Most research in El Salvador focuses on chronic kidney disease (CKD) in rural coastal populations. Our aim was to determine the prevalence of CKD, diabetes, hypertension and hyperuricemia and associations to CKD in an urban adult population.
MethodsPopulation-based, cross-sectional. A representative sample of adults from an urban community in San Salvador was randomly selected (80.6% participation, N=121, 65% female, mean age 52yo). A questionnaire with socio-demographic information was applied; blood and urine samples were collected. Subjects with low estimated glomerular filtration rate (eGFR, MDRD equation) or spot proteinuria were reexamined 3 months later to confirm CKD. Gender, age, educational level, income, tobacco smoking, alcohol consumption, analgesics use, hypertension, diabetes, and hyperuricemia were evaluated as predictors for CKD, diagnosed and staged by KDIGO guidelines.
ResultsPrevalence of CKD: 12.6% (N=15, CI 95%, 7.23–19.94), 14.2% in males and 11.4% in females, all in stages G2-4. Prevalence of eGFR<60mL/min/1.73m2: 9%. Most, 73%, were previously undiagnosed. Prevalence of diabetes: 11.6%; hypertension: 34.7%; hyperuricemia: 24.8%. CKD was present in 42.9%, 21% and 23.3% of diabetic, hypertensive and hyperuricemic patients, respectively. From all predictors, only diabetes (OR 8.1, p=0.0002), hypertension (OR 3.17, p=0.03) and hyperuricemia (OR 3.1, p=0.02) showed increased risk for CKD.
Discussion and conclusionsGeneral prevalence of CKD is not increased in this population, but prevalence in stages G3-4 is slightly increased. Most cases were previously undiagnosed. Diabetes, hypertension, and hyperuricemia increase the risk for CKD. Preventive measures and early screening is recommended, especially for those with risk factors.
Las investigaciones en El Salvador se enfocan mayormente en enfermedad renal crónica (ERC) en poblaciones rurales costeras. Nos propusimos determinar la prevalencia de ERC, diabetes, hipertensión e hiperuricemia, y sus asociaciones, en una población urbana adulta.
Materiales y métodosRealizamos un estudio poblacional transversal en una muestra representativa de adultos seleccionada aleatoriamente (80,6% participación, n=121, 65% femenino, edad promedio 52años) en una comunidad urbana en San Salvador. Recogimos información sociodemográfica mediante cuestionario; tomamos muestras de sangre y orina. Aquellos con tasa de filtración glomerular (TFG) baja o proteinuria por tira reactiva fueron reevaluados 3meses después para confirmar ERC. Evaluamos sexo, edad, nivel educativo, ingreso, tabaquismo, alcoholismo, analgésicos, hipertensión, diabetes e hiperuricemia como predictores para ERC, definida y clasificada según guías KDIGO.
ResultadosPrevalencia de ERC: 12,6% (n=15, IC95%: 7,23-19,94), 14,2% en varones, 11,4% en mujeres, todos en estadios G2-4, 73% sin diagnóstico previo. Prevalencia de TFG <60ml/min/1,73m2: 9%. Prevalencia de diabetes: 11,6%; hipertensión: 34,7%; hiperuricemia: 24,8%. Encontramos ERC en el 42,9, el 21 y el 23,3% de los pacientes diabéticos, hipertensos e hiperuricémicos, respectivamente. De los predictores estudiados, solamente diabetes (OR:8,1, p=0,0002), hipertensión (OR:3,17, p=0.03) e hiperuricemia (OR:3,1, p=0,02) incrementaron el riesgo de ERC.
Discusión y conclusionesLa prevalencia general de ERC no está aumentada en esta población urbana, pero la prevalencia en estadios G3-4 sí, discretamente. La mayoría no tenían diagnóstico previo. Diabetes, hipertensión e hiperuricemia incrementan el riesgo de ERC. Sugerimos medidas preventivas y detección precoz, especialmente en quienes presentan factores de riesgo.
