The MitraClipTM system has been recently approved for clinical use in Brazil for percutaneous treatment of mitral valve regurgitation. This device is based on the Alfieri surgical procedure, creating a double orifice by bringing together the central segments of the two mitral valve cusps. This report describes the first two procedures performed in Brazil using this device. Two female patients considered to be at high surgical risk due to advanced age and presence of comorbidities were treated, with degenerative mitral regurgitation due to prolapse/flail, associated with chordae tendineae rupture. In both cases, significant mitral regurgitation intensity reduction was obtained using the MitraClipTM, demonstrating the great potential of this innovative technology for the percutaneous treatment of mitral valve regurgitation.
O sistema MitraClip® foi recentemente aprovado para uso clínico no Brasil para o tratamento percutâneo da insuficiência valvar mitral. Esse dispositivo se baseia na cirurgia de Alfieri, criando um orifício duplo pela união central das duas cúspides da valva mitral. Descrevemos aqui os dois primeiros procedimentos realizados em nosso meio utilizando esse dispositivo. Tratam-se de duas pacientes do sexo feminino, consideradas de alto risco cirúrgico pela idade avançada e pela presença de comorbidades, portadoras de insuficiência mitral degenerativa por prolapso/flail associado à rotura de cordoalhas. Nos dois casos, obteve-se redução expressiva da intensidade da regurgitação mitral com a utilização do MitraClip®, demonstrando o grande potencial dessa tecnologia inovadora para o tratamento percutâneo da insuficiência valvar mitral.
