Candida auris is an emerging multidrug-resistant and highly virulent yeast that spreads easily among patients.
AimsTo describe the characteristics of candidemia caused by C. auris in the southeast of Spain (Autonomous Community of Valencia – ACV) through a 5-year population-based study.
MethodsAn analysis of all the episodes of candidemia diagnosed in the ACV, with approximately 4,500,000 inhabitants, during 2013–2017, was done. Data were obtained from the Epidemiological Surveillance Valencian Network, a network that collects all the microbiological data from the hospitals in the study region.
ResultsBased on the records, 1.9% of the isolates recovered from the positive blood cultures (corresponding to 1789 patients) were yeasts. This implies an annual rate of 7.09 cases/100,000 inhabitants. Of the 23 yeast species isolated, Candida albicans was the most frequent (37.3%), showing a higher frequency than Candida parapsilosis (28.4%) and Candida glabrata (15.6%) (p<0.0001). It is remarkable the emergence of C. auris during 2016 and 2017, as this species became the fourth more prevalent in 2016 (9.2%), and the third in 2017 (15.7%). Fungemia was more common in hospitals with >500 beds (63.3% versus 36.7% in small hospitals) (p<0.0001), and C. auris was mostly isolated in large hospitals (8.5% versus 0.3%); its incidence was higher in autumn and among the age group of 65–84 years.
ConclusionsThe information about the local epidemiology of candidemia is essential in order to decide the best empirical treatment approach. This study reports the novel presence of C. auris in large hospitals. This pathogen has usually resistance to several antifungals and causes severe fungemia, so the results of this work reveal the need to monitor the presence of this species systematically.
Candida auris es una levadura multirresistente y virulenta que puede propagarse fácilmente entre los pacientes.
ObjetivosDescribir las características de las candidemias causadas por C. auris en el sureste de España (Comunidad Valenciana) a través de un estudio poblacional de 5 años.
MétodosSe realizó el análisis de todos los episodios de candidemia diagnosticados en la Comunidad Valenciana, con aproximadamente 4.500.000 de habitantes, durante los años 2013-2017. Los datos se obtuvieron de la Red de Vigilancia Microbiológica de la Comunidad Valenciana, que recoge los datos de todos los hospitales de la región.
ResultadosSegún los datos estudiados, un 1,9% de los aislamientos recuperados en hemocultivos (correspondientes a 1.789 pacientes) eran levaduras. Esto supone una tasa anual de 7,09 casos/100.000 habitantes. Se aislaron 23 especies de levaduras, Candida albicans fue la más frecuente (37,3%), con una frecuencia significativamente mayor que Candida parapsilosis (28,4%) y Candida glabrata (15,6%) (p<0,0001). Es importante remarcar la aparición de C. auris, que se convirtió en la cuarta más prevalente en 2016 (9,2%), y la tercera en 2017 (15,7%). Las fungemias fueron más comunes en los hospitales grandes (63,3%>500 camas versus 36,7%<500 camas) (p<0,0001). C. auris fue aislada en hospitales grandes (8,5 versus 0,3%), y su incidencia fue más alta en otoño y en el grupo de edad de 65-84 años.
ConclusionesDisponer de información sobre la epidemiología de las candidemias es esencial para establecer el mejor tratamiento de forma empírica. Este estudio pone de manifiesto la presencia de C. auris en grandes hospitales. Este patógeno causa fungemias graves y suele presentar multirresistencia a los antifúngicos. Los resultados de este trabajo revelan la necesidad de evaluar su presencia en otras comunidades.
Invasive fungal infection is a serious illness that continues to change over time, and the emergence of Candida auris aggravates this health problem. Fungemia carries a high mortality rate, especially among vulnerable patients admitted to the intensive care units, but also among those using prostheses, catheters or other intravascular devices, and even those receiving immunosuppressant treatments, chemotherapy or transplant recipients.14 Candidemia represents a threat to public health systems considering the large numbers of cases and the enormous expenditure. It has been estimated that, in the USA, hospitalizations due to Candida infections cost $1.4 billion.1
In this study, we describe the epidemiology of candidemia in the southeast of Spain, by the Mediterranean coast, through a 5-year population-based study. Besides, this work analyses the impact of the emergence of C. auris in several hospitals in the study region.
