This study evaluates the prognostic role of different [18F]FDG PET/CT metabolic response criteria in metastatic breast cancer (MBC) patients treated with cyclin-dependent kinase 4/6 inhibitors (CDK 4/6).
Materials and methodsWe retrospectively evaluated the data of MBC patients treated with CDK 4/6 inhibitors who underwent an [18F]FDG PET/CT scan before starting and during treatment. [18F]FDG PET/CT response was assessed with the European Organization for Research and Treatment of Cancer (EORTC), PET Response Criteria in Solid Tumors (PERCIST), and whole-body total lesion glycolysis (WBTLG) criteria. Fleiss kappa was computed to assess the agreement between metabolic response criteria. The endpoint of the study was progression-free survival (PFS). PFS data were analyzed by the Kaplan–Meier method and compared using the log-rank test.
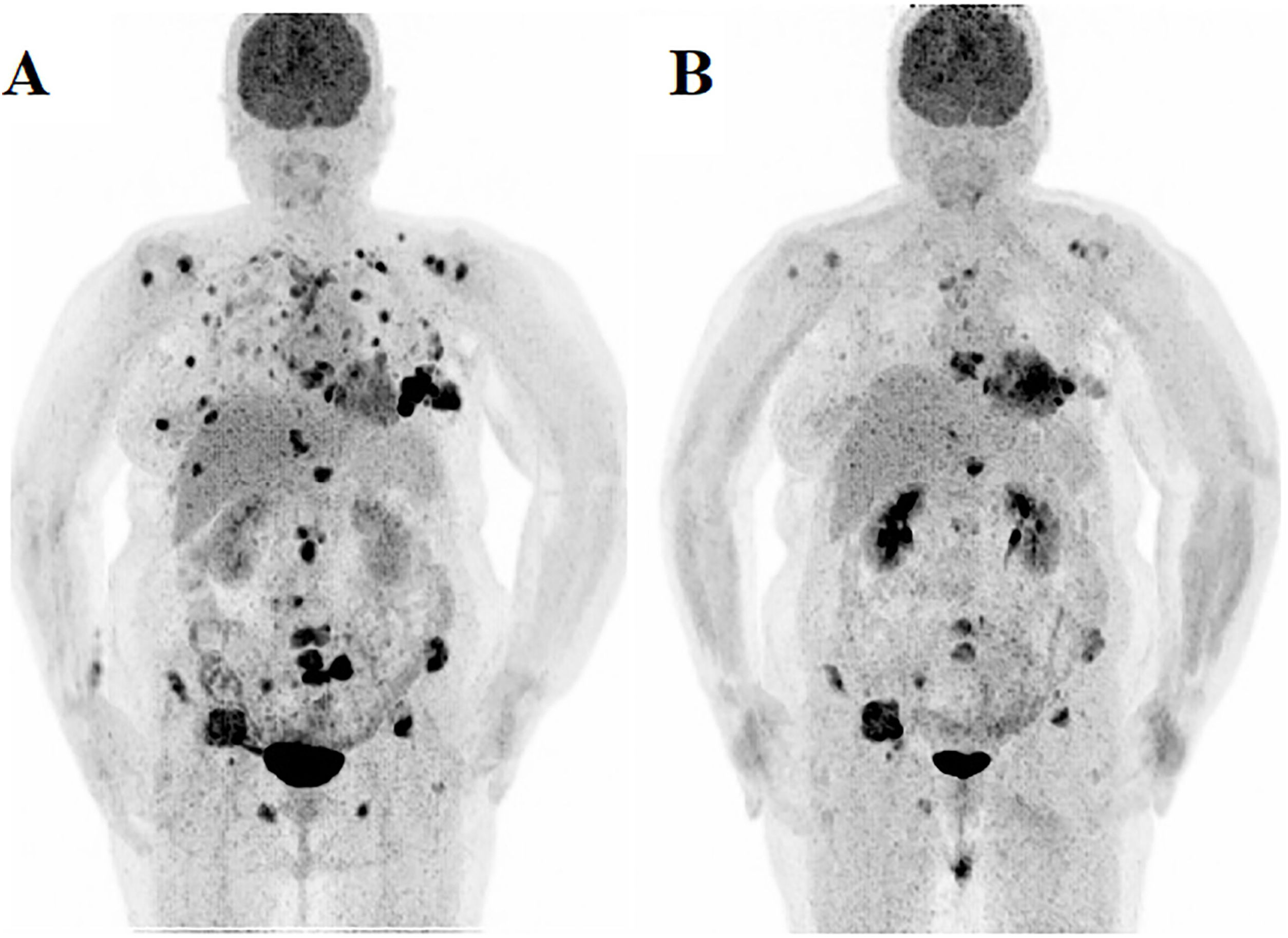
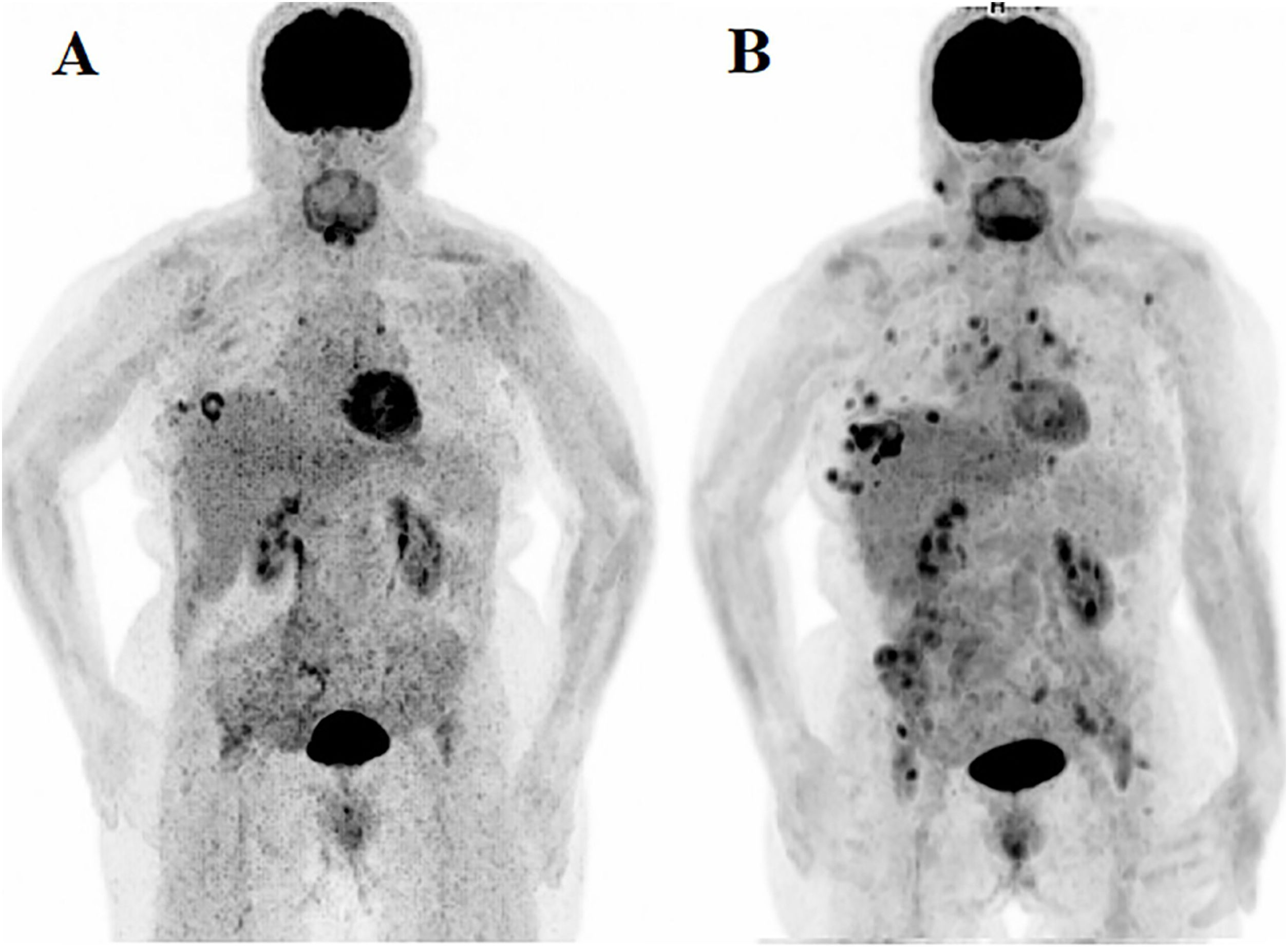
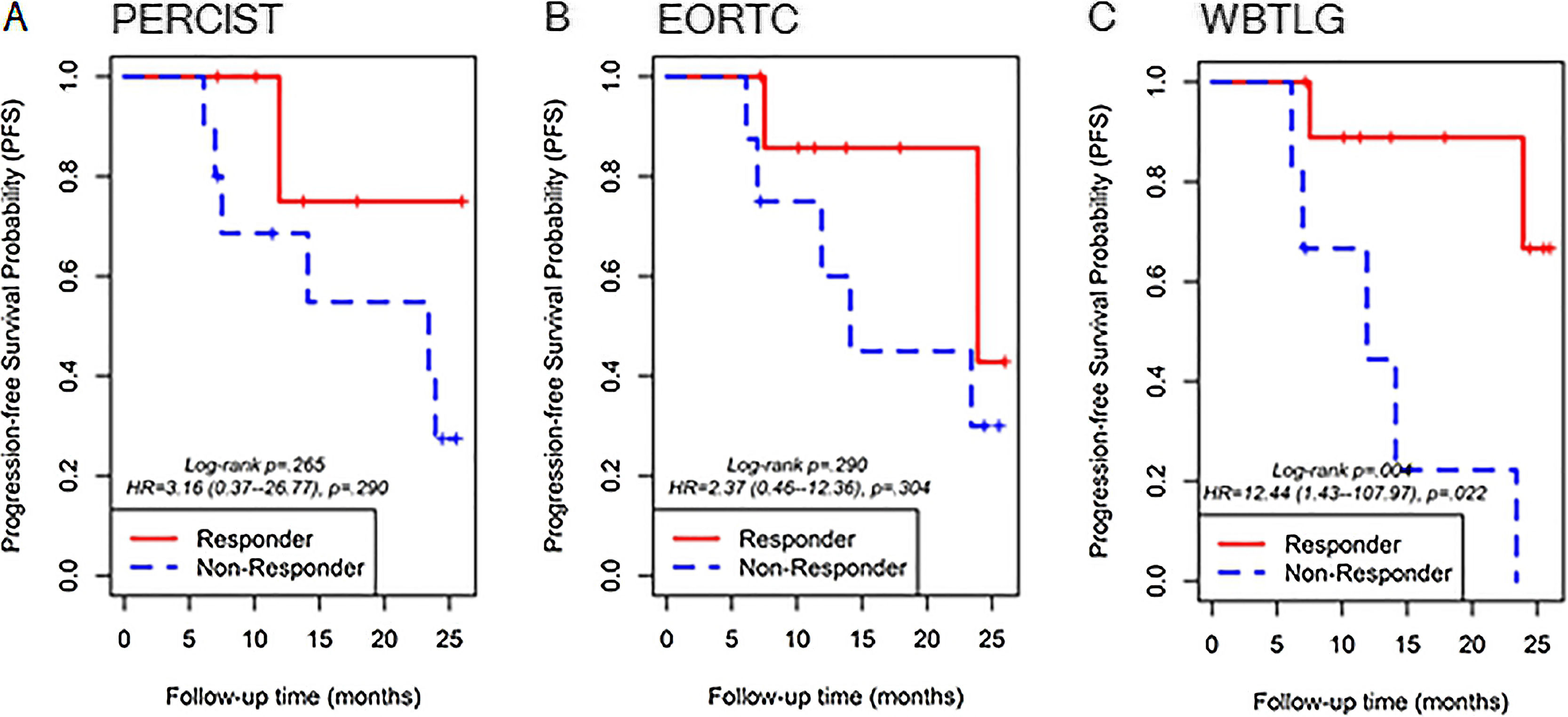
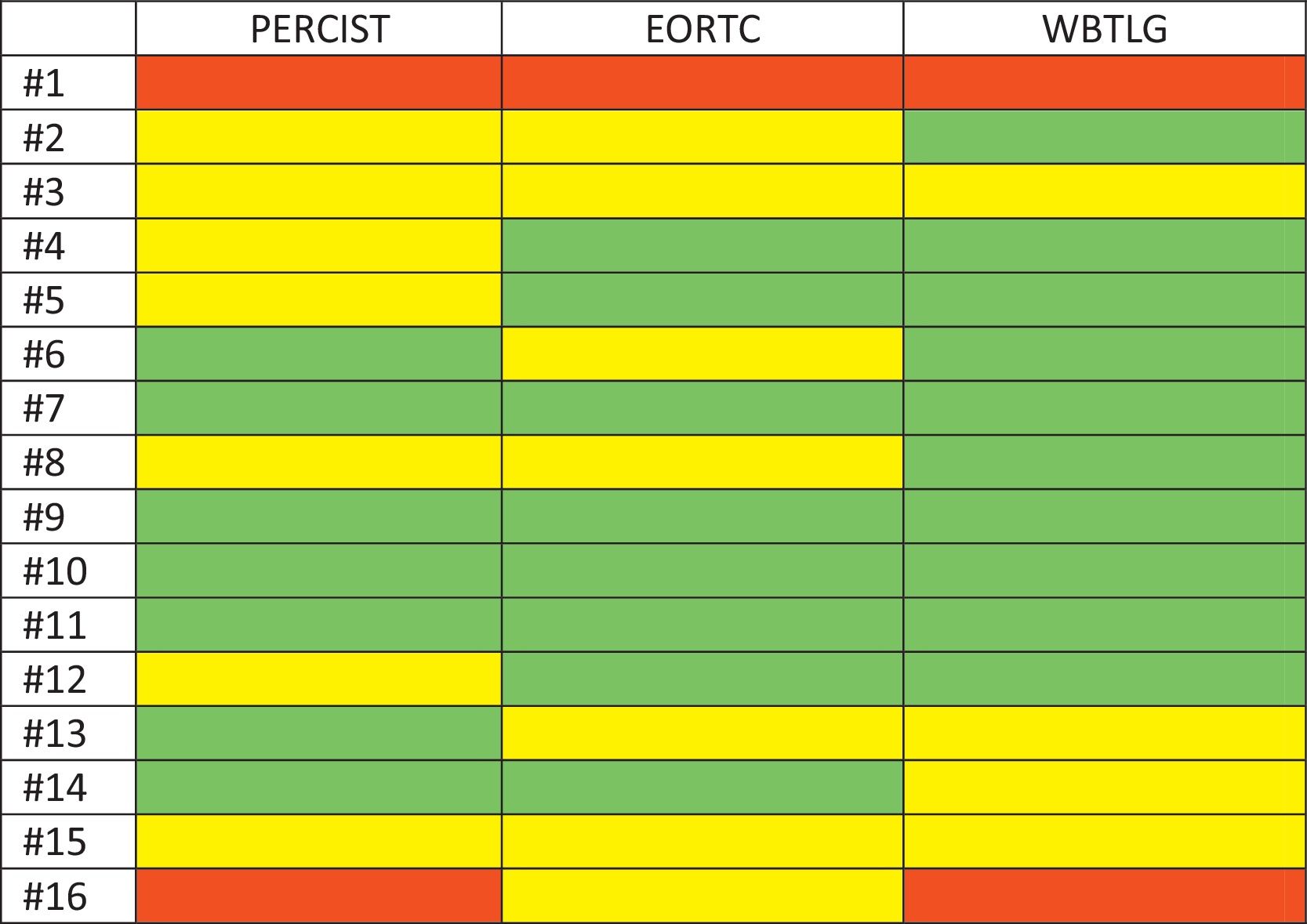
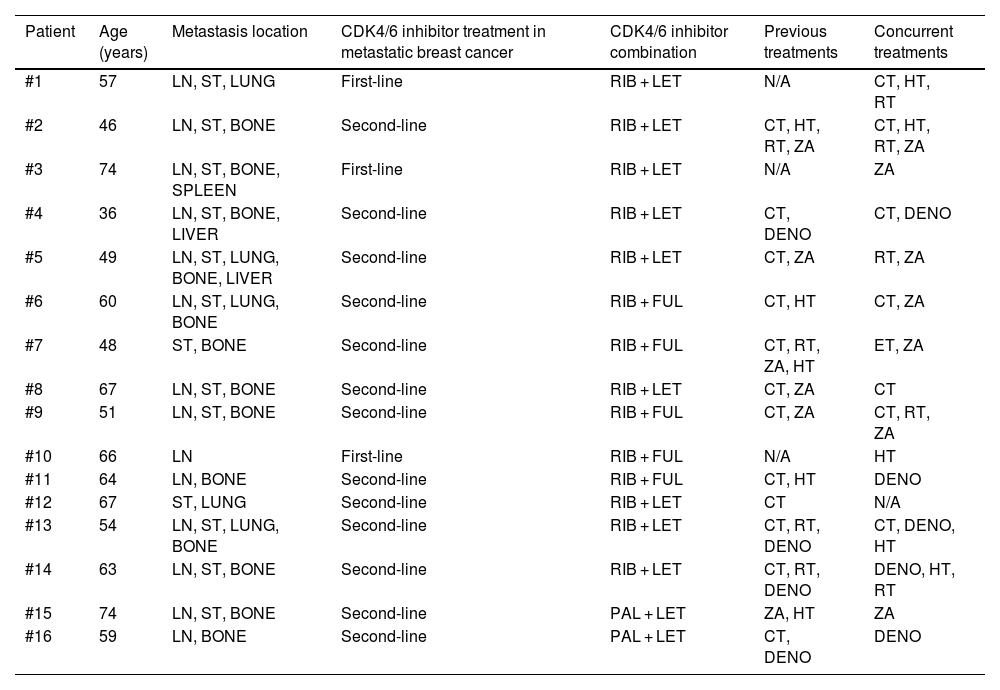
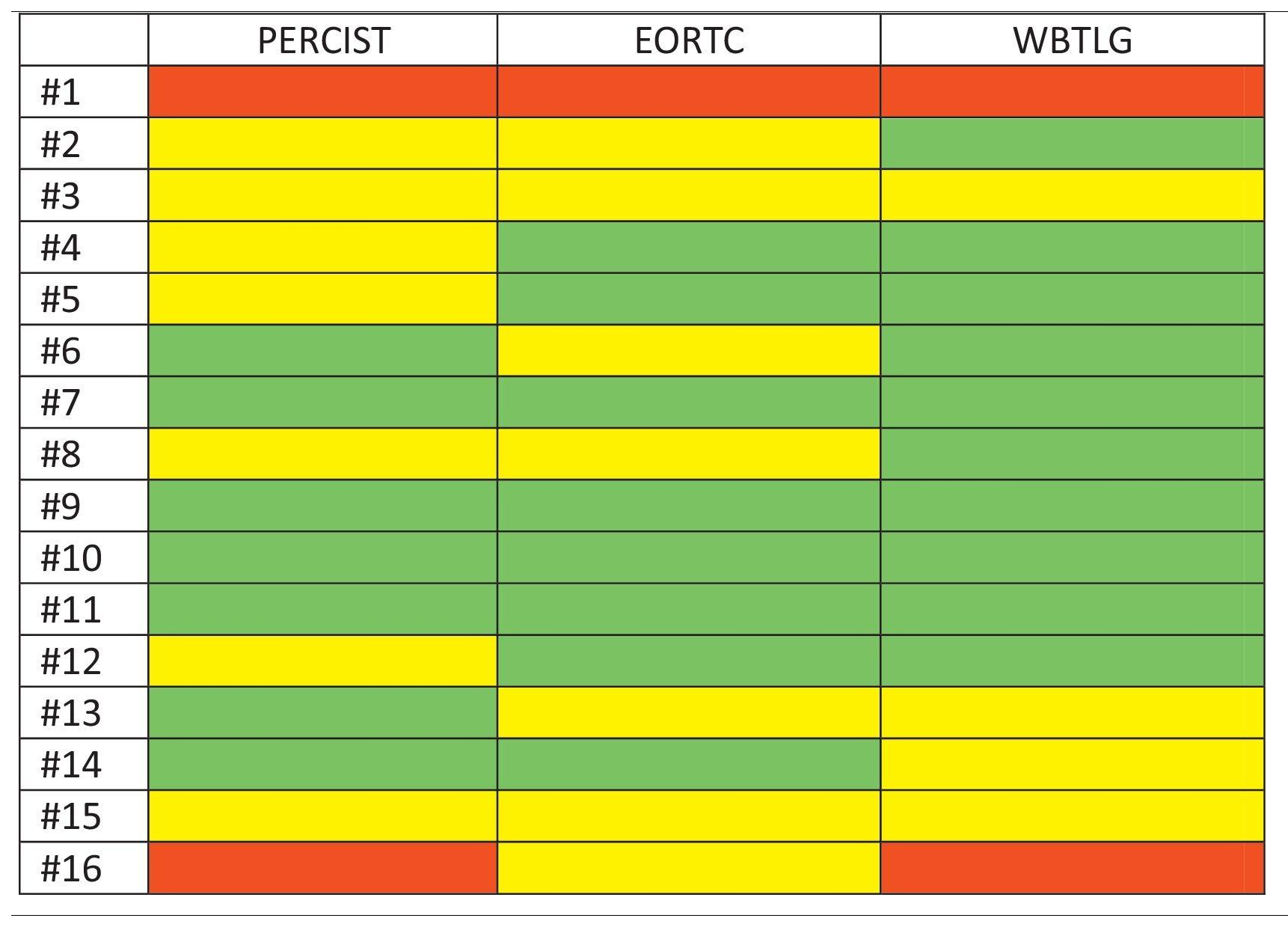
ResultsThe study included sixteen MBC patients who received CDK 4/6 inhibitors therapy. According to PERCIST, partial metabolic response (PMR) was found in seven patients, stable metabolic disease (SMD) in seven patients, and progressive metabolic disease (PMD) in two patients. According to