Mitral valve regurgitation is one of the most common acquired valvular diseases, with a prevalence of approximately 7 to 10% in the population aged > 75 years.1,2 The treatment of mitral regurgitation is traditionally based on clinical management with medications, resynchronization, and especially valve repair or valve replacement surgery.3 However, despite guideline recommendations, approximately 50% of the patients are not treated surgically, due to the presence of high surgical risk caused by advanced age, left ventricular dysfunction, or comorbidities.4 For this reason, more recently the focus of research in this area has changed to the development of new and less invasive percutaneous devices for the treatment of mitral valve regurgitation. Among them, the MitraClipTM system (Abbott Vascular, Redwood City, USA) (Fig. 1) is one of the most promising. This device was approved for clinical use in Europe in March 2008, and in the United States in October 2013. To date, more than 25,000 patients have already benefited from this new treatment modality, predominantly used in individuals at high surgical risk. In Brazil, the MitraClipTM was approved for use in the end of 2014. This report describes the first two procedures performed in Brazil for percutaneous treatment of mitral valve regurgitation using the MitraClipTM device.
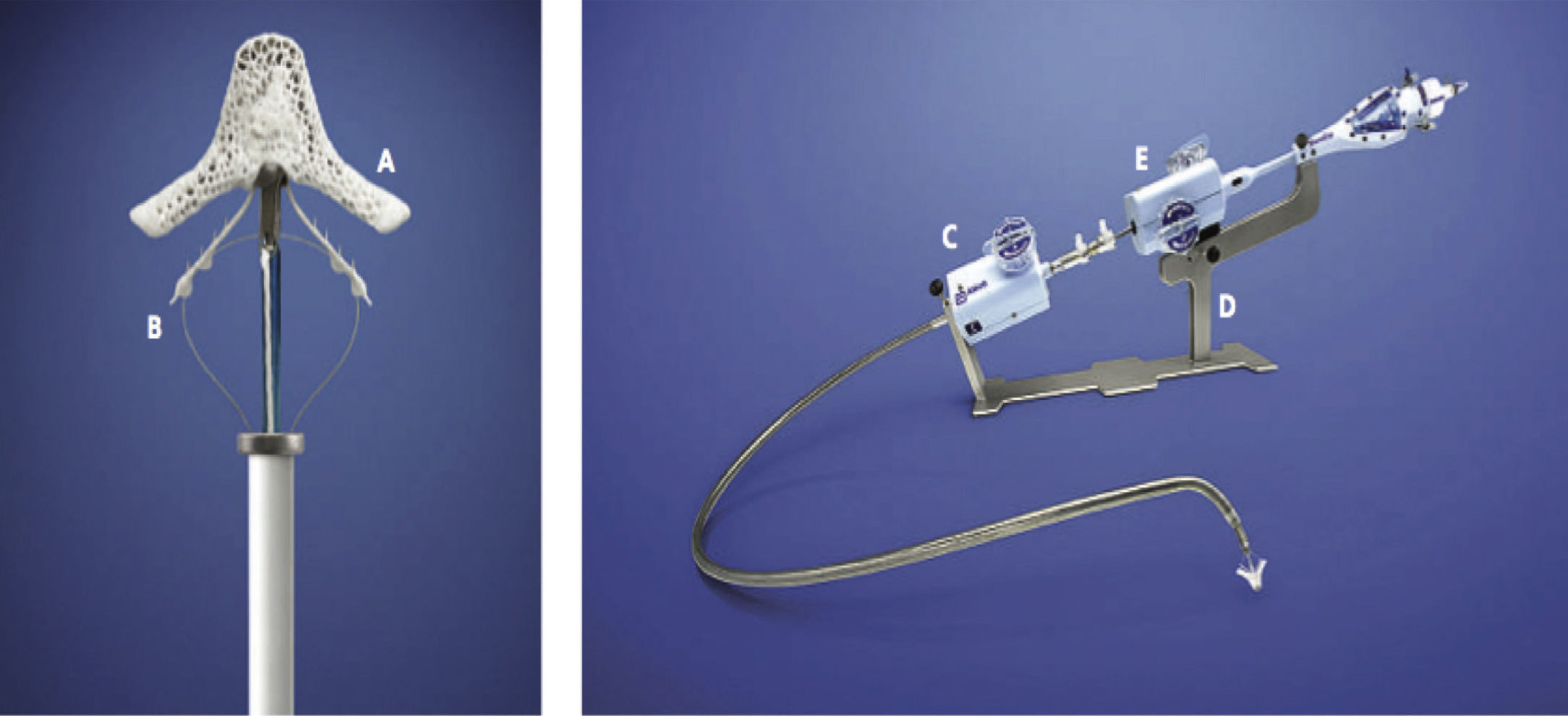
MitraClipTM Device (left) and its delivery system (right). Each arm (A) of the clip is 4mm wide and 8mm long. The clips (B) are used to hold the cusps of the mitral valve in the clip arms. The steerable catheter is a 24 F and has shunters (C and E) to guide and correctly position the clip. The stabilizer (D) is used to support the MitraClipTM delivery system.
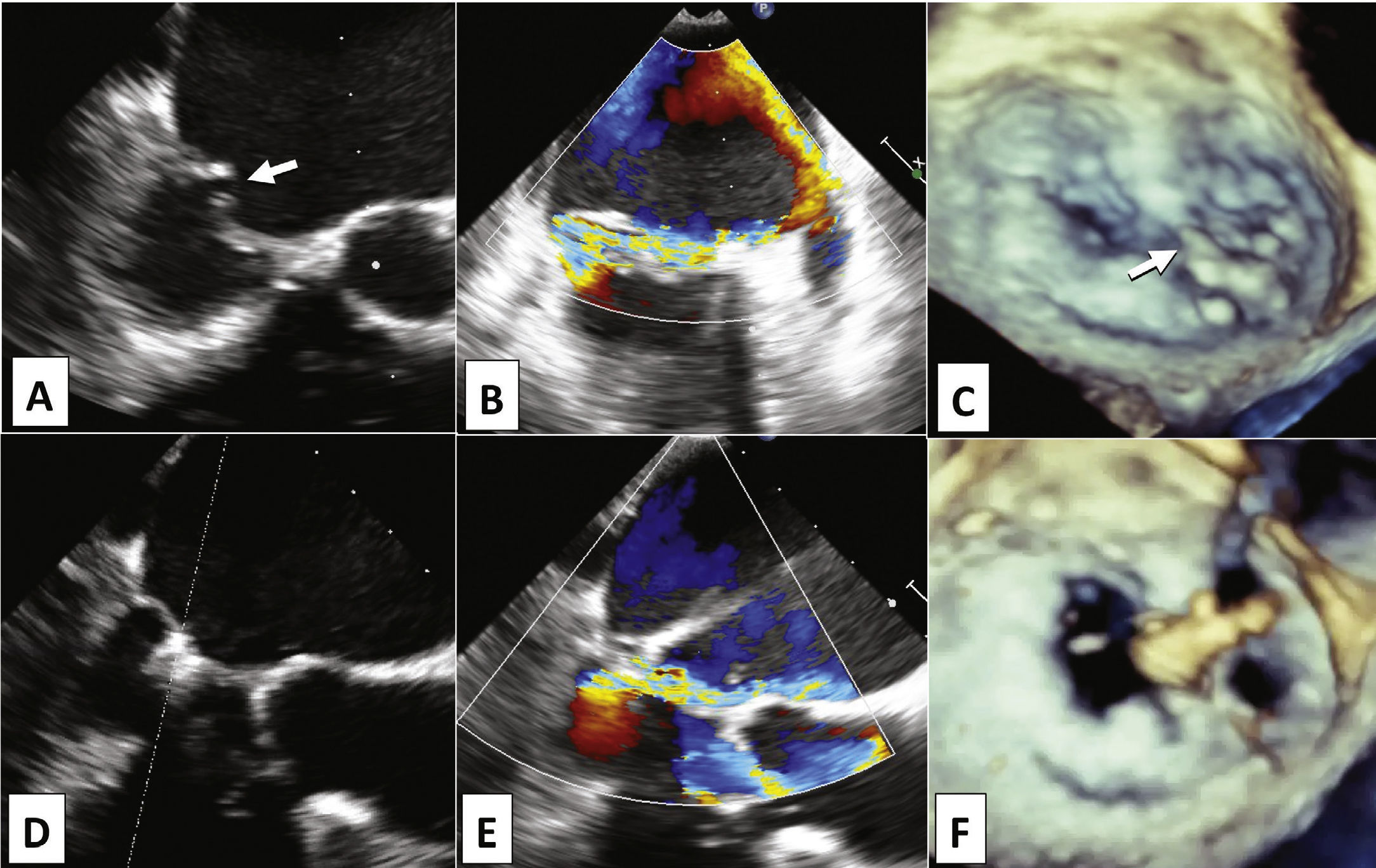
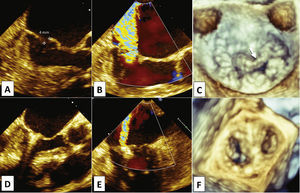
