric surface. The hepaticojejunostomy was performed using continuous 3/0 double barbed suture, and the gastrojejunostomy was done using a mechanical anastomosis with a 60-mm robotic endostapler, all in a single antecolic loop.
Statistical analysisThe descriptive analysis uses measures of frequency (percentage) for the qualitative variables. For quantitative variables, measures of central value, mean and median are used depending on whether or not the variable follows normal distribution, and the range, with which we also express the maximum and minimum values of the results obtained.
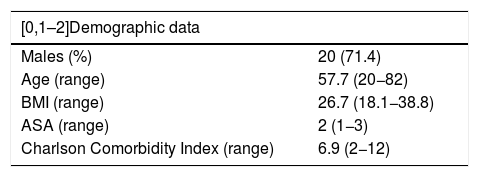
ResultsLiver resectionsThirty-five patients underwent liver surgery, with a total of 45 lesions, predominantly ASA 2 patients, with a Charlson Comorbidity Index (CCI) of moderate comorbidity and a mean age of 57.7 years (Table 1).
Demographic data of patients treated with hepatic surgery.
| [0,1–2]Demographic data | |
|---|---|
| Males (%) | 20 (71.4) |
| Age (range) | 57.7 (20−82) |
| BMI (range) | 26.7 (18.1−38.8) |
| ASA (range) | 2 (1−3) |
| Charlson Comorbidity Index (range) | 6.9 (2−12) |
ASA: American Society of Anesthesiologists Classification; BMI: body mass index.
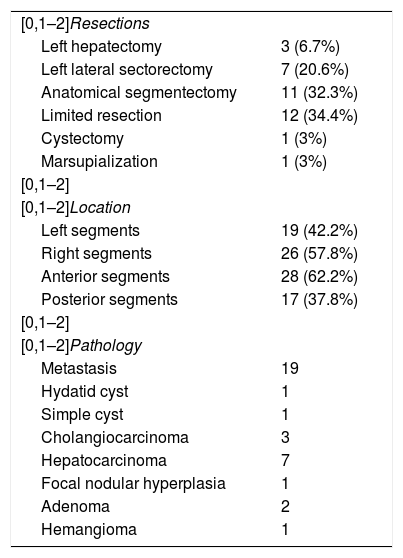
The procedures performed included: anatomical segmentectomies (32.3%), limited resections (34.4%), left lateral sectorectomies (20.6%), left hepatectomies (6.7%), one cystectomy (3%), and one marsupialization (3%). In 80% of cases, the lesions were solitary, and 37.8% were located in posterior segments; 80% of the interventions were due to oncological pathology (Table 2).
Resections, location and pathology of the hepatic lesions.
| [0,1–2]Resections | |
| Left hepatectomy | 3 (6.7%) |
| Left lateral sectorectomy | 7 (20.6%) |
| Anatomical segmentectomy | 11 (32.3%) |
| Limited resection | 12 (34.4%) |
| Cystectomy | 1 (3%) |
| Marsupialization | 1 (3%) |
| [0,1–2] | |
| [0,1–2]Location | |
| Left segments | 19 (42.2%) |
| Right segments | 26 (57.8%) |
| Anterior segments | 28 (62.2%) |
| Posterior segments | 17 (37.8%) |
| [0,1–2] | |
| [0,1–2]Pathology | |
| Metastasis | 19 |
| Hydatid cyst | 1 |
| Simple cyst | 1 |
| Cholangiocarcinoma | 3 |
| Hepatocarcinoma | 7 |
| Focal nodular hyperplasia | 1 |
| Adenoma | 2 |
| Hemangioma | 1 |
The mean surgical time was 204.4 min (range 100−265 min). Mean blood loss was 167 mL (100−300 mL), with no cases of perioperative transfusion. Mean hilar clamping time was 41.5 min (0−76 min). There were no conversions. There was one case (2.8%) of a Clavien-Dindo postoperative complication >2 due to reoperation of a loop injury caused by deserosalization during previous laparoscopic adhesiolysis maneuvers. There were also five cases of Clavien-Dindo 2 complications (14.3%). There were no readmissions. No cases of 90-day mortality were recorded. The median hospital stay was four days (range 2–14 days). The pathological study of the oncological margin of patients with malignant pathology (29) reported free surgical margins in 26 patients (89.6%), with a separation of 5.3 mm on average (0–30), and a parenchymal margin less than 1 mm in three patients (10.4%). Mean lesion size was 41.8 mm (10−125 mm).