Patients and methodsDesignThe work is a cross-sectional study to estimate the prevalence of candidemia. The results of the positive blood cultures reported from all public hospitals in the Autonomous Community of Valencia (ACV) (population: 4,397,476) between January 2013 and December 2017 were analysed.
Source of informationThe data were obtained from “RedMIVA”, a network connecting information from the microbiology laboratories of public hospitals in the ACV. One isolate per patient was studied. To estimate the annual incidence rate per 100,000 inhabitants, the information about the overall population was obtained from the official statistic census published by Valencian Autonomous Community Government (Generalitat valenciana – www.gva.es)
Statistical analysesThe categorical variables were expressed as counts (percentage) together with 95% confidence intervals (95% CI), and continuous variables as the mean and standard deviation (SD) or median and InterQuartile Range (IQR), as appropriate. The statistical differences among the groups were assessed using Chi-square or Fisher's exact test for categorical variables. For the continuous variables, the t-Student's test was applied. The alpha error was set at 0.05, and p values were two-tailed. All statistical analyses were conducted using the SPSS Statistics (IBM, version 22.0).
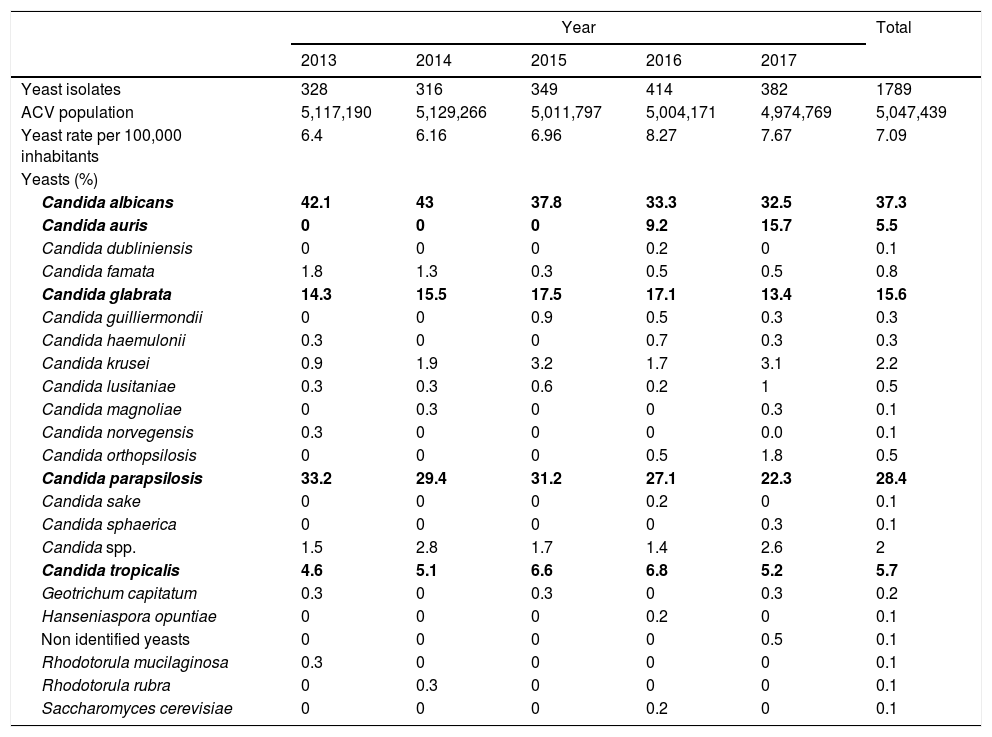
ResultsDuring 2013–2017, yeasts were isolated from 1789 patients, accounting for 1.9% (95% CI 1.85–2.0) of the total positive blood cultures in the ACV. Twenty-three different species were identified; the five species more frequently isolated were Candida albicans (37.3%; 95% CI 35.1–39.6), Candida parapsilosis (28.4%; 95% CI 26.3–30.5), Candida glabrata (15.6%; 95% CI 13.9–17.3), Candida tropicalis (5.7%; 95% CI 4.6–6.8) and C. auris (5.5%; 95% CI 4.4–6.6) (p<0.0001). (Table 1). C. auris was the fifth species more frequently isolated, although it was found only in the last two years. In 2016 its incidence was 9.2% (95% CI 6.3–12.1), and in 2017 it raised to 15.7% (95% CI 11.9–19.5).