Female patient, 97 years old, admitted to Hospital Israelita Albert Einstein, in São Paulo (SP), due to sudden onset of congestive heart failure, functional class IV. Evaluations by transthoracic echocardiography (TTE) and subsequently by two-dimensional transesophageal echocardiography (TEE) showed severe mitral valve regurgitation (4+/4+), due to mitral valve posterior leaflet prolapse (segments P2 and P3) associated with chordae tendineae rupture (P2 segment), resulting in extensive coaptation gap (Figs. 2A to 2C). The left atrium measured 48mm, and systolic blood pressure in the pulmonary artery was estimated at 53mmHg. Left ventricular function estimated by ejection fraction was normal. Despite the prolonged hospital stay (45 days) for drug treatment optimization, the patient had repeated episodes of acute pulmonary edema, requiring intermittent intensive care and characterizing treatment resistance. Due to the advanced age and comorbidities such as renal failure, the medical team considered the surgical risk to be unacceptable. Estimates of surgical mortality by logistic EuroSCORE and Society of Thoracic Surgeons (STS) risk score were 21.1 and 18.4%, respectively. For this reason, the percutaneous transseptal mitral valvuloplasty with MitraClipTM device was indicated.
Transesophageal echocardiography pre- (A, B, and C) and post-procedure (D, E, and F). Note the coaptation gap between the cusps (A, arrow) due to prolapse of segments P2 and P3, and chordae tendineae rupture in P2 (C, arrow) causing eccentric anterolateral regurgitation (B). After MitraClipTM implantation in the medial border of P2 (D and F), there was significant reduction of regurgitation (E) and formation of double mitral orifice, with the characteristic “8” image in the three-dimensional echocardiography (F).
