Cutaneous congenital candidiasis (CCC) is a rare condition consisting of invasive fungal infection of the epidermis and dermis that mostly affects preterm infants. Maternal vaginal candidiasis is present in half of the cases, although the occurrence of invasive candidiasis during pregnancy or peripartum period is exceptional.
Case reportWe present the case of a full-term infant that was born by vacuum-assisted vaginal delivery to an apparently healthy 33 year-old woman with no history of intravenous drug use or vaginal candidiasis during pregnancy. The newborn showed a diffuse maculopapular rash with respiratory distress and bilateral interstitial lung infiltrates, requiring nasal continuous positive airway pressure support. Blood cultures obtained from the mother due to intrapartum fever yielded Candida albicans. Cultures of vaginal discharge and neonate skin also yielded C. albicans with the same in vitro susceptibly pattern. No alternative source for candidemia was identified. The clinical course after starting a systemic antifungal therapy was favorable in both the mother and the neonate, with clearance of candidemia and resolution of the skin lesions.
ConclusionsCCC must be considered in full-term newborns with maculopapular rash at birth or during the first days of life. The absence of alternative sources for bloodstream infection in the present case suggests a potential etiopathogenic relationship between CCC and maternal candidemia. It is reasonable to rule out postpartum candidemia when CCC is suspected.
La candidiasis congénita cutánea (CCC) es una entidad infrecuente que consiste en una infección invasiva de la epidermis y dermis, fundamentalmente en neonatos pretérmino. La candidiasis vaginal materna puede estar presente en la mitad de los casos, si bien el desarrollo de candidiasis invasiva durante el embarazo o el periodo post-parto es excepcional.
Caso clínicoPresentamos el caso de un recién nacido a término mediante parto vaginal asistido con ventosa de una mujer de 33 años aparentemente sana y en la que no se recogían antecedentes de uso de drogas por vía parenteral o candidiasis vaginal durante el embarazo. El neonato presentaba un exantema maculopapular difuso asociado a dificultad respiratoria e infiltrados pulmonares intersticiales bilaterales, por lo que precisó de soporte ventilatorio con presión positiva nasal contínua. Los hemocultivos realizados a partir de muestras de sangre de la madre debido a la presencia de fiebre intraparto fueron positivos para Candida albicans. Los cultivos de secreción vaginal y de la piel del neonato también revelaron C. albicans con idéntico perfil de sensibilidad in vitro. No se identificó ninguna fuente alternativa de candidemia. La evolución clínica fue favorable tanto en la madre como en el recién nacido tras el inicio de un tratamiento antifúngico, con aclaramiento de la candidemia y resolución de las lesiones cutáneas.
ConclusionesEl diagnóstico de CCC debe ser considerado en el recién nacido a término con exantema maculopapular al nacimiento o en los primeros días de vida. La ausencia de un origen alternativo de infección en nuestro caso sugiere una asociación etiopatogénica potencial entre la CCC y la candidemia materna. En escenarios sugerentes de CCC resulta razonable descartar la presencia de candidemia materna en el periodo post-parto.
Cutaneous congenital candidiasis (CCC) is a rare condition consisting of invasive fungal infection of the epidermis and dermis, which usually presents as an extensive maculopapular rash with occasional placental involvement and funisitis.3 CCC has been recently estimated to occur in 0.1% of neonatal intensive care unit (NICU) admissions, with most cases affecting preterm infants younger than 37 weeks’ gestational age.8 Dissemination to the bloodstream, central nervous system or other end organs is not uncommon in the neonate.3,8 Although maternal vaginal candidiasis is present in half of the cases, the report of invasive Candida infection during pregnancy or peripartum period is exceptional. Herein, we present an uncommon case of CCC in a full-term newborn associated with maternal peripartum candidemia.