Chronic kidney disease (CKD) has emerged as a global public health burden for several reasons, including an increasing number of patients (8–16% of global population), progression to end-stage renal disease (ESRD), high costs to public health systems, and its morbidity and mortality, particularly associated to cardiovascular disease [1–3]. During the last two decades there have been disconcerting reports of an excessive prevalence of CKD in the Pacific coast of Central America, mainly from El Salvador, Nicaragua, Guatemala, and Costa Rica [4]. In El Salvador, CKD is the second cause of hospital mortality and the first cause for males only. Also, a 50% increase in hospitalization rate for CKD was noted from 2005 to 2012 [5]. Despite being a mayor public health issue, there is no surveillance system capable of detecting CKD at any stage [6]. In addition to the well-known causes of CKD – diabetes and hypertension – a new form of CKD named Mesoamerican nephropathy (MeN) or chronic kidney disease of undetermined cause (CKDu) is responsible for many cases in the country, mainly young male workers from the Pacific coastland [7]. Noticeably, several studies have reported an excess in prevalence of hyperuricemia in these populations and in patients with MeN [8,9]. Since all previous studies on CKD have focused on the epidemic coastal region, the urban situation has not been described; only one study included an urban population as a control group [10]. On the other hand, the urban prevalence of other non-communicable diseases (NCDs) associated to CKD has not been previously reported either.
Our aim was to determine the prevalence of CKD in adults from an urban population in El Salvador, the prevalence of other NCDs such as diabetes, hypertension, and hyperuricemia, and to describe associations with CKD.
MethodsStudy designA population-based, cross-sectional study was conducted during the last quarter of 2015. Approval from the National Ethics Committee for Health Research of El Salvador was obtained. All participants signed an informed consent.
SettingAccording to the Salvadoran Institute of Municipal Development, the Municipality of Antiguo Cuscatlán, comprised in the metropolitan area of San Salvador city, has an extension of 19.41 square kilometers, 33,698 inhabitants and a human development index of 0.87. A mid-income community from Antiguo Cuscatlán was selected convenience-wise, since it was the workplace for most of the researchers. A house-to-house census of all adults was completed with assistance from the board of community directors prior to the study, covering 100% of households, reporting 741 inhabitants>18 years old (yo), 57% female (N=422), 43% male (N=319).
ParticipantsThe study was limited to adult residents and participation was voluntary. We planned to exclude people with fever, dehydration signs or symptoms, eating disorders, active alcohol consumption, extreme body mass index (BMI; <18.5 or >40kg/m2 of body surface) or high performance athletes, but no participant met these criteria.
VariablesAge and gender; past and present history of intake of nonsteroidal anti-inflammatory drugs (NSAIDs); educational level categorized as primary, high school, technical or university; lifetime tobacco smoking and alcohol consumption; and history of previously diagnosed and treated CKD, diabetes, and hypertension, were recorded with a verbal questionnaire administered to the participants during the study baseline.
CKD was defined and staged by KDIGO guidelines [11], measuring proteinuria and estimating the glomerular filtration rate (eGFR) with the MDRD equation [12]. Proteinuria and hematuria were determined using urine test strips and microscopy urine analysis. Proteinuria was categorized as absent/mild (none or +−), moderate (+) or severe (≥ ++).
Hypertension was defined by self-report and being medically treated or blood pressure ≥140/90mmHg at examination point. Diabetes was defined by self-report and being medically treated or by serum glucose>126mg/dL. Hyperuricemia was defined as serum acid uric>7mg/dL for males and >6 for females. Overweight and obesity were classified according to the World Health Organization (WHO) definition as BMI≥25kg/m2 and 30kg/m2, respectively.
Data sources and measurementsA two-phase study was conducted. At baseline, the selected participants were invited to attend the Municipal Clinic of the Ministry of Health after a 12-h fasting on selected weekend days. An oral questionnaire was applied, anthropometric measures were obtained and urine and blood samples were collected. Three months later, all participants showing abnormal serum creatinine, low eGFR, and/or moderate/severe proteinuria were invited again to confirm CKD.
The research team was trained to uniformly administer the baseline oral questionnaire, recording participant's age, gender, socioeconomic status, lifestyle questions, personal and family medical history data such as previous diagnosis of CKD, diabetes or hypertension. Each participant's height in cm and weight in kg was measured with minimal clothing using DETECTO model 439 weigh beam eye-level scales. Blood pressure was measured after 5min of rest with the patient sitting with an OMRON wrist blood pressure monitor model BP629 electronic device. Spot urine samples were collected in plastic sterile bottles; proteinuria and hematuria were determined in situ using semiquantitative MARANTAH 10 V dipsticks scored visually against a colored chart. Blood samples were collected in vacuum tubes from vein puncture in the left arm of the participants in sitting position by the clinic's trained laboratory professionals. Blood samples were immediately transported in cooler containers to the Ministry of Health's Central Laboratory facility for analyses. Serum creatinine was analyzed using the Jaffe method. The eGFR was calculated using the MDRD equation.