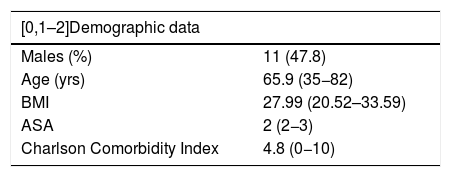
Pancreatic and duodenal resectionsTwenty-nine patients underwent surgery, predominantly ASA 2, with a low CCI and a mean age of 65.9 years (Table 3).
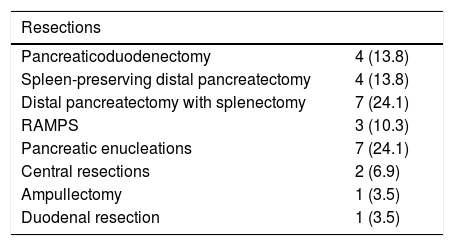
Major resections were performed in 18 patients (62.1%), including 14 distal pancreatectomies (48.3%) and four PD (13.8%). Eleven patients (37.9%) underwent parenchyma-sparing surgeries, including 7 enucleations (24.1%), two central resections (6.9%), one ampullectomy (3.5%), and one resection of the second part of the duodenum (3.5%). In total, 65.5% of the procedures were for oncological pathology (Table 4).
Resections and pathology of pancreatic lesions.
| Resections | |
|---|---|
| Pancreaticoduodenectomy | 4 (13.8) |
| Spleen-preserving distal pancreatectomy | 4 (13.8) |
| Distal pancreatectomy with splenectomy | 7 (24.1) |
| RAMPS | 3 (10.3) |
| Pancreatic enucleations | 7 (24.1) |
| Central resections | 2 (6.9) |
| Ampullectomy | 1 (3.5) |
| Duodenal resection | 1 (3.5) |
| Pathology | |
|---|---|
| Malignant (%) | 19 (65.5) |
| Ductal adenocarcinoma | 8 |
| Neuroendocrine tumor | 6 |
| Non-colorectal metastasis (2 renal, 1 ovarian) | 3 |
| Signet ring cell carcinoma | 1 |
| Cholangiocarcinoma | 1 |
| Benign (%) | 10 (34.5) |
| Cystic mass | 5 |
| Mucinous cyst | 3 |
| Pseudocyst | 1 |
| Ovarian ectopic stromal | 1 |
RAMPS: radical antegrade modular pancreatosplenectomy.
Mean operative time was 243 min (range 125−460 min), and mean blood loss was 202 mL (100−500 mL), with no cases of perioperative transfusion. The median Clavien-Dindo index for postoperative complications was 2 (10.3%), and 5 cases (17.2%) had Clavien-Dindo complications >2. There were three cases of intra-abdominal collection, one case of pancreatic fistula type ISGPS B that required percutaneous drainage, and one reoperation due to perforation of the stomach with the robotic clamp. Two other cases presented biochemical leaks, and the drains were able to be removed in the outpatient consultation with no other therapeutic measures or clinical repercussions. There were 4 associated readmissions (13.8%) and a single case of conversion due to an intraoperative complication (bleeding). Another 4 conversions were due to a greater extension of the disease than initially expected, 3 of which were due to lack of progression, and the remaining one due to a change in surgical indication (converting from ampullectomy to PD). The median hospital stay was seven days (range 3–23 days). There was no perioperative mortality (within 90 days).
The pathological study revealed a mean oncological margin of 8.4 mm (0−70 min) and a margin of less than 1 mm in three patients (15.8%); thus, R0 margins were achieved in 16 out of the 19 patients (84.2%). Mean tumor size was 23.9 mm (8−90 mm).
