This study aimed to establish basal biomarkers in patients with bone metastatic castration-resistant prostate cancer (mCRPC) treated with 223Ra to predict better overall survival (OS), and assess hematologic toxicity and treatment response.
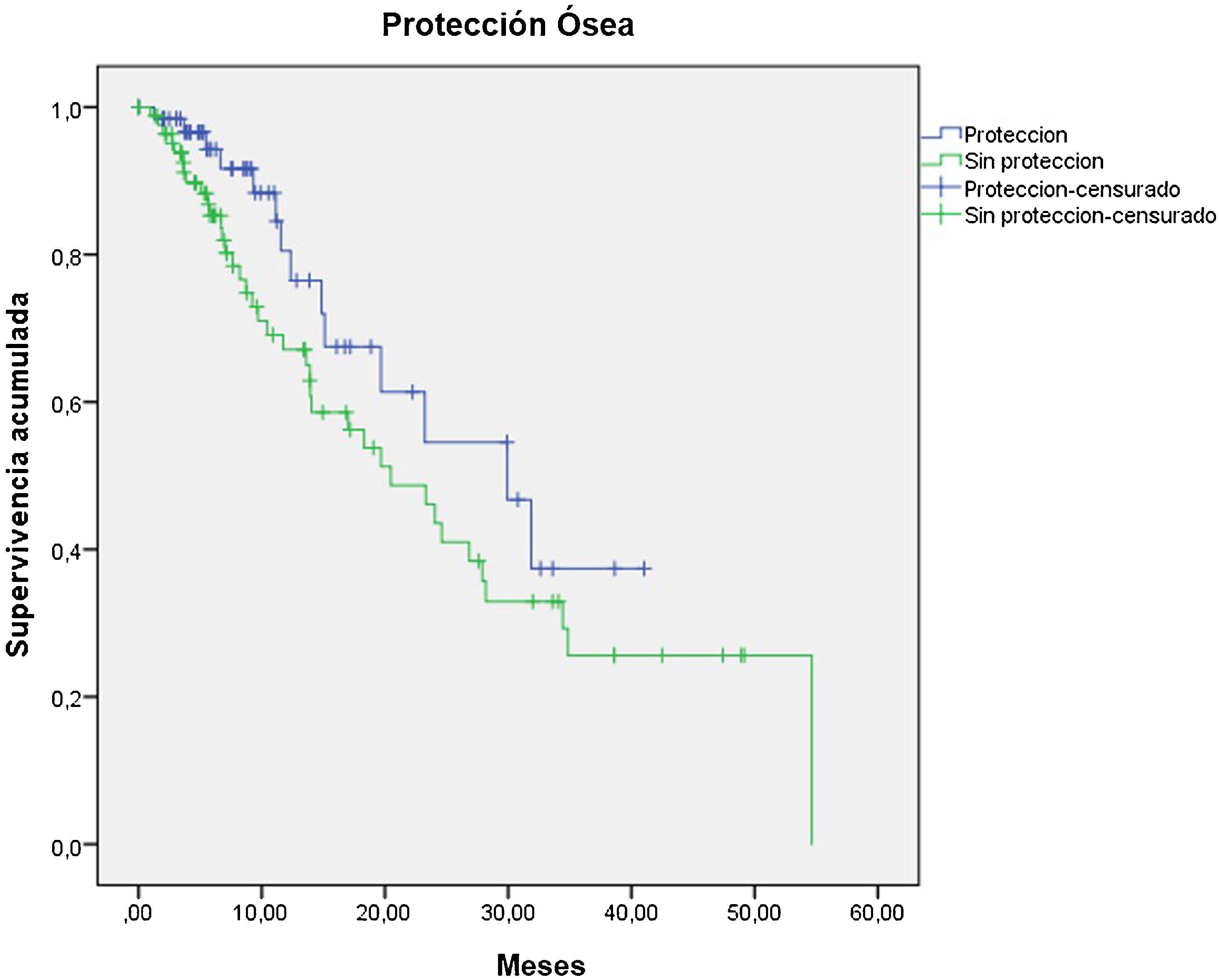
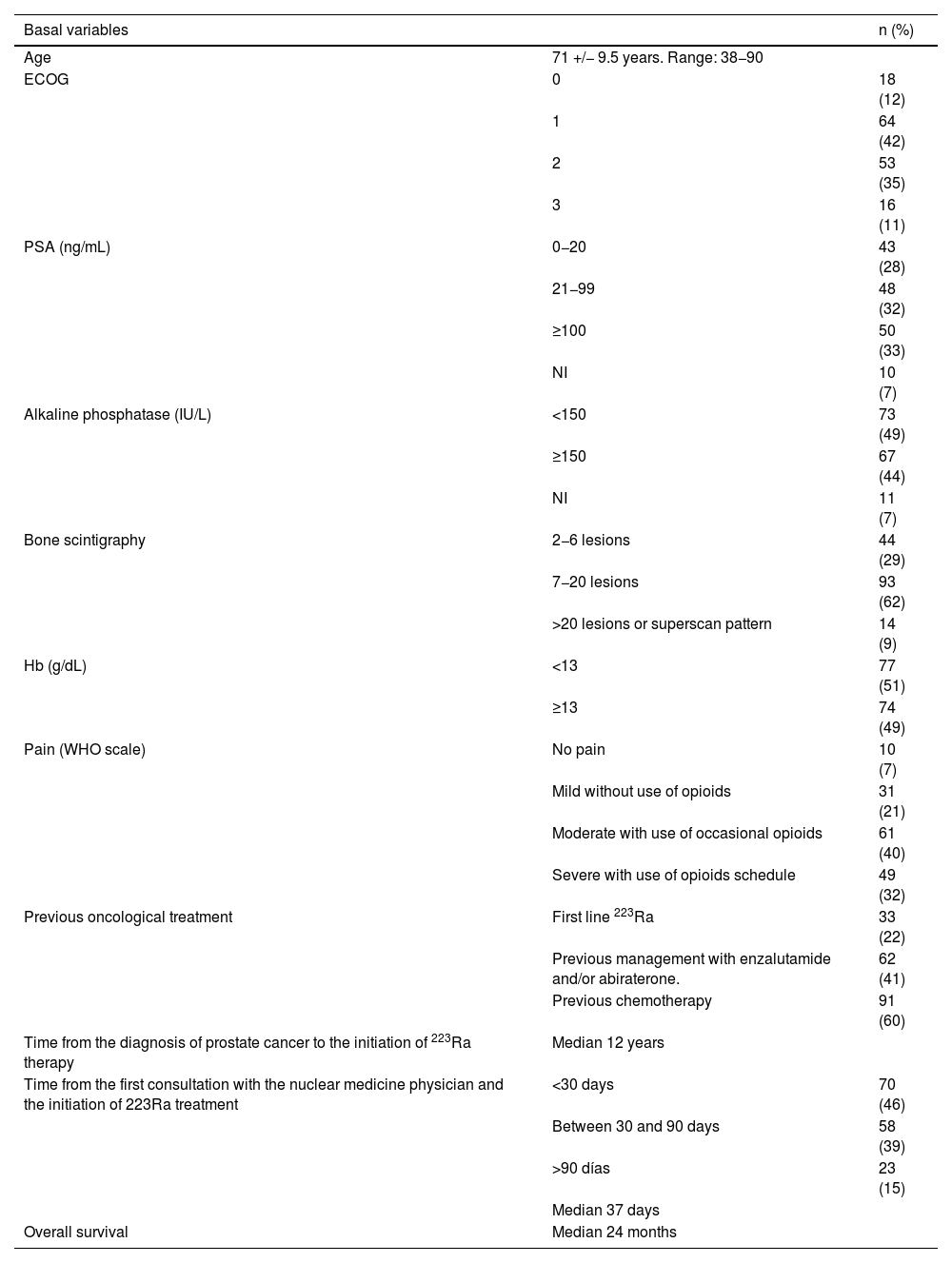
Materials and methodsThis was a retrospective multicenter study including 151 patients with mCRPC between 2013 and 2020. OS was assessed according to basal hemoglobin (Hb), prostate-specific antigen (PSA), and alkaline phosphatase (AP) values, the World Health Organization pain scale, the Eastern Cooperative Oncology Group (ECOG) performance status scale, the number of metastatic lesions on bone scintigraphy (BS), and the use of protective bone agents and the dose received. The grade of hematological toxicities was evaluated as well as treatment response based on changes in AP and pre- and post-treatment pain.
