This study was designed to detect the potential association of a nonfunctional adrenal incidentaloma (NFAI) with insulin resistance and associated metabolic disturbances, with a subsequent increase in cardiovascular risk factors.
MethodsEighty-three NFAI patients and 56 volunteers (controls) without any adrenal abnormalities on computed tomography (CT) were included. Fasting blood glucose (FBG), fasting insulin, lipid profiles, uric acid, homocysteine, fibrinogen, high sensitivity C-reactive protein (hs-CRP), and adiponectin levels were measured in both groups. Blood pressure (BP), waist circumference, body mass index (BMI), and carotid intima media thickness (CIMT) were evaluated in both the patients and volunteers.
ResultsThere were no significant difference between the NFAI and control groups with respect to age, sex, BMI, waist circumference, systolic and diastolic BP, smoking, concomitant disease, and medications. Fasting insulin and glucose levels and homeostasis model of assessment-insulin resistance (HOMA-IR) scores were significantly higher in the NFAI group as compared with those in the control group (p<0.01). The frequency of metabolic syndrome in the NFAI group was higher than that in the control group (p<0.01). All the lipid fractions, except triglyceride (TG), (p<0.05), homocysteine (p=0.01), and fibrinogen levels (p<0.001), were significantly higher in the NFAI group as compared with the levels in the control group. There were no significant differences between the NFAI and control groups in terms of uric acid, hs-CRP, and adiponectin levels. The CIMT values in the NFAI group were significantly higher than those in the control group (0.74±0.14 vs. 0.53±0.09, p<0.001). The mean CIMT value showed a statistically positive correlation with age (r=0.245, p=0.004); the HOMA-IR score (r=0.490, p<0.001); and FBG (r=0.521, p<0.001), fasting insulin (r=0.432, p<0.001), total cholesterol (TC) (r=0.267, p=0.002), and fibrinogen (r=0.398, p<0.001) levels in the NFAI group.
ConclusionsThe results indicated that the NFAI patients had an elevated risk of insulin resistance, with metabolic syndrome and increased CIMT values. Long-term follow-up studies should be designed to evaluate postsurgical alterations in metabolic parameters and cardiovascular risk factors in NFAI patients.
Este estudio se diseñó para detectar la posible asociación del incidentaloma suprarrenal no funcionante (ISNF) con resistencia a la insulina y trastornos metabólicos asociados, con un incremento subsecuente en los factores de riesgo cardiovascular.
MétodosSe incluyó a 83 pacientes con ISNF y a 56 voluntarios (controles) sin anomalías suprarrenales en la tomografía computarizada (TC). Se determinaron en ambos grupos los valores de glucemia en ayunas (GA), insulina en ayunas, perfiles lipídicos, ácido úrico, homocisteína, fibrinógeno, proteína C reactiva de alta sensibilidad (PCRas) y adiponectina. Se evaluaron la presión arterial (PA), el perímetro de la cintura, el índice de masa corporal (IMC) y el grosor íntima-media carotídea (GIMC) tanto en los pacientes como en los voluntarios.
ResultadosNo había una diferencia significativa entre los grupos con ISNF y de control en cuanto a edad, sexo, IMC, perímetro de la cintura, PA sistólica y diastólica, tabaquismo, enfermedades concomitantes y medicamentos. Las concentraciones de insulina y glucosa en ayunas y las puntuaciones del modelo homeostático de evaluación de la resistencia a la insulina (HOMA-IR) fueron significativamente mayores en el grupo con ISNF que en el de control (p<0,01). La frecuencia de síndrome metabólico fue mayor en el grupo con ISNF que en el de control (p<0,01). Los valores de todas las fracciones lipídicas, excepto los de triglicéridos (TG) (p<0,05), homocisteína (p=0,01) y fibrinógeno (p<0,001), fueron significativamente mayores en el grupo con ISNF que en el de control. No hubo diferencias significativas entre los grupos con ISNF y de control en las concentraciones de ácido úrico, PCRas y adiponectina. Los valores del GIMC en el grupo con ISNF fueron significativamente mayores que los del grupo de control (0,74±0,14 frente a 0,53±0,09; p<0,001). El valor medio del GIMC mostró una correlación estadísticamente positiva con la edad (r=0,245; p=0,004); la puntuación del HOMA-IR (r=0,490; p<0,001), y la GA (r=0,521; p<0,001), la insulina en ayunas (r=0,432; p<0,001), el colesterol total (CT) (r=0,267; p=0,002) y el fibrinógeno (r=0,398; p<0,001) en el grupo con ISNF.