DiscussionLaparoscopic surgery has been shown to reduce morbidity in most procedures compared to open surgery12, and robotic surgery reports good results in surgeries with complex access, such as prostate or rectal surgery1.
The anatomical complexity of BPH surgery, which requires manipulation of deep structures close to major vessels, has led to cautious and heterogenous adoption of the minimally invasive approach, initially laparoscopic and now robotic. As a result, there are very few publications about robotic BPH surgery, and these are not apt for comparison5,9.
In order to set up a robotic HBP surgery program, it is essential to have experience in open and laparoscopic hepatic and pancreatic surgery, while also having a thorough understanding of how the robotic platform (DaVinci®) functions.
Furthermore, three phases are required to learn how to use the platform. First, get to know the robot and incorporate the surgical habits of the system itself. To do this, start with online courses and specific in-person instruction for the console and surgical field. This is followed by a training period supervised by an accredited tutor. It is also helpful to have a virtual DaVinci system training platform, which allows surgeons to practice and makes it easier to control the system.
The second step is to start on the learning curve with non-complex surgery. In our case, 25 cholecystectomies and 3 splenectomies were included, with results that were similar to the laparoscopic approach. There were no cases of conversion or reintervention.
Lastly, hepatic and pancreatic resections are started in selected cases. In our series, for liver resections, we selected cases with solitary lesions or those that required a single resection located in both lobes, excluding major hepatectomies, with the exception of the left hepatectomy. In pancreatic surgery, we selected patients with limited parenchyma-sparing resections, distal pancreas, and then we finally include PD. All patients were candidates for laparoscopic surgery. The presence of previous surgery or liver cirrhosis was not a contraindication, although morbidly obese patients were ruled out.
By analyzing our series, it is evident that robotic surgery has certain advantages over laparoscopic and open surgery. Nonetheless, the technology is continuously evolving, and it still has some drawbacks.
In liver surgery, robotic procedures share the same benefits of the minimally invasive approach provided by laparoscopic surgery13. However, it offers some advantages. For instance, 37.2% of the resections we performed were located in posterior segments, considered hidden and difficult to access. This type of laparoscopic resection can be complex due to the limited movement caused by the rigidity and structure of the instruments, which may even require the use of intercostal trocars in certain instances14. Robotic approaches, with instruments that offer a wide range of movements (thanks to their articulated arms, which also eliminate the associated physical effort), greatly facilitates these surgeries. Another factor of interest is the greatly improved vision offered by the robotic platform, based on high-definition 3D optics; this results in an immersive surgical experience that facilitates the identification of tissues and structures. Together with the elimination of physiological tremor and the translation of the surgeon’s hand movement, it allows for high-precision movements in confined spaces, reducing the risk of injuring structures adjacent to the intervention area. For example, in three patients requiring surgery for metastasis, major resection had been planned preoperatively due to the proximity of the tumors with vascular structures. However, thanks to the advantages described, it was possible to free them from the affected vessel, being able to offer limited resections with an R1 vascular margin, whose oncological results have been shown to be similar to those obtained with an R0 margin15. Therefore, it is likely that robotic surgery may favor parenchyma-sparing resections compared to open or laparoscopic surgery. These improvements may also enable us to perform more anatomical surgeries, based on the extrahepatic approximation of the Glissonian pedicles, through the space between them and the Laennec capsule. In addition to extensive knowledge of liver anatomy, this surgical technique requires delicate tissue manipulation, a quality that the robot can provide.