A full-term (38 gestational weeks) infant weighing 3300g was born to a 33 year-old woman with irregular prenatal follow-up care. She had previously undergone uneventful vaginal delivery three years ago and two induced abortions. There was no previous or current history of intravenous drug use. She had been diagnosed with vaginal trichomoniasis and cervicitis due to Neisseria gonorrhoeae at week 18 of pregnancy, and received treatment with metronidazole (500mg PO twice daily for 7 days) and ceftriaxone (250mg IM in single dose). Otherwise her pregnancy course had been uncomplicated, although the patient failed to attend the third-trimester visit. Of note, there had not been any clinical or microbiological evidence of vaginal candidiasis. The baby was born by vacuum-assisted vaginal delivery due to prolonged second labor stage. No macroscopic alterations were observed in the placenta or umbilical cord. The mother developed intrapartum fever (38.4°C) without hemodynamic instability, after which two sets of blood were obtained for culture purposes, and a single dose of ampicillin (2g IV) and gentamicin (240mg) were administered. She later required episiotomy and uterine curettage under antibiotic therapy with cefazolin (1000mg IV every 8h) and metronidazole (500mg IV every 8h) due to the presence of retained blood clots. Blood samples obtained during the delivery yielded in culture C. albicans. The isolate exhibited susceptibility to all the tested agents according to a standardized colorimetric microdilution method (Sensititre™ YeastOne™, Thermo Fisher Scientific, Waltham, MA) with the exception of flucytosine (minimal inhibitory concentration [MIC] of 4mg/ml). By the time these results became available, at 48h post-partum, the patient had been afebrile and clinically stable. A culture of vaginal discharge yielded also a C. albicans isolate with the same susceptibility pattern. The mother had not been placed a central venous catheter, urine culture was sterile, and no other alternative sources of candidemia were suspected. Antifungal therapy with anidulafungin (200mg loading dose followed by 100mg daily) was started. A transthoracic echocardiogram and a funduscopic examination ruled out endocarditis or ocular involvement. Follow-up blood cultures at 48h were sterile, and a 14-day course with fluconazole (400mg PO daily) was completed.
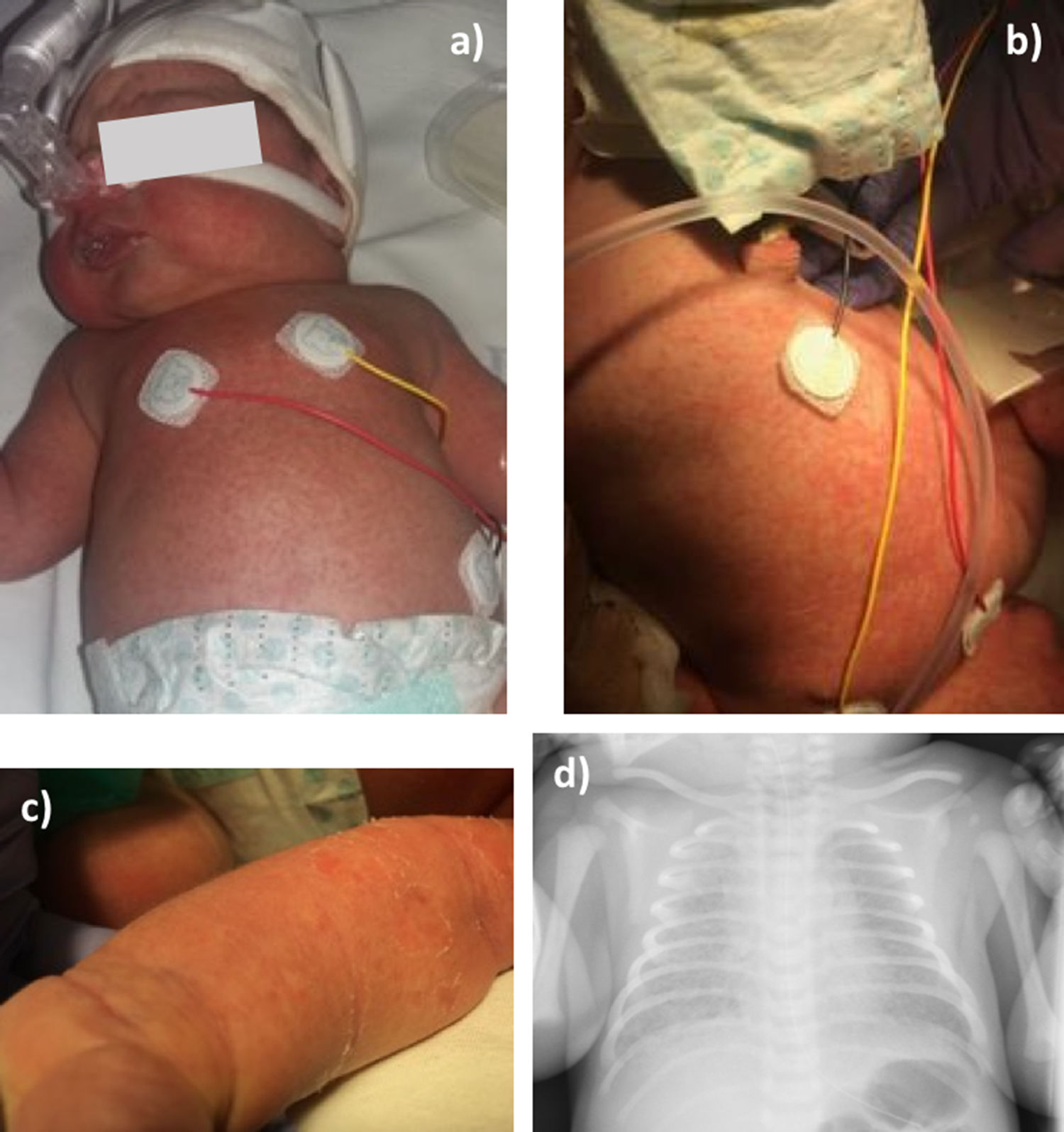
At birth the newborn showed a maculopapular rash involving the chest, neck, axillae, abdomen and upper and lower limbs, including palms and soles (Fig. 1a–c). The presence of respiratory distress led to NICU admission, where cefotaxime (50mg/kg IV every 8h) was empirically started in addition to a single dose of IV gentamicin (5mg/kg). A chest X-ray revealed bilateral interstitial lung infiltrates (Fig. 1d). Blood count was within the normal range, and C-reactive protein concentration was 1.66mg/dl (normal: 0.1–0.5mg/dl). Due to the clinical suspicion of CCC, liposomal amphotericin B (L-AmB) (5mg/kg IV daily) was started and nasal continuous positive airway pressure support was set with a maximum fraction of inspired oxygen of 0.5. Blood and cerebrospinal fluid cultures were negative for bacteria and fungi, whereas C. albicans was isolated in skin cultures from ears, axillae and chest, with no other microorganisms found. The neonatal strain showed an identical in vitro antifungal susceptibility pattern than that of the mother. After a 5-day course of L-AmB, the treatment was changed to fluconazole (6mg/kg PO daily). Respiratory support was discontinued at third day of life and the child remained afebrile and hemodynamically stable during the in-hospital period. Cranial and abdominal ultrasonography was normal, as well as the transthoracic echocardiogram and the funduscopic examination. Antifungal therapy was maintained for 14 days, with complete resolution of the skin lesions.