ConclusiónLos resultados indicaban que los pacientes con ISNF tenían un riesgo elevado de resistencia a la insulina, con síndrome metabólico y aumento de los valores del GIMC. Deben diseñarse estudios de seguimiento a largo plazo para evaluar los cambios posquirúrgicos de los parámetros metabólicos y los factores de riesgo cardiovascular en pacientes con ISFN.
An adrenal incidentaloma (AI) refers to a mass detected incidentally on radiological imaging performed for reasons other than adrenal pathology. The European Society of Endocrinology clinical practice guideline recommends that all patients with AIs should be tested for cortisol excess using the overnight 1mg dexamethasone suppression test, hyperaldosteronism in the presence of hypertension or unexplained hypokalemia, and the presence of a pheochromocytoma.1 Previous data showed that among incidentalomas, the frequency of nonfunctioning adenomas, subclinical Cushing's syndrome (CS), pheochromocytomas, aldosteronomas, and adrenocortical carcinomas were 80%, 5%, 5%, 1%, and <5%, respectively.2
Previous research suggested that the presence of an AI was related to metabolic syndrome and that hyperinsulinemia was a predisposing factor in adrenal tumorigenesis.3 On the other hand, a number of studies reported that a nonfunctional adrenal incidentaloma (NFAI) may cause hypertension, insulin resistance, obesity, and hyperlipidemia, with a subsequent increase in cardiovascular risk.4,5
This study was designed to detect the potential association of NFAIs with insulin resistance, with associated metabolic disturbances and cardiovascular risk factors.
2Subjects and methodsEighty-three patients with NFAIs and 56 individuals (control group) without any adrenal lesions were included in this study. The control group consisted of volunteers who underwent abdominal computed tomography (CT) for an indication other than adrenal pathology and were found to have normal adrenals. All the volunteers were free of any diseases that could affect metabolic parameters. The exclusion criteria were patients younger than 18y; patients with diabetes mellitus or malignancies; and patients with a history of myocardial infarctions and/or heart failure, strokes or peripheral vascular disease, acute or chronic inflammatory diseases, and suspected or known psychiatric disorders. Patients who were pregnant or who used glucocorticoids were also excluded.
The adrenocorticotropic hormone (ACTH) and plasma cortisol levels of the patients with AIs were tested in the morning (08:00h). The patients’ aldosterone and plasma renin activity in a standing position were tested, in addition to their creatinine, dopamine, vanillylmandelic acid, metanephrine, and normetanephrine levels in 24-h urine and dehydroepiandrosterone sulfate levels in serum. The overnight low-dose dexamethasone suppression test (1mg, orally at 23:00h, with measurement of fasting serum cortisol at 08:00h the following morning) was performed to detect adrenal hyperfunctioning. Suppression was accepted as adequate if the morning cortisol levels were lower than 1.8μg/dL. The 24-h urinary free cortisol (24-h UFC) level was also measured to exclude subclinical Cushing's syndrome (CS). Patients with post-dexamethasone suppression test (PDST) cortisol levels of <1.8μg/dL, normal 24-h UFC findings [reference range (RR): 38–208nmol/day], and ACTH levels ≥10pg/ml were included. Only biochemically proven nonfunctional tumors were included.