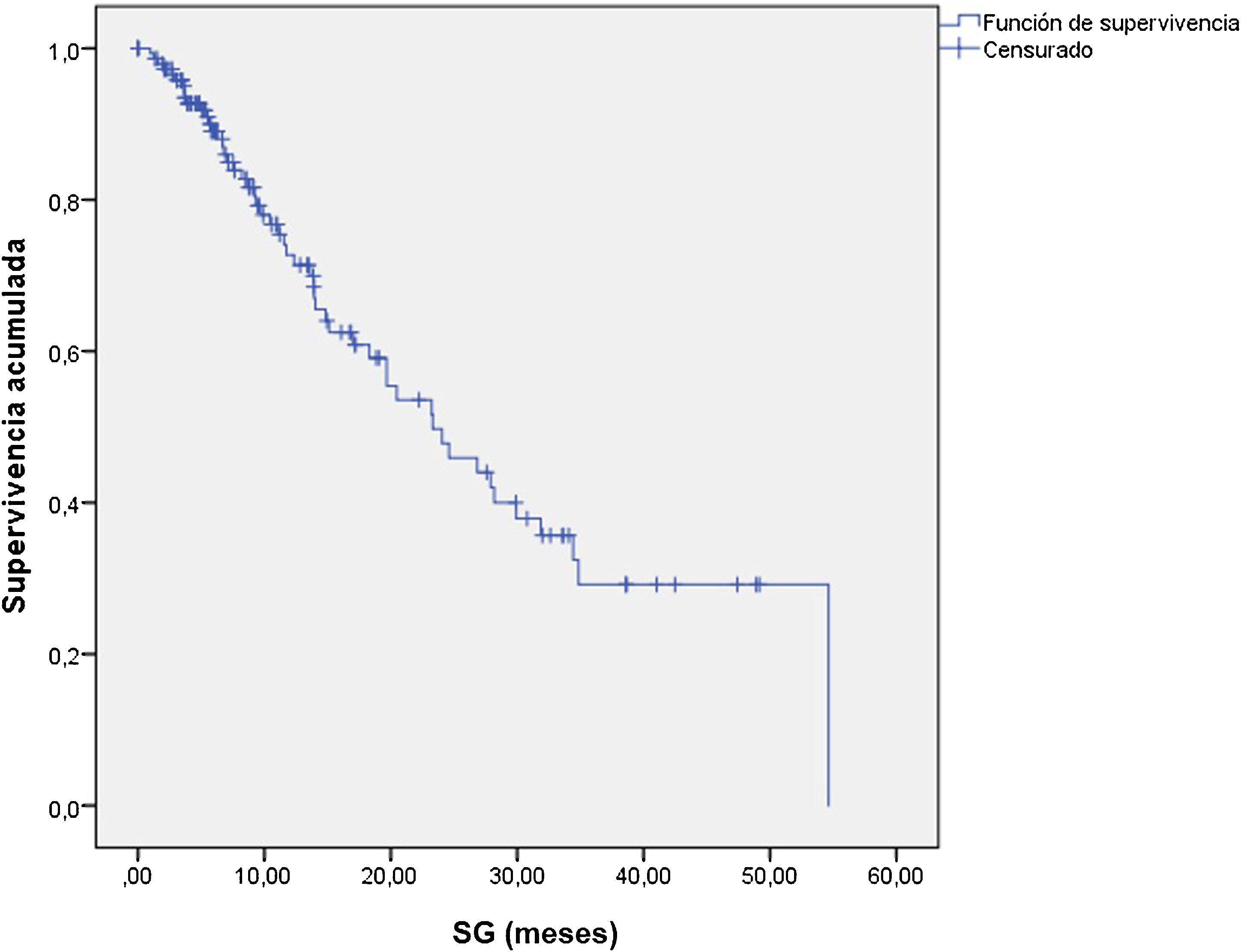
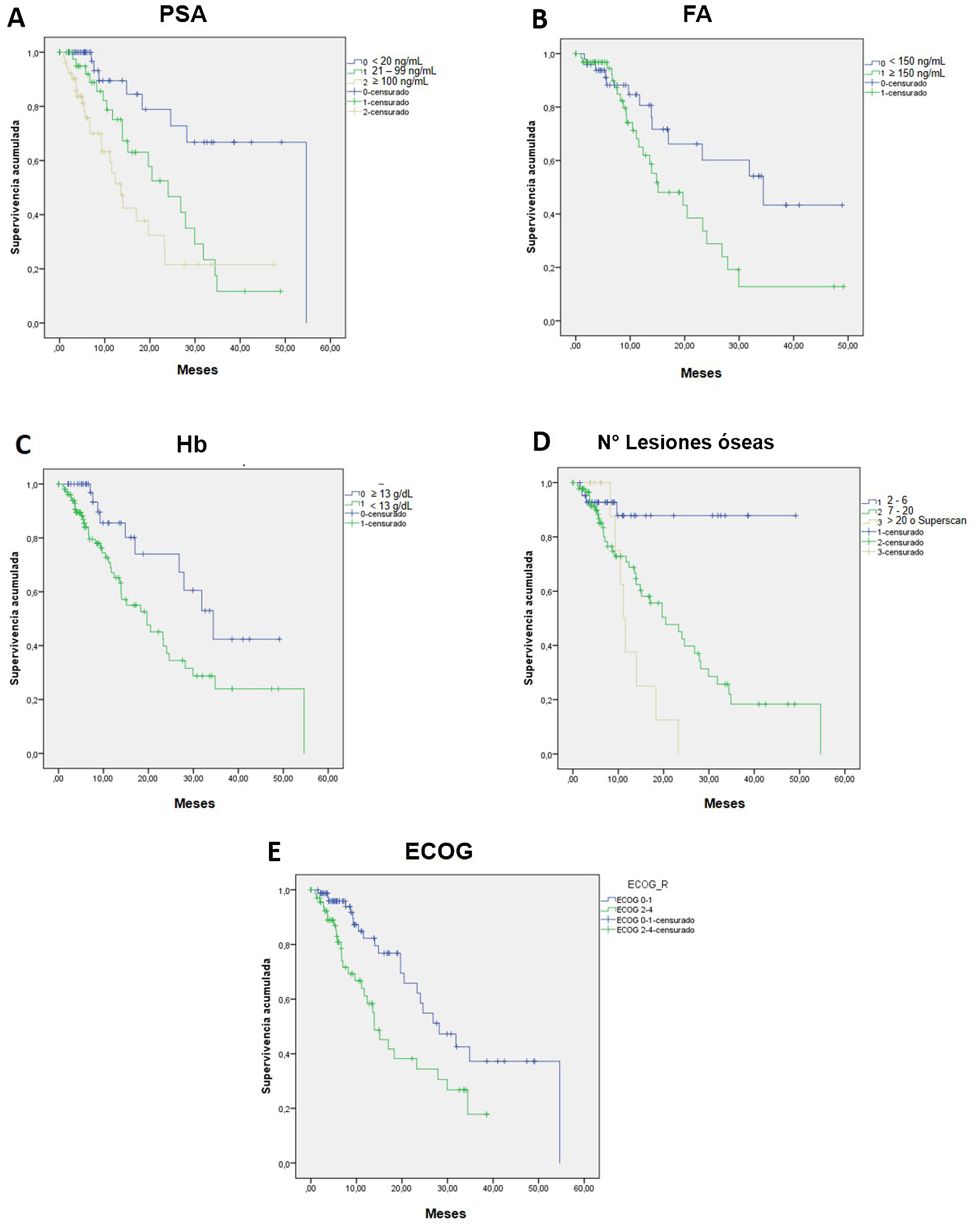
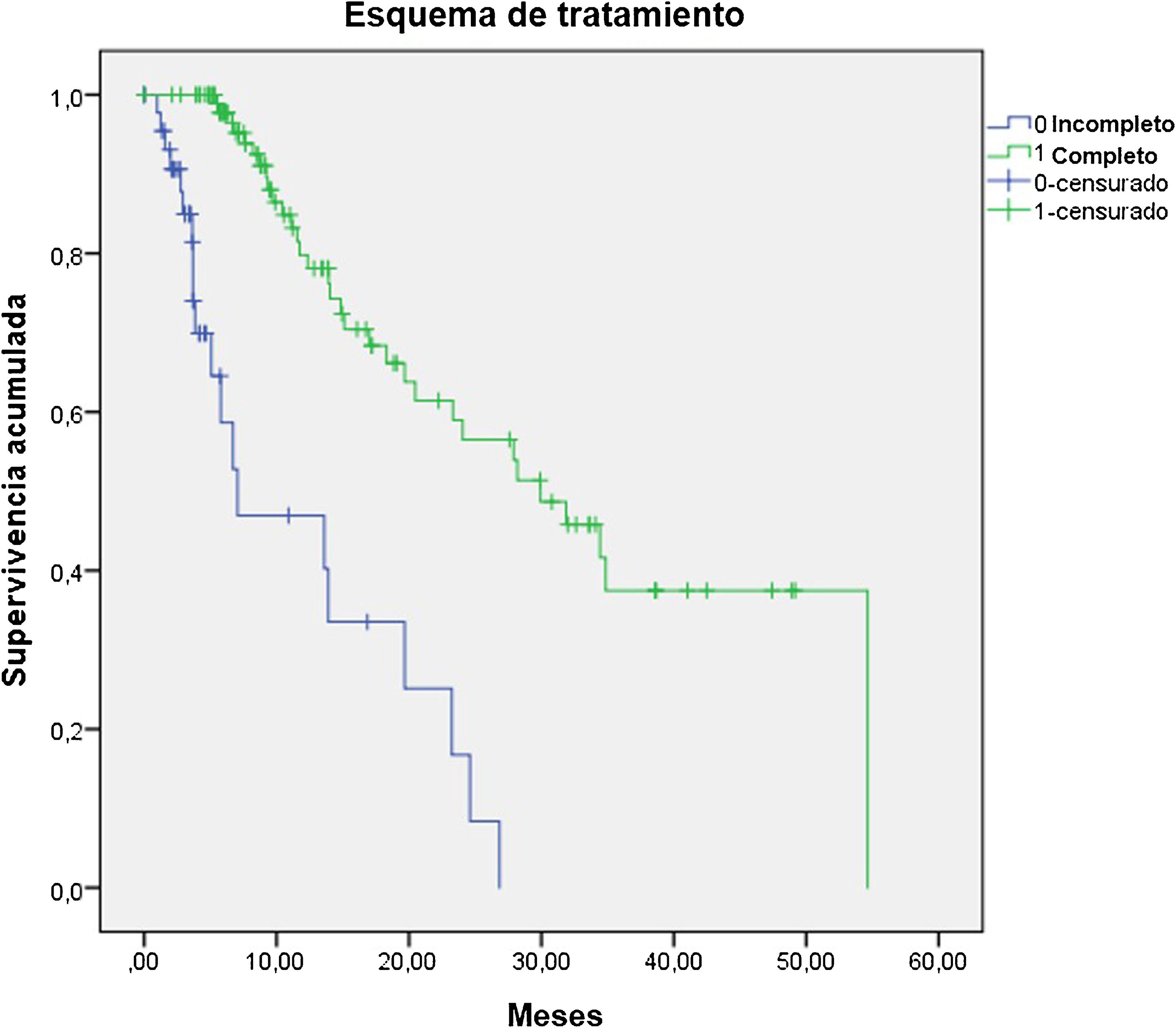
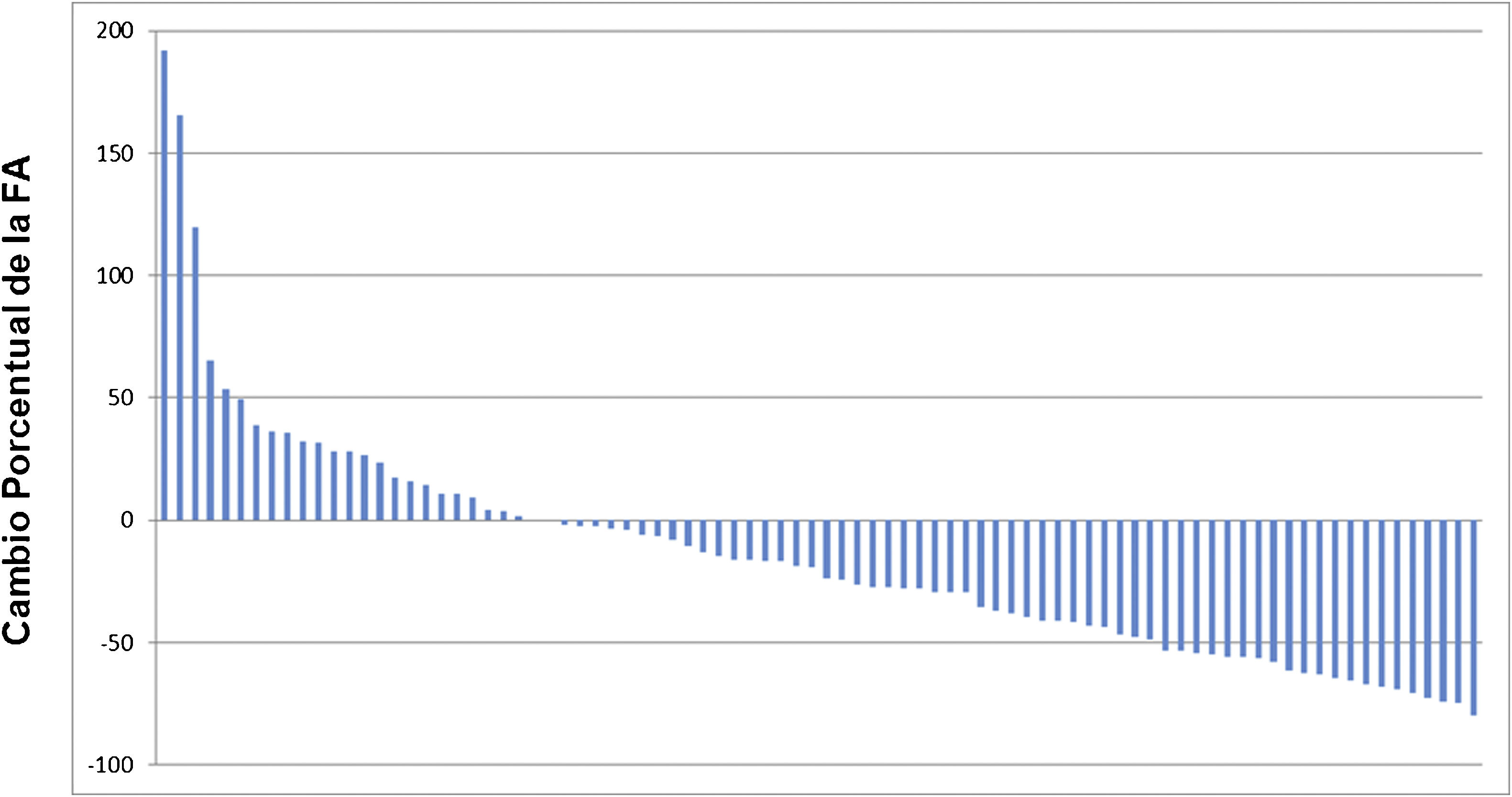
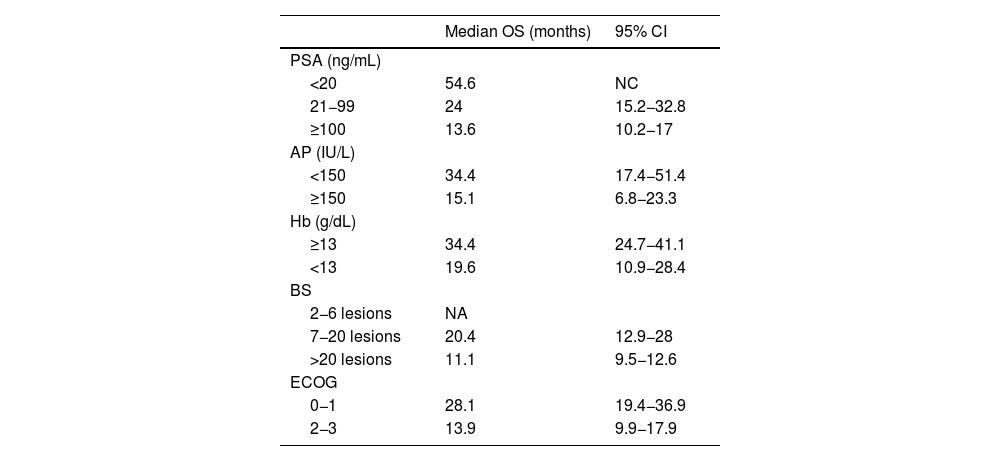
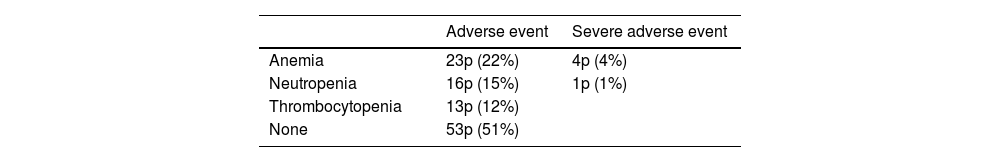
ResultsThe median OS was 24 months (95% confidence interval 16.5–31). The OS in 70% of patients who received complete (5–6 doses) versus incomplete (1–4 doses) 223Ra treatment was 34.9 vs. 5.8 months, respectively, being longer in patients with lower PSA and AP values, Hb >13 g/dl, lesser bone metastasis on bone scan and with an ECOG 0−1. 