Yeast species per year (the most isolated are in bold type).
| Year | Total | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | ||
| Yeast isolates | 328 | 316 | 349 | 414 | 382 | 1789 |
| ACV population | 5,117,190 | 5,129,266 | 5,011,797 | 5,004,171 | 4,974,769 | 5,047,439 |
| Yeast rate per 100,000 inhabitants | 6.4 | 6.16 | 6.96 | 8.27 | 7.67 | 7.09 |
| Yeasts (%) | ||||||
| Candida albicans | 42.1 | 43 | 37.8 | 33.3 | 32.5 | 37.3 |
| Candida auris | 0 | 0 | 0 | 9.2 | 15.7 | 5.5 |
| Candida dubliniensis | 0 | 0 | 0 | 0.2 | 0 | 0.1 |
| Candida famata | 1.8 | 1.3 | 0.3 | 0.5 | 0.5 | 0.8 |
| Candida glabrata | 14.3 | 15.5 | 17.5 | 17.1 | 13.4 | 15.6 |
| Candida guilliermondii | 0 | 0 | 0.9 | 0.5 | 0.3 | 0.3 |
| Candida haemulonii | 0.3 | 0 | 0 | 0.7 | 0.3 | 0.3 |
| Candida krusei | 0.9 | 1.9 | 3.2 | 1.7 | 3.1 | 2.2 |
| Candida lusitaniae | 0.3 | 0.3 | 0.6 | 0.2 | 1 | 0.5 |
| Candida magnoliae | 0 | 0.3 | 0 | 0 | 0.3 | 0.1 |
| Candida norvegensis | 0.3 | 0 | 0 | 0 | 0.0 | 0.1 |
| Candida orthopsilosis | 0 | 0 | 0 | 0.5 | 1.8 | 0.5 |
| Candida parapsilosis | 33.2 | 29.4 | 31.2 | 27.1 | 22.3 | 28.4 |
| Candida sake | 0 | 0 | 0 | 0.2 | 0 | 0.1 |
| Candida sphaerica | 0 | 0 | 0 | 0 | 0.3 | 0.1 |
| Candida spp. | 1.5 | 2.8 | 1.7 | 1.4 | 2.6 | 2 |
| Candida tropicalis | 4.6 | 5.1 | 6.6 | 6.8 | 5.2 | 5.7 |
| Geotrichum capitatum | 0.3 | 0 | 0.3 | 0 | 0.3 | 0.2 |
| Hanseniaspora opuntiae | 0 | 0 | 0 | 0.2 | 0 | 0.1 |
| Non identified yeasts | 0 | 0 | 0 | 0 | 0.5 | 0.1 |
| Rhodotorula mucilaginosa | 0.3 | 0 | 0 | 0 | 0 | 0.1 |
| Rhodotorula rubra | 0 | 0.3 | 0 | 0 | 0 | 0.1 |
| Saccharomyces cerevisiae | 0 | 0 | 0 | 0.2 | 0 | 0.1 |
Bold values indicate the five species more frecuently isolated, including C. auris in the last years.
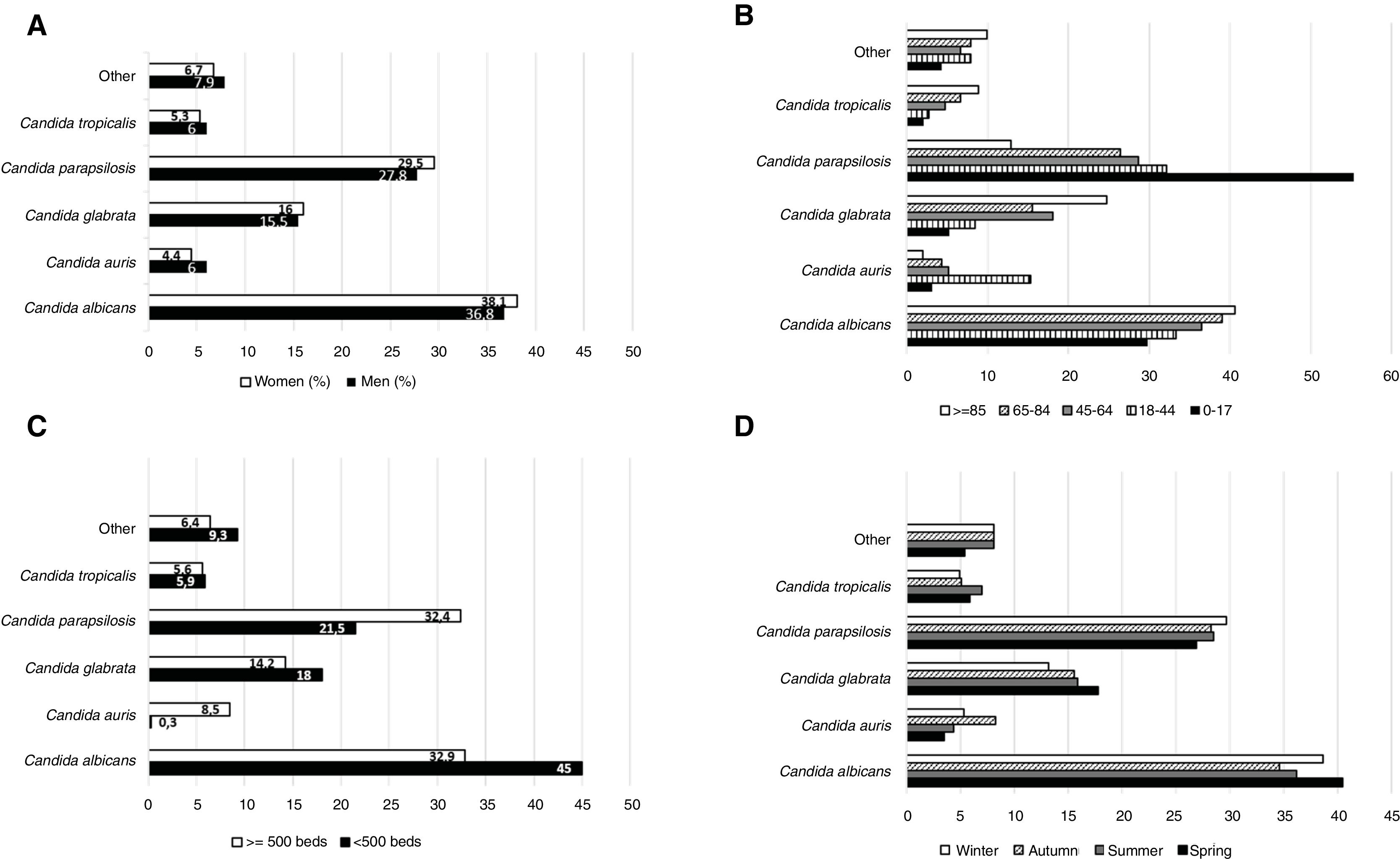
The percentage of C. albicans gradually decreased along the years: 42.1% of the total yeast isolates in 2013 (95% CI 36.6–47.6), 43% in 2014 (95% CI 37.4–48.7), 37.8% in 2015 (95% CI 32.6–43.1), 33.3% in 2016 (95% CI 28.7–38.) and 32.5% in 2017 (95% CI 27.6–37.3). The 63.1% (n=1126) (95% CI 60.9–65.4) of the total yeast isolates were recovered from men and 36.9% (n=658) (95% CI 34.6–39.2) from women (p<0.0001). The differences in the prevalence of the yeast species according to gender were minimal and not statistically significant (Fig. 1A).
Considering the age, the analysis revealed that many cases of candidemia occurred in patients aged 65–84 years (50.1% of the total yeast isolates; 95% CI 47.8–52.5), followed by those aged 45–64 years (29.1%; 95% CI 26.9–31.2), 18–44 years (9.9%; 95% CI 8.5–11.3), >85 years (5.6%; 95% CI 4.5–6.7), and <18 years (5.3%; 95% CI 4.2–6.3) (p<0.0001). C. parapsilosis predominated in patients with less than 18 years (55.3%; 95% CI 44.7–65.9), while C. albicans did so in elderly patients (40.6%; 95% CI 30.5–50.7). C. auris was most frequent in patients of 18–44 years (15.3%; 95% CI 9.7–20.8) (Fig. 1B).
When considering the hospital size, the results revealed that 63.3% (95% CI 61.1–65.6) of the fungal species were isolated from hospitals with at least 500 beds, while 36.7% (95% CI 34.4–38.9) were isolated from smaller hospitals (p<0.0001). In large hospitals, the prevalence of C. albicans and C. parapsilosis was similar (32.9% [95% CI 30.1–35.7] versus 32.4% [95% CI 29.6–35.2], respectively), while in small hospitals the frequency of C. albicans was twice that of C. parapsilosis (45%; 95% CI 41.1–48.9 versus 21.5%; 95% CI 18.3–24.7, respectively). Additionally, C. auris was primarily isolated in large hospitals, being the fourth-most prevalent species (8.5% of all; 95% CI 6.8–10.1), while it was scarcely found in small hospitals (0.3%; 95% CI 0.0–1.1) (Fig. 1C). At the end, the study showed that the prevalence of fungemia was not related to the season (Fig. 1D).
DiscussionA European study reported a candidemia incidence of approximately 79 cases/day; it was estimated that 29 patients had fatal outcomes on day 30.7 In our study area, the annual prevalence of fungemia was 7.09 cases/100,000 inhabitants, which is higher than the European average (3.88 cases/100,000). These values highlight the importance of a better knowledge of this pathology in order to improve the empirical treatment.
C. albicans is the yeast most frequently isolated in candidemia,6 and there are discrepancies in the prevalence of the other species. Some studies find that C. glabrata is the most common non-C. albicans Candida species among the high-risk units and across all geographic regions, except Latin America, where C. parapsilosis and C. tropicalis are more common.13,16 Other studies reveal that after C. albicans, C. parapsilosis and C. glabrata are the species most frequently isolated, which agrees with our data.2,4,17
A decrease in the incidence of C. albicans but the increase of other yeast species incidence has been reported from other geographical areas, a fact probably due to a major use of azole drugs and a trend towards an increased antifungal resistance. Finally, the emergence of multi-resistant species, such as C. auris or C. glabrata, is a big threat that needs global surveillance of candidemia cases.9,15 Yeast species other than C. albicans cause a long term candidemia (median 3 days vs. 1 day) after an effective antifungal regime, bringing to light a slower response to the antifungal treatment and a higher treatment failure rate than that for C. albicans candidemia.10
C. parapsilosis is the most prevalent species (55.3%) found among children, whereas C. albicans is the most frequent isolated in other patients. Moreover, the prevalence of C. glabrata among the elderly (age>85 years) is high, although that of C. albicans is the highest (40.6%). The difference in the prevalence based on the age-group has also been reported for other geographical areas.17 Our data differ from those of other geographical areas in which C. albicans was the most common pathogen causing invasive candidiasis among neonates and children (47.8% vs. 44.1% cases).5
C. auris has been reported as the fourth-most prevalent species in large hospitals of the study region, and its presence is associated with hospitals with more than 500 beds. This data radically modified the approach of this pathology as the pathogen is resistant to several antifungal agents, is highly virulent and spreads easily among patients.18 A similar phenomenon has been reported for the rest of Europe during 2013–2017, with 620 cases of C. auris reported in the European Union/European Economic Area (110 cases of bloodstream infections), which is generally associated with large outbreaks.3,8
The severity of this pathology, its heterogeneity and the appearance of new species of yeasts is often associated with resistance to multiple antifungal agents, which makes a fast identification of the microorganism involved in each process critical. Therefore, the development of a mixed expert panel comprising specialists in clinical microbiology and infectious diseases is mandatory for the appropriate management of such serious pathology, based on the information sourced about the local epidemiology of these pathogens.11,12
FundingThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestsAuthors declare no conflict of interest.