All the participants were screened for diabetes using glycated hemoglobin (HbA1c) and fasting blood glucose (FBG) tests. Patients with FBG ≥126mg/dL (7.0mmol/L) and HbA1C ≥6.5% were excluded. To exclude diabetes in patients with impaired fasting glycemia (100–125mg/dL), the oral glucose tolerance test was performed. Twenty-one patients with impaired fasting glycemia or glucose tolerance were included after exclusion of diabetes using both, the HbA1C test and oral glucose tolerance test.
Ethical approval was obtained from the local ethics committee of Ankara University Faculty of Medicine, Ankara (06-176-12, March 2012).
The indications for CT imaging in the AI patients and controls included the evaluation of ureteral and renal stones; an incidentally found adrenal mass in chest CT during follow-up for pulmonary nodules (included when the nodule was stable and considered benign); differential diagnosis of a focal hepatic mass found on ultrasound (included if the lesion was considered as a hemangioma or focal spared area in a fatty liver); follow-up for a pancreatic pseudocyst; undifferentiated abdominal pain (usually performed for differential diagnosis of irritable bowel syndrome from structural causes); and adrenal lesions incidentally found on abdominal ultrasound in patients with abdominal pain (included when no vascular, malignant, infectious, or inflammatory etiology was found and the pain was not attributable to an adrenal mass).
Medical histories were obtained, and physical examinations were performed by physicians who were specifically trained in endocrinology and metabolism diseases. Arterial hypertension was diagnosed in patients with systolic blood pressure (BP) ≥140mmHg and/or diastolic BP ≥90mmHg and in patients receiving antihypertensive therapy.6 The patient's waist circumference was measured using a flexible tape placed on a horizontal plane at the level of the iliac crest.7 The body mass index (BMI) was defined as the weight in kilograms divided by the square of the height in meters. Metabolic syndrome was defined according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III.8 Systolic and diastolic BPs were measured twice in the sitting position, with an interval of 15min using standard sphygmomanometers of appropriate sized adult cuffs, following a rest period for 30min.
Blood samples were centrifuged for 15min at 3000cycle/min, and the plasma on the top was extracted. The extracted plasma samples were preserved in Eppendorf tubes (volume: 1.5ml) and kept frozen at −80°C. FBG, fasting insulin, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), uric acid, homocysteine, fibrinogen, C-reactive protein (CRP), and adiponectin levels were evaluated.
The FBG level was measured with the aid of a Roche P800 device using an enzymatic method (RR: 74–100mg/dL.) Fasting insulin levels were measured with the Roche E170 electrochemiluminescence immunoassay (ECLIA) (RR: 74–16μIU/ml). The levels of TC, LDL, HDL, and TG were measured with Roche P800 using the photometric method. A cholesterol level of <200mg/dL was defined as normal whereas levels of 200–239mg/dL and >240mg/dL were considered borderline high and high, respectively. For LDL, a reference value of <100mg/dL was defined as optimal, whereas values of 100–129mg/dL, 130–159mg/dL, 160–189mg/dL, and >190mg/dL were considered acceptable, borderline high, high, and very high, respectively. For HDL and TG, the reference values were defined as 40–60mg/dL and <150mg/dL, respectively. High sensitive CRP levels are measured using the photometric method (RR: 0–3mg/L). Fibrinogen levels were measured using an Amax 190 (Trinity, USA) device using the coagulometric method (RR: 2.38–4.98g/Dl). Homocysteine levels were measured with the aid of an Immulite 2 (Siemens, Germany) device using the Clia method (RR: 5–14μmol/L). Adiponectin levels were measured with the aid of an RIA device using an enzyme-linked immunoassay (Biovendor, Czech Republic), and the lower reference value was established as 9μg/dL.
In both groups, homeostasis model of assessment-insulin resistance (HOMA-IR) scores were calculated using the formula (FBG [mg/dL]×fasting insulin [mIU/ml])/405.9
The carotid intima media thickness (CIMT) values of the patients and volunteers were determined by the same investigator using an ultrasonography device with a 7.5-mHz linear probe. In the first 2cm distal to the carotid bulbus, three measurements of a 1-cm segment were obtained. The measurements were obtained where the CIMT was greatest and mean of the values were accepted as the CIMT.