EORTC, PMR was detected in eight patients, SMD in seven patients, and PMD in one patient. According to WBTLG, PMR was found in 10 patients, SMD in four patients, and PMD in two patients. There was a fair agreement between the three criteria. While progression was detected in seven of the patients during follow-up, no progression was detected in nine of them. Kaplan–Meier analysis revealed that the responders according to WBTLG showed significantly longer PFS than non-responders.
ConclusionTreatment response according to WBTLG criteria during treatment appears to be associated with prolonged PFS in patients treated with CDK 4/6 inhibitors for MBC.
Este estudio evalúa el papel pronóstico de los diferentes criterios de respuesta metabólica con [18F]FDG PET/CT en pacientes con cáncer de mama metastásico (CMM) tratados con inhibidores de la cinasa dependiente de ciclina 4/6 (CDK 4/6).
Material y métodoEvaluamos retrospectivamente los datos de pacientes con CMM tratados con inhibidores de CDK 4/6 que se sometieron a una exploración PET/TC [18F]FDG antes de comenzar y durante el tratamiento. La respuesta de la PET/TC con [18F]FDG se evaluó con los criterios de la Organización Europea para la Investigación y el Tratamiento del Cáncer (EORTC), los Criterios de respuesta de PET en tumores sólidos (PERCIST) y los criterios de gicólisis de lesión total de cuerpo entero (WBTLG). Para