The procedure was performed in a hybrid operating room on January 6, 2015, with the patient under general anesthesia, and guided by three-dimensional TEE. Heparin was administered at a dose of 10,000 IU, aiming to achieve an activated clotting time of 300 to 350seconds. Right femoral venipuncture was performed, followed by transseptal puncture to obtain access to the left atrium. A 24 F MitraClipTM system catheter was introduced in the left atrium, being directed to the mitral valve aided by fluoroscopy and three-dimensional TEE. Several two- and three-dimensional TEE images were used to achieve adequate positioning of the clip, perpendicular to the mitral commissure and over the regurgitation jet. The clip was then advanced into the left ventricle, with open arms. Small additional adjustments in the clip position were guided by two- and three-dimensional TEE.
When optimal positioning was obtained, the clip was closed, capturing equivalent portions of the mitral valve cusps (Fig. 2D). An immediate reduction was observed in mitral regurgitation intensity, from 4+/4+ to 2+/4+, observing the characteristic image of double mitral orifice (Figs. 2E and 2F). The mean transvalvular pressure gradient was 4mmHg.
The clip was released from the delivery system. The catheter was removed, and hemostasis was achieved with a single Perclose ProGlideTM (Abbott Vascular, Redwood City, USA) device applied to the puncture site in the femoral vein. The procedure time (clip time) was 95minutes. A total of 50mL of iodinated contrast was used to perform the baseline left ventriculography, and after the procedure, the patient was extubated and taken to the intensive care unit. Worsening of renal function ensued, requiring hemodialysis. There was significant improvement in heart failure symptoms and the TTE performed 1 and 4 days after the procedure disclosed only mild mitral regurgitation (1+/4+). On the fourth day after the intervention, when the patient was progressing with significant heart failure symptom improvement, she suddenly developed cardiogenic shock and respiratory failure due to massive pulmonary embolism, and subsequently died.