However, there are also some drawbacks to be aware of. On one hand, the existing instrumentation for robotic surgery is still limited, so there is no specific material for use in hepatic surgery. The lack of an ultrasonic dissector (CUSA) makes it necessary to perform liver transection with mono and bipolar coagulation. Although it is very effective thanks to the detailed vision and robotic movements, the process is laborious and time-consuming at the beginning of the learning curve. Afterwards, our mean operative time was 204 min, with very low blood loss (167 mL), which are results similar to or lower than reports in open surgery and even in laparoscopic surgery16,17. Another factor that must be taken into account when starting this type of surgery is that the lack of sensitivity when manipulating the tissues, especially liver tissue, can lead to potentially serious injuries. Therefore, extreme precautions must be taken, especially during mobilization of the liver. Last of all, it is important to emphasize that it is impossible to modifying the position of the patient during surgery, unless the table is synchronized with the robotic platform, which is expensive. This inconvenience, which is shared by both liver and pancreatic surgery, makes resection of bilateral lesions difficult, especially if lesions in left segments coexist with lesions in posterior right segments. In the event of performing surgeries that require position changes, the robot must be uncoupled and then re-docked, which takes time. For this reason, we preferably selected patients with single lesions (82.3%).
With regards to pancreatic surgery, there are also obvious advantages over laparoscopic surgery. The robotic approach allowed us to perform a large number of parenchyma-sparing surgeries, such as central resections, which are rare due to the technical difficulty involved. Overall, they accounted for 37.9% of the procedures performed, which far exceeds the usual range of these surgeries in existing series of 13%–30%18. These resections present a high risk of injury to the gland or the Wirsung duct, possible appearance of a fistula in the postoperative period, or injury to the splenic vessels that could result in the need for associated splenectomy. The described vision and accuracy of movements of the robotic platform facilitated these procedures, with no increase in intra- or postoperative complications. Another more evident benefit is found in the reconstructive phase of PD19. This includes three anastomoses and is physically and technically very demanding to perform laparoscopically because it is necessary to perform delicate sutures in fields that are difficult to access. The versatility of the robotic instruments and the ergonomics the platform offers facilitate this procedure, thereby reducing surgical time and shortening the learning curve20,21. Despite the time spent on docking, our surgical times for PD (mean 339 min) do not differ from the times described in laparoscopic or open surgery22,23. However, the implementation of robotic surgery in these procedures is associated with the difficult resolution of any possible iatrogenic injuries to large vessels. In laparoscopic surgery, it is established that the problem should be solved laparoscopically and convert to open surgery only when resolution is not possible. This sequence is much more complex in robotic surgery because it is more laborious. Therefore, it is necessary to be skilled in undocking and removing the robot quickly.
With all this, we achieved the good results described. There were no significant differences compared to our laparoscopic surgical times, despite docking, which has been described as a determining factor24. There was also no greater blood loss or need for transfusions. On the other hand, technical, clinical and oncological factors, such as surgical time, R0 percentage, blood loss or complication rates were similar to those described in the literature for robotic surgery5,25–27.
Furthermore, robotic surgery offers functionalities that facilitate surgical procedures. In our case, we have used integrated intraoperative ultrasound or indocyanine green to locate lesions or areas of ischemia, and 3D virtual models to have a detailed reference of the patient's anatomy. In the future, it is likely that new technologies will be added, including augmented reality, intraoperative navigation, or aids based on artificial intelligence28.
For all these reasons, and despite the complexity of performing minimally invasive HBP surgery, we believe that robotic surgery will play an important role. This is especially true in parenchyma-sparing surgery, as it enables us to perform very limited resections in difficult locations with reduced fields, while it can also be of great help in reconstruction phases by facilitating sutures.
Under appropriate conditions, robotic HBP surgery is safe and feasible. It provides short-term clinical results that are comparable to the laparoscopic approach and results that are better than open surgery in terms of oncological standards, frequency and severity of complications, and 90-day mortality6,8,16,17.
However, its general application cannot be recommended at this time due to the lack of quality scientific evidence to support it, as current data are restricted to specialized high-volume medical centers. Furthermore, additional studies are required to explore the cost-effectiveness29 and oncological efficacy of the platform.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Cugat Andorrà E, Cremades Perez M, Navinés López J, Matallana Azorín C, Zárate Pinedo A, Pardo Aranda F, et al. Desafío y futuro de la cirugía robótica hepática y pancreática. Análisis de 64 casos en una unidad especializada. Cir Esp. 2022;100:154–160.