Maternal candidemia is a rare event during the intrapartum and postpartum period. An 8-year single-center study that included 3797 obstetric patients with peripartum fever revealed no cases of Candida bloodstream infection.12 A retrospective study encompassing 78,781 pregnant women, 1295 of whom were taken samples for blood cultures during the peripartum period, found one single case of candidemia (0.5% of all episodes of true bloodstream infections) in a patient with polymicrobial urosepsis.2 Cases of Candida glabrata bloodstream infection associated with chorioamnionitis have been reported in in vitro fertilization-assisted pregnancies.7 Other risk factors for candidemia or Candida chorioamnionitis during pregnancy and the postpartum period include prolonged antibiotic exposure, intrauterine devices and presence of cervical cerclage.10
Of note, no apparent sources for bloodstream infection were identified in the present case, posing the question whether a potential etiopathogenic relationship may be established between the occurrence of CCC and maternal candidemia. The precise pathogenesis of CCC remains to be fully elucidated. It has been shown that C. albicans is able to penetrate the amniotic sac without obvious membrane rupture. In accordance with previous reports,4,8 prolonged rupture of membranes was not observed in the present experience. The visual inspection of the placenta and umbilical cord revealed no alterations, although it has been reported that the presence of white microabscesses may be considered pathognomonic for the diagnosis of CCC.5,11 It has been also proposed an ascending mechanism via the birth canal, through microscopic fissures as a portal entry.1 No history of vaginal candidiasis during the pregnancy was retrieved, although this diagnosis may be lacking in CCC.8 Finally, it seems theoretically plausible that the fungus infects the neonate through hematogenous dissemination via maternal circulation,6 although such a mechanism seems to be highly unlikely in the present case. To our knowledge, this is the first reported case of CCC in which the presence of candidemia has been concurrently demonstrated in the mother. It could be hypothesized that the bloodstream infection may have been secondary to C. albicans chorioamnionitis, a common finding in the setting of CCC.8 This alternative causal direction would be supported by the rapid clearance of candidemia following delivery. A clonal relatedness between the isolates obtained from the mother and the neonate was not investigated by molecular typing. Nevertheless, the concordance found in their antifungal susceptibility patterns, which exhibited a MIC value to flucytosine higher than the epidemiological cut-off value (a feature observed in less than 1% of C. albicans strains in Spain9) reinforces the possibility of a common pathogenic link.
In conclusion, CCC must be ruled out in full-term newborns with maculopapular rash at birth or during the first days of life, even when being born from a healthy mother with no apparent risk factors. In addition, the possibility of concurrent maternal candidemia should be considered. Although the pathogenesis of such a clinical scenario remains to be clarified, it seems reasonable to systematically obtain maternal blood cultures during the immediate postpartum if CCC is suspected.
Funding sourcesM.F.R. holds a research contract “Miguel Servet” (CP18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science, Innovation and Universities.
Conflict of interestThe authors declare that there are no conflicts of interest associated with the publication of this manuscript.