2.1Statistical analysisStatistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software, version 20.0 (IBM Corp., NY, USA). Categorical data were compared using a chi-square test and Fisher's exact test. Group data with a normal distribution were compared using the Student's t test or an analysis of variance, and nonparametric data were compared using the Mann–Whitney U test or Kruskal–Wallis test. Values were expressed as mean±standard deviation or median as appropriate. Spearman's correlation test was performed for continuous variables without a normal distribution, and Pearson's correlation test was performed for variables with a normal distribution. Independent linear factors were determined using linear regression, with backwards elimination. For results, a p value of <0.05 was considered statistically significant.
3ResultsEighty-three NFAI patients (26 males [31.3%] and 57 females [68.7%]) and 56 volunteers (24 males [42.9%] and 32 females [57.1%]) were enrolled.
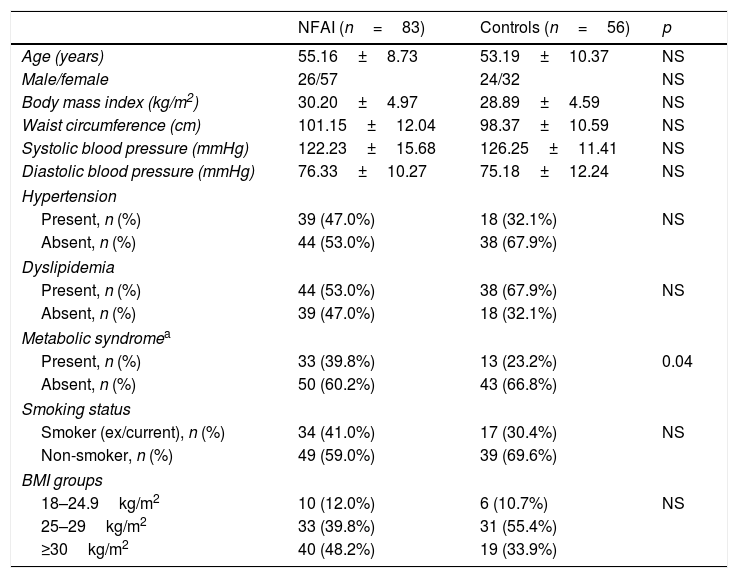
As shown in Table 1, there were no differences between the NFAI and control groups with respect to age, sex, BMI, waist circumference, systolic and diastolic BP, smoking history, presence of hypertension, and dyslipidemia.
Demographic and clinical characteristics of NFAI and control groups.
| NFAI (n=83) | Controls (n=56) | p | |
|---|---|---|---|
| Age (years) | 55.16±8.73 | 53.19±10.37 | NS |
| Male/female | 26/57 | 24/32 | NS |
| Body mass index (kg/m2) | 30.20±4.97 | 28.89±4.59 | NS |
| Waist circumference (cm) | 101.15±12.04 | 98.37±10.59 | NS |
| Systolic blood pressure (mmHg) | 122.23±15.68 | 126.25±11.41 | NS |
| Diastolic blood pressure (mmHg) | 76.33±10.27 | 75.18±12.24 | NS |
| Hypertension | |||
| Present, n (%) | 39 (47.0%) | 18 (32.1%) | NS |
| Absent, n (%) | 44 (53.0%) | 38 (67.9%) | |
| Dyslipidemia | |||
| Present, n (%) | 44 (53.0%) | 38 (67.9%) | NS |
| Absent, n (%) | 39 (47.0%) | 18 (32.1%) | |
| Metabolic syndromea | |||
| Present, n (%) | 33 (39.8%) | 13 (23.2%) | 0.04 |
| Absent, n (%) | 50 (60.2%) | 43 (66.8%) | |
| Smoking status | |||
| Smoker (ex/current), n (%) | 34 (41.0%) | 17 (30.4%) | NS |
| Non-smoker, n (%) | 49 (59.0%) | 39 (69.6%) | |
| BMI groups | |||
| 18–24.9kg/m2 | 10 (12.0%) | 6 (10.7%) | NS |
| 25–29kg/m2 | 33 (39.8%) | 31 (55.4%) | |
| ≥30kg/m2 | 40 (48.2%) | 19 (33.9%) | |
NS: not significant.