Study sizeThe sample size was calculated using Statcalc of the Centers for Disease and Control Prevention (CDC) statistical program, Epi info 7.1.5. Based on the population census of 741 adult inhabitants and estimating a frequency of CKD of 10% with a 5% confidence limit, design effects 1.0 and confidence level 95%, a sample size of 117 participants was calculated. A 22% nonresponse rate was expected; thus, 150 inhabitants were invited to participate.
Statistical methodsStatistical analyses were performed using CDC-Epi Info 7.1.5 software. Qualitative variables are reported as percentages and quantitative variables as means. Univariate analysis of age, sex, age range and family income variables was performed using contingency tables by calculating the proportions and single frequencies as well as a bivariate analysis of hypertension, diabetes mellitus, obesity and overweight, hyperuricemia, tobacco smoking, chronic NSAIDS use, family history of CKD. Odds ratio (OR) with a confidence interval (CI) of 95% was established and a p value<0.05 was considered as statistically significant.
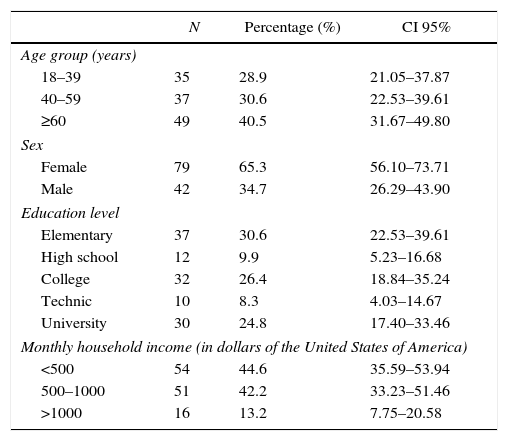
ResultsParticipants and descriptive dataOut of 150 people invited, 121 attended at baseline (80.6% response rate), 42 (34.71%) male and 79 (65.29%) female, average age 52 yo. Seventy-two (59.5%) participants were <60 yo, 40 (33.1%) had university degree, and 105 (86.8%) reportedTable 1). Forty (33%) participants had overweight and 42 (35%) had obesity.
Socio-demographic characteristics of a representative sample of adults from an urban population of El Salvador.
| N | Percentage (%) | CI 95% | |
|---|---|---|---|
| Age group (years) | |||
| 18–39 | 35 | 28.9 | 21.05–37.87 |
| 40–59 | 37 | 30.6 | 22.53–39.61 |
| ≥60 | 49 | 40.5 | 31.67–49.80 |
| Sex | |||
| Female | 79 | 65.3 | 56.10–73.71 |
| Male | 42 | 34.7 | 26.29–43.90 |
| Education level | |||
| Elementary | 37 | 30.6 | 22.53–39.61 |
| High school | 12 | 9.9 | 5.23–16.68 |
| College | 32 | 26.4 | 18.84–35.24 |
| Technic | 10 | 8.3 | 4.03–14.67 |
| University | 30 | 24.8 | 17.40–33.46 |
| Monthly household income (in dollars of the United States of America) | |||
| <500 | 54 | 44.6 | 35.59–53.94 |
| 500–1000 | 51 | 42.2 | 33.23–51.46 |
| >1000 | 16 | 13.2 | 7.75–20.58 |
Fifteen participants had abnormal serum creatinine or low eGFR at baseline, four with a previous diagnosis of CKD. The 11 remaining participants were invited again 3 months later to confirm CKD. The response and confirmation rates were 100%.
The prevalence of CKD was 12.6% (15/121, CI 95% 7.23–19.94), 73% of CKD cases (11/15, CI 95% 47.47–90.90) were diagnosed during the study. The prevalence of CKD by stages was: stage G2 3%, stage G3 7% and stage G4 2%. We did not find any participants in stages G1 or G5. The prevalence of eGFR<60mL/min/1.73m2 was 9% (11/121). The prevalence of moderate/severe proteinuria was 8.3% (10/121), half moderate and half severe.