52/151 patients (34%) died during follow-up. Pain reduced in nearly 70% of patients and 66% presented a reduction in AP values. Half of the patients presented mild and 5 % severe hematological adverse effects.
ConclusionsmCRPC patients treated with 223Ra with Hb values >13 g/mL, an ECOG 0−1, low AP values, PSA < 20 ng/mL and lesser bone metastasis on BS presented a better OS with an adequate safety profile.
Establecer biomarcadores basales en pacientes (p) con cáncer de próstata metastático resistente a la castración (CPMRC) tratados con Ra-223 que predigan una mejor supervivencia global (OS), valorar la toxicidad hematológica y la respuesta.
Materiales y métodosEstudio retrospectivo multicéntrico en 151p con CPMRC tratados con Ra-223 entre 2013−2020. Se valoró la OS de acuerdo a: los niveles basales de hemoglobina (Hb), antígeno prostático específico (PSA), fosfatasa alcalina (FA), escala de dolor de la OMS, Eastern Cooperative Oncology Group (ECOG), el número de lesiones en gammagrafía ósea(GO), el uso de agentes de protección ósea y las dosis recibidas. Se determinó el grado de toxicidad hematológica y la respuesta basada en los cambios de la FA y el dolor pre y post-tratamiento.
ResultadosMediana de OS de 24 meses (IC 95% 16,5–31). En el 70% que recibió tratamiento completo (5–6 dosis) la mediana de OS fue de 34.9 meses versus 5.8 en el tratamiento incompleto (1–4 dosis). La OS fue mayor en los pacientes con menor PSA, FA, Hb>13gr/dl, menor número de metástasis óseas y ECOG 0−1. 52/151p (34%) fallecieron durante el seguimiento. Cerca del 70% de los pacientes presentaron disminución del dolor y 66% reducción de la FA. La mitad de los pacientes presentaron eventos adversos hematológicos leves y solo un 5% severos.
ConclusionesLos pacientes con CPMRC tratados con Ra-223 que presentan biomarcadores basales como Hb >13gr/mL, ECOG 0-1, PSA < 20 ng/mL y menor número de lesiones en GO muestran mejor OS con un adecuado perfil de seguridad.
Article

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)