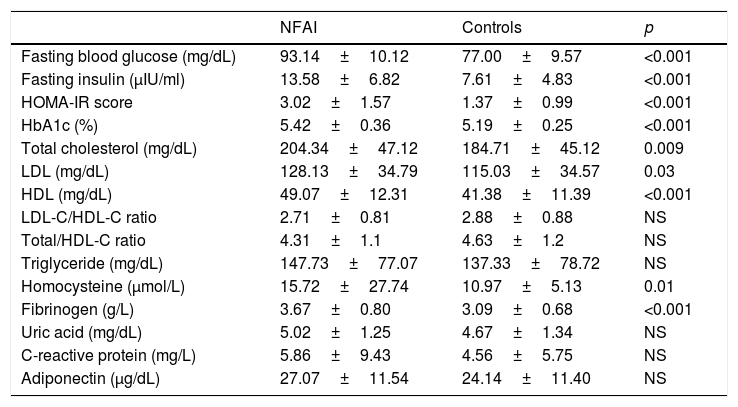
Fasting insulin levels, FBG, HbA1C, and HOMA-IR scores were significantly higher in the NFAI group as compared with those in the control group (p<0.001). The FBG level was not associated with fasting morning (08:00h) ACTH and cortisol levels. The TC (p=0.009), LDL-C (p=0.03), HDL-C (p<0.001), homocysteine (p=0.01), and fibrinogen levels (p<0.001) were all higher in the NFAI group as compared with those in the control group. The prevalence of metabolic syndrome was increased in the NFAI group (p=0.04). There were no significant between-group differences in the LDL-C/HDL-C and TC/HDL-C ratios or the TG, uric acid, CRP, and adiponectin levels (Table 2). Eighteen of the 21 (85%) patients with impaired fasting glucose or glucose tolerance were in the NFAI group (p<0.01).
Biochemical data of NFAI and control group.
| NFAI | Controls | p | |
|---|---|---|---|
| Fasting blood glucose (mg/dL) | 93.14±10.12 | 77.00±9.57 | <0.001 |
| Fasting insulin (μIU/ml) | 13.58±6.82 | 7.61±4.83 | <0.001 |
| HOMA-IR score | 3.02±1.57 | 1.37±0.99 | <0.001 |
| HbA1c (%) | 5.42±0.36 | 5.19±0.25 | <0.001 |
| Total cholesterol (mg/dL) | 204.34±47.12 | 184.71±45.12 | 0.009 |
| LDL (mg/dL) | 128.13±34.79 | 115.03±34.57 | 0.03 |
| HDL (mg/dL) | 49.07±12.31 | 41.38±11.39 | <0.001 |
| LDL-C/HDL-C ratio | 2.71±0.81 | 2.88±0.88 | NS |
| Total/HDL-C ratio | 4.31±1.1 | 4.63±1.2 | NS |
| Triglyceride (mg/dL) | 147.73±77.07 | 137.33±78.72 | NS |
| Homocysteine (μmol/L) | 15.72±27.74 | 10.97±5.13 | 0.01 |
| Fibrinogen (g/L) | 3.67±0.80 | 3.09±0.68 | <0.001 |
| Uric acid (mg/dL) | 5.02±1.25 | 4.67±1.34 | NS |
| C-reactive protein (mg/L) | 5.86±9.43 | 4.56±5.75 | NS |
| Adiponectin (μg/dL) | 27.07±11.54 | 24.14±11.40 | NS |
Data were expressed as mean±SD.
HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; LDL: low density lipoprotein; HDL: high density lipoprotein.
The median size of the adenomas (maximal diameter) was 20mm (12–50mm). The maximal diameter of the adenoma was not associated with FBG, insulin, HOMA-IR scores, lipid profiles, adiponectin, CRP, fibrinogen, homocysteine, ACTH, basal cortisol, PDST cortisol levels, BMI, waist circumference, or CIMT values.