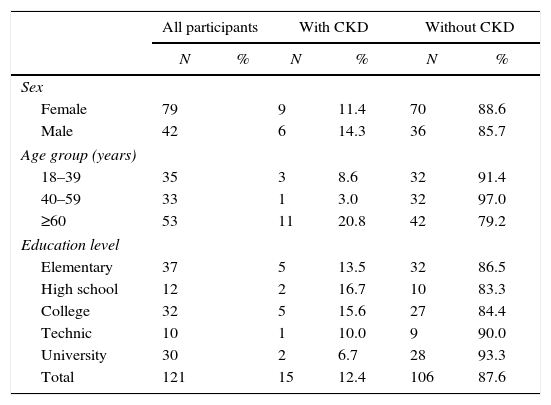
The prevalence of CKD in the male and female population was 14.3% (6/42, CI 95%, 6.00–27.36) and 11.4% (9/79, CI 95% 5.70–19.87), respectively. Participants>60 yo had the highest prevalence (21.2%, 11/52, CI 95% 11.66–33.79). Subjects with higher educational level had lower CKD prevalence (6.9%, 2/29, CI 95% 1.17–20.97) (Table 2). Five of the studied risk factors showed an OR>1 but only hypertension, diabetes and hyperuricemia showed statistical significance (Table 3).
Socio-demographic characteristics of a representative sample of adults from an urban population of El Salvador, categorized by presence or absence of chronic kidney disease (CKD).
| All participants | With CKD | Without CKD | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex | ||||||
| Female | 79 | 9 | 11.4 | 70 | 88.6 | |
| Male | 42 | 6 | 14.3 | 36 | 85.7 | |
| Age group (years) | ||||||
| 18–39 | 35 | 3 | 8.6 | 32 | 91.4 | |
| 40–59 | 33 | 1 | 3.0 | 32 | 97.0 | |
| ≥60 | 53 | 11 | 20.8 | 42 | 79.2 | |
| Education level | ||||||
| Elementary | 37 | 5 | 13.5 | 32 | 86.5 | |
| High school | 12 | 2 | 16.7 | 10 | 83.3 | |
| College | 32 | 5 | 15.6 | 27 | 84.4 | |
| Technic | 10 | 1 | 10.0 | 9 | 90.0 | |
| University | 30 | 2 | 6.7 | 28 | 93.3 | |
| Total | 121 | 15 | 12.4 | 106 | 87.6 | |
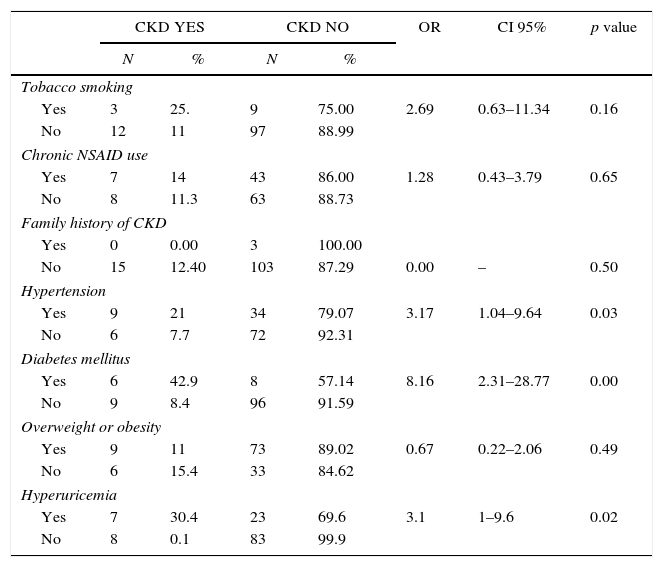
Evaluated predictors of chronic kidney disease (CKD) on a representative sample of adults from an urban population of El Salvador.