evaluar la concordancia entre los criterios de respuesta metabólica se calculó el Fleiss kappa. El criterio de valoración del estudio fue la supervivencia libre de progresión (SLP). Los datos de SLP se analizaron mediante el método de Kaplan–Meier y se compararon mediante la prueba de rango logarítmico.
ResultadosEl estudio incluyó a dieciséis pacientes con CMM que recibieron terapia con inhibidores de CDK 4/6. Según PERCIST, se encontró respuesta metabólica parcial (RMP) en siete pacientes, enfermedad metabólica estable (EME) en siete pacientes y enfermedad metabólica progresiva (EMP) en dos pacientes. Según la EORTC, se detectó RMP en ocho pacientes, EME en siete pacientes y EMP en un paciente. Según WBTLG, se encontró RMP en 10 pacientes, EMP en cuatro pacientes y EMP en dos pacientes. Hubo una buena concordancia entre los tres criterios. Si bien se detectó progresión en siete de los pacientes durante el seguimiento, no se detectó progresión en nueve de ellos. El análisis de Kaplan–Meier reveló que los que respondieron según WBTLG mostraron una SLP significativamente más larga que los que no respondieron.
ConclusiónLa respuesta al tratamiento según los criterios WBTLG durante el tratamiento parece estar asociada con una SLP prolongada en pacientes tratados con inhibidores de CDK 4/6 para CMM.
Article

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)