The median PDST cortisol level in the NFAI group was 0.9μg/dL (0.45–1.47μg/dL). There was a positive correlation between the PDST cortisol level and FBG (r=0.69, p<0.001). However, fasting insulin levels, HOMA-IR scores, lipid profiles, adiponectin, CRP, fibrinogen, homocysteine, BMI, waist circumference, and CIMT values were not associated with morning cortisol, ACTH, or PDST cortisol levels.
The mean 24-h UFC level was 77.9±20.6nmol/day. The 24-h UFC level was not associated with FBG, insulin, HOMA-IR scores, lipid profiles, adiponectin, CRP, fibrinogen, homocysteine, BMI, waist circumference, or CIMT values.
The HOMA-IR scores showed a positive correlation with the CIMT value (r=0.533, p<0.001) and FBG (r=0.582, p<0.001), fasting insulin (r=0.975, p<0.001), TG (r=0.225, p=0.008), fibrinogen (r=0.282, p=0.001), and uric acid (r=0.209, p=0.013) levels.
The CIMT in the NFAI group was significantly higher than that in the control group (0.74±0.14 vs. 0.53±0.09, p<0.001) (Table 3 and Fig. 1).
In the NFAI group, the CIMT showed a statistically positive correlation with age (r=0.245, p=0.004), FBG levels (r=0.521, p<0.001), fasting insulin levels (r=0.432, p<0.001), HOMA-IR scores (r=0.490, p<0.001), TC levels (r=0.267, p=0.002), and fibrinogen (r=0.398, p<0.001) levels. Cortisol, ACTH, 24-h UFC, and PDST cortisol levels were not associated with the CIMT values. When age, sex, BMI, smoking behaviors, waist circumference, LDL-C, uric acid, adiponectin, CRP, homocysteine, fibrinogen levels, and HOMA-IR variables were evaluated using the linear regression method with backwards elimination, age (β=0.004 [0.001–0.06]), HOMA-IR scores (β=0.037 [0.22–0.52]), and fibrinogen (β=0.052 [0.020–0.83]) levels were independent risk factors for CIMT.
In the control group, age (r=0.23, p=0.01), TC (r=0.26, p=0.002), and LDL-C (r=0.21, p=0.01) levels were positively correlated with the CIMT.
4DiscussionIn the present study, we observed that fasting insulin levels, FBG levels, HOMA-IR scores, and the frequency of metabolic syndrome were significantly higher in NFAI patients when compared with age-, sex-, and BMI-matched controls. In addition, TC, LDL-C, and HDL-C levels were elevated in the NFAI group as compared with that in the control group. There were no significant between-group differences in TG levels.
Previous studies reported the presence of insulin resistance in patients with NFAIs.4,10,11 In one study, the area under the curve for glucose after an oral glucose tolerance test was significantly higher in patients with CS and NFAIs as compared with that of a control group.12 Their data indicated that NFAIs may represent an intermediate state between normal and pathological and that they may be caused by subtle cortisol hypersecretion.12 Consistent with previous data, insulin resistance was associated with the PDST cortisol level in the AI patients in the study of Papanastasiou et al.13 Another study also reported insulin resistance in patients with NFAIs, even when PDST cortisol levels were lower than 1.8μg/dL.14 A significant improvement in plasma glucose and systolic BP levels after an adrenalectomy was reported in NFAI patients and patients with subclinical CS (SCS).10
Although debate surrounds the screening and diagnosis of SCS,15–19 the overnight 1mg PDST is widely recommended as the first-line screening test.15–19 A second confirmatory test, including late night salivary cortisol, 24-h UFC, or late night serum cortisol, was recommended in a number of guidelines.17,19 As a strength of our study, we evaluated morning ACTH levels, PDST cortisol levels, and 24-h UFC levels to exclude SCS in the NFAI group. The FBG level was positively correlated with the PDST cortisol level, but no cortisol secretion-related parameters were associated with fasting insulin levels or HOMA-IR scores. However, we did not assess the adrenal function in the control group. Thus, a subtle difference in cortisol metabolism between-groups may have been overlooked.