Case 2Female patient, 93 years old, with hypertension, diabetes, obesity, and hypothyroidism, admitted to the emergency department of Hospital Pro-Cardíaco, in Rio de Janeiro (RJ), with functional class IV progressive congestive heart failure, which had started 45 days before. On admission, atrial fibrillation of unknown duration was observed.
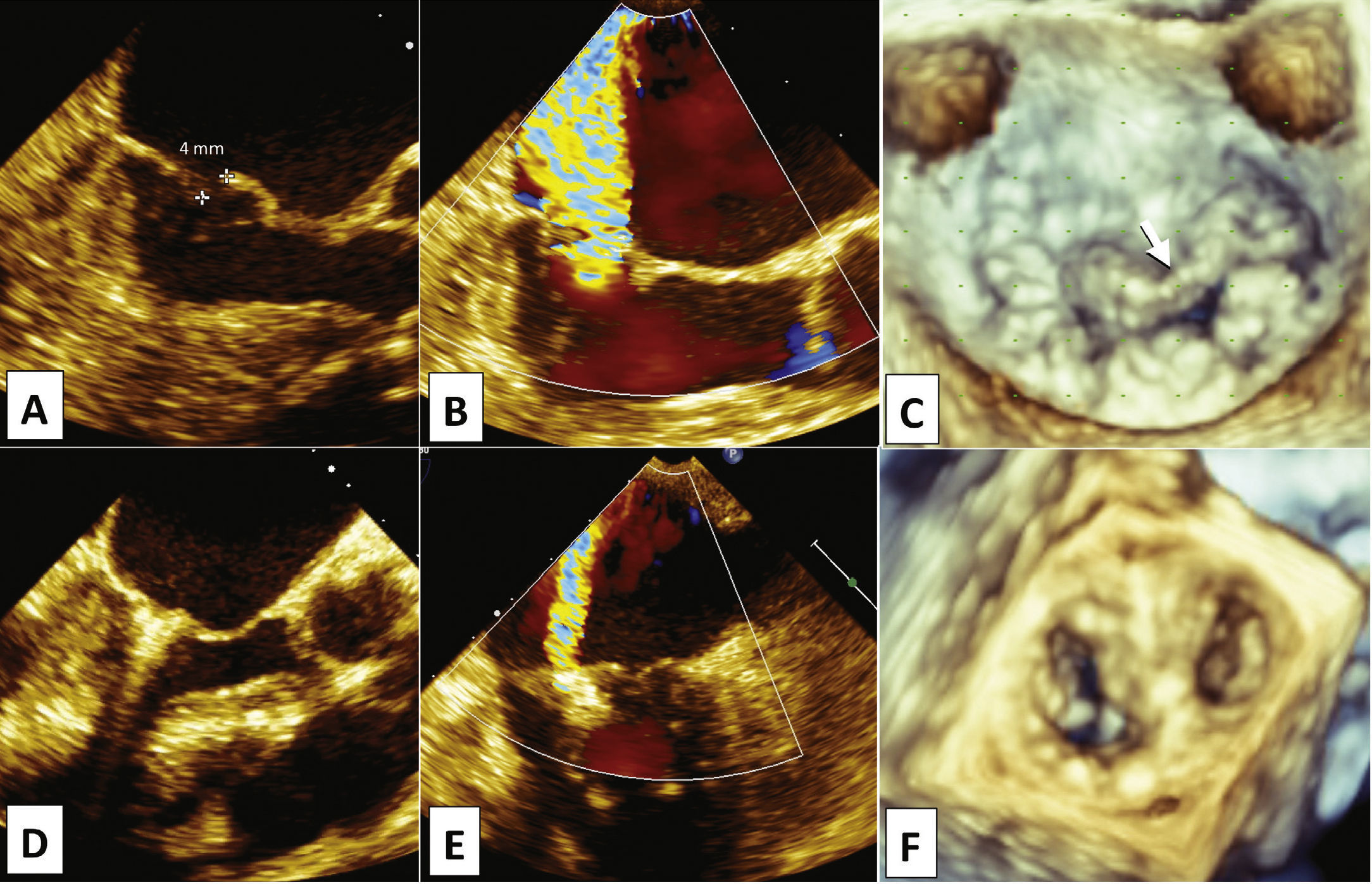
Since 2006, the patient had a diagnosis of mild mitral valve regurgitation due to prolapse. Evaluations by TTE and subsequently by TEE performed during hospitalization disclosed the presence of severe mitral valve regurgitation (4+/4+) with Coanda effect, due to prolapse of both cusps, associated with chordae tendineae rupture and A2 flail (Figs. 3A to 3C). The left atrium measured 50mm, and mean systolic blood pressure in the pulmonary artery was estimated at 50mmHg. Left ventricular function estimated by ejection fraction was normal.
Transesophageal echocardiography pre- (A, B, and C) and post-procedure (D, E, and F). Prolapse of both cusps, associated with chordae tendineae rupture and A2 flail (A and C, arrow), generating significant posterior eccentric regurgitation (B), due to 4-mm flail gap. After the implantation of one clip (D), significant reduction in regurgitation (E) and formation of the double mitral orifice (F) were observed.
She was submitted to several unsuccessful attempts at clinical compensation, despite the use of furosemide in continuous infusion, in addition to inotropic (milrinone) and vasodilators. The patient developed anasarca, severe hyponatremia, and hypokalemic metabolic alkalosis, with continuous ultrafiltration and non-invasive ventilation being indicated. Due to her advanced age and comorbidities such as renal failure, she was considered as a high surgical risk patient by the medical team, with mortality estimated by logistic EuroSCORE and STS risk score of 49.3 and 48%, respectively. For this reason, percutaneous transseptal mitral valvuloplasty with the MitraClipTM device was indicated.
The procedure was performed in a hybrid operating room on January 16, 2015, with the patient under general anesthesia, and guided by TEE. The same technique described for the previous case was employed, using a single clip. An immediate reduction in mitral regurgitation intensity was obtained, from 4+/4+ to 2+/4+, creating the double mitral orifice (Figs. 3D to 3F).
The mean transvalvular pressure gradient was 4mmHg. After catheter removal