| CKD YES | CKD NO | OR | CI 95% | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Tobacco smoking | |||||||
| Yes | 3 | 25. | 9 | 75.00 | 2.69 | 0.63–11.34 | 0.16 |
| No | 12 | 11 | 97 | 88.99 | |||
| Chronic NSAID use | |||||||
| Yes | 7 | 14 | 43 | 86.00 | 1.28 | 0.43–3.79 | 0.65 |
| No | 8 | 11.3 | 63 | 88.73 | |||
| Family history of CKD | |||||||
| Yes | 0 | 0.00 | 3 | 100.00 | |||
| No | 15 | 12.40 | 103 | 87.29 | 0.00 | – | 0.50 |
| Hypertension | |||||||
| Yes | 9 | 21 | 34 | 79.07 | 3.17 | 1.04–9.64 | 0.03 |
| No | 6 | 7.7 | 72 | 92.31 | |||
| Diabetes mellitus | |||||||
| Yes | 6 | 42.9 | 8 | 57.14 | 8.16 | 2.31–28.77 | 0.00 |
| No | 9 | 8.4 | 96 | 91.59 | |||
| Overweight or obesity | |||||||
| Yes | 9 | 11 | 73 | 89.02 | 0.67 | 0.22–2.06 | 0.49 |
| No | 6 | 15.4 | 33 | 84.62 | |||
| Hyperuricemia | |||||||
| Yes | 7 | 30.4 | 23 | 69.6 | 3.1 | 1–9.6 | 0.02 |
| No | 8 | 0.1 | 83 | 99.9 | |||
The prevalence of diabetes was 11.6% (N=14) and 6 also had CKD (42.9%, OR: 8.1, CI 95% 2.31–28.77 p=0.0002). Nine (8.4%) of the 107 non-diabetic participants had CKD.
The prevalence of hypertension was 35.5% (N=43). Eight participants (18.6%) had high blood pressure at examination and 9 had CKD (20.6%, OR: 3.17, CI 95% 1.04–9.64 p=0.03). Six (7.7%) of the 78 non-hypertensive participants had CKD.
The prevalence of hyperuricemia was 24.8% (N=30). Seven out of 15 CKD patients had hyperuricemia (46.7%, OR 3.1, CI 95% 1–9.6). Eight (8.8%) of the 91 non-hyperuricemic participants had CKD.
DiscussionThe prevalence of CKD we observe in this adult urban population of El Salvador (12.6%) is not increased as it is in the coastal rural communities of the country. The observed prevalence is in line with the worldwide reports for CKD on any stage (8–16%) [1,2] and similar to data from Chile (12.1%) with slightly higher prevalence in females (14.5%) and elders (23.3%, >60 yo) [13]. The urban prevalence of CKD in this population from El Salvador showed a similar trend (21.1%>60 yo), although with a slightly higher prevalence in males (14.3% vs 11.4%). Likewise, the observed prevalence of eGFR<60mL/min/1.73m2 (9%) is similar to the urban prevalence reported in Mexico (80,788 per million population, 8%) [14]. In contrast, in community-based surveys in rural populations in the coastland of El Salvador, prevalence of CKD is 12.7% for stages G3-5 only, and 17.9% for any stage [15,16]. Similar data is reported in rural populations from other Central American countries such as Nicaragua, with prevalences up to 40% [17]. Such a remarkable difference may be associated to the absence of Mesoamerican nephropathy in this urban region located at 700m above sea level, fresher and away from the coast, and to the higher socioeconomic status, as shown in our results. Low socioeconomic status has been linked to a higher risk of CKD [18].
We found CKD stage G3 to be the most common, accounting for half of the diagnosed cases. This finding differs from the international prevalence in which stages G1-2 are the most common, adding up 11.1% of cases, while stage G3 accounts only for 5.4% [19]. Nevertheless, our study could have missed some early-stage cases because we relied on eGFR and urine analysis only as kidney damage markers, and eGFR was estimated with the MDRD equation. The age group with the highest CKD prevalence was the one >60 yo; this is also consistent with data reports from the United States, in which the age group>60 yo accounts for 39.4%, followed by the group 40–59 yo with 12.6% [20]. By gender, we observed a quite even distribution between males and females, with a slight predominance of males. This urban population distribution differs from what has been described in coastal rural populations affected by the Mesoamerican nephropathy, where the prevalence is strikingly higher in males between 20 and 49 yo [15,16].
A higher educational level has been associated with lower prevalence of NCDs such as hypertension, diabetes, and reduced GFR [21,22]. We observed a similar trend in this population, since 72% subjects with hypertension and 64% with diabetes reported a low educational level. The number of subjects with CKD was 4 times higher within those with low educational level as well.