In the present study, fibrinogen and homocysteine levels were elevated in the NFAI group as compared with those in the control group, and HOMA-IR showed a positive association with fibrinogen levels. This data may support the presence of a proinflammatory state in NFAI patients, which may be associated with insulin resistance. Our results suggest that subtle cortisol secretion may not be the only factor responsible for insulin resistance in NFAI patients.
It is well known that high levels of LDL-C are atherogenic, whereas high HDL-C levels are cardioprotective.20,21 Previous studies suggested that high LDL-C/HDL-C and TC/HDL ratios were predictors of increased cardiovascular risk.22,23 Some studies reported controversial results regarding the levels of lipid fractions in NFAIs.24–26 In our study, TC, LDL-C, and HDL-C levels were all significantly increased in the NFAI group as compared to controls. On the other hand, the LDL-C/HDL-C and TC/HDL ratios of the two groups were not different. The CIMT showed a statistically positive correlation with TC levels.
An important finding of our study was the higher CIMT values in the NFAI group as compared with those in the control group. A limited number of previous studies evaluated CIMT, which is an indicator of premature atherosclerosis, in NFAI patients.24,26–29 In one study, epicardial fat thickness, the left ventricular mass index, and CIMT were significantly higher in NFAI patients when compared with those in controls.26 The authors suggested that subclinical inflammation, insulin resistance, and altered cytokine levels in these patients may represent possible mechanisms of increased cardiovascular risk in this population. In another study, the frequency of hypertension in NFAI patients was higher than that in controls, and CIMT was increased among NFAI patients as compared with that in controls.27 In other research, CIMT was not significantly different between NFAI and BMI-matched controls.29 In the same study, CIMT was correlated with age, BMI, HOMA-IR scores, waist circumference, morning cortisol, and uric acid levels in all the participants. Consistent with previous data, our results showed that NFAIs were associated with insulin resistance, which may give rise to an increase in cardiovascular risk. We found no relationship between CIMT, PDST cortisol, 24-h UFC, and ACTH levels. However, as mentioned already, the possibility of a subtle difference between the two groups in cortisol secretion status cannot be ruled out.
In the present study, the plasma fibrinogen level was significantly higher in the NFAI group as compared with that in the controls, and it was an independent risk factor for increased CIMT. A previous study showed that elevated fibrinogen levels were associated with an increased risk of thromboembolic events and cardiovascular diseases in overt CS patients due to increased cortisol secretion.30 Consequently, fibrinogen levels of NFAI patients may serve as an indicator of cardiovascular risk.
Previous studies reported decreased plasma adiponectin levels in clinical situations related to insulin resistance.31,32 Increased levels of adiponectin were reported in AI patients, independent of visceral obesity.33 However, in another study, adiponectin levels of female CS patients and controls were not significantly different.19 In our study, there were no differences in the levels of adiponectin in the NFAI patients and controls and no relationship between the CIMT and adiponectin levels.
A major limitation of our study was the absence of data about fat distribution, although the BMI and waist circumference were comparable in the two groups. In addition, we did not assess adrenal functions in the control group. Thus, a subtle difference in cortisol metabolism between the groups may have been overlooked.
In summary, we demonstrated that patients with NFAIs had an increased risk of insulin resistance and metabolic syndrome, in addition to elevated CIMT values. Patients with NFAIs merit closer follow-up in terms of insulin resistance and cardiovascular risks. When evaluating surgical indications for patients with NFAIs, the presence of metabolic syndrome, lipid profiles, glucose levels, fibrinogen levels, and CIMT may be used as indicators of the risk of cardiovascular diseases. Long-term follow-up studies should be designed to evaluate postsurgical alterations in metabolic parameters and cardiovascular risk factors in NFAI patients.
5Ethical approvalAll procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
6FundingThis research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
7Conflict of interestThere is no conflict of interest for each author and there is no source of any support for the study.