Hypertension and diabetes are associated with higher prevalence of CKD and an increased risk of progression to ESRD. Hypertension is both a risk and a consequence of CKD and is the second leading cause of ESRD in the United States [23]. As expected, in this study we observed a relevant association between CKD and hypertension (OR: 3.17, CI 95% 1.04–9.64, p=0.03). Thus, an adequate control of blood pressure to avoid the development or progression of CKD is extremely important.
Diabetes is considered the main risk factor for CKD. The worldwide increase in CKD prevalence is directly related to the number of CKD cases attributed to diabetes, and keeps rising [24]. Nearly half of patients undergoing dialysis have diabetic nephropathy and it has been estimated that 23% of diabetic patients already have CKD stages G3-5 [25]. We observed that 42% of diabetic patients had CKD stages G3-4, with a significant association (OR: 8.1, CI 95% 2.31–28.77, p=0.0002). It is important to acknowledge the impact both diseases, hypertension and diabetes, have on CKD, mainly due to the increased risk of cardiovascular comorbidity and mortality, in comparison with patients without those diseases combined [26].
Obesity is a major health concern worldwide and a risk factor for diabetes, cardiovascular disease and CKD [27]. Noticeably, 68% of our studied population had overweight or obesity, a prevalence higher than that reported by the World Health Organization (46% in population>20 yo). Several studies have identified an association between obesity and CKD [28], however, we did not find an association in this population (OR=0.67, CI 95% 0.22–2.66), maybe because overweight was ubiquitous.
The use of counter drugs is widespread in the general population [29]. Such is the case of NSAIDs, which can affect kidney function [30,31]. Noticeably, half of patients with CKD reported chronic use of NSAIDs, although an association was not observed (OR: 1.28, CI 95%: 0.43–3.79, p=0.65).
In recent years, hyperuricemia has been proposed as an independent risk factor for the development of albuminuria, which is associated with cardiovascular diseases and CKD [32]. Hyperuricemia is both a risk factor and a consequence of CKD that can or cannot manifest with gout [33]. We found a relevant prevalence of hyperuricemia in these CKD patients, 46.7% (7/15 OR 3.1, CI 95% 1–9.6), showing a strong association with CKD. Such high prevalence of hyperuricemia in CKD patients is consistent with other reports [34], but a high prevalence was also noticed in the non-CKD population and deserves further research.
LimitationsIDMS-traceable creatinine assays were not available; the study relied on conventional serum creatinine methods. Thus, eGFR was estimated using the MDRD equation instead of the CKD-EPI equation suggested by KDIGO guidelines. Due to financial constrains no other markers of early kidney damage detection were used. Also, the use of semiquanitative reagent strip urinalysis for total protein with manual reading is less optimal compared to other methods. Likely, all these limitations may have caused some loss of early-stage CKD cases and the prevalence could be discretely higher than reported. Prevalences described in this convenience sample cannot be inferred as representative of the whole urban population.
Conclusions and recommendationsTo our knowledge, this is the first study aimed at reporting the prevalence of CKD and other NCDs in urban adult populations of El Salvador. Nevertheless, and despite the limitations, at least 1 out of every 10 subjects had CKD, and most of them (73%) were previously unaware of the diagnosis. While our results are consistent with international reports, it is worrisome that so many cases of CKD are occult.
The prevalence and demographic pattern of CKD is completely different between urban and coastal rural populations in the country. The behavior of CKD in this urban population of El Salvador matches the worldwide picture; meanwhile, in the coastal rural areas, young males prevail without an association to diabetes and hypertension, the classic risk factors. Health providers in the primary public system should insist on early screening, especially in patients with diabetes, hypertension, and hyperuricemia, to ensure early access to medical control and nephroprotection to prevent further kidney damage, delay progression to end-stage renal disease and prevent complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study did not receive any direct funding. Data collection and laboratory analyses were provided by the Department of Investigation of the Central Region of the Ministry of Public Health of El Salvador.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the dedication and cooperation of Dr. David Saúl Rodriguez Araujo, Dra. Adela Méndez Chicas, the team of the Municipal Clinic of Antiguo Cuscatlán, and the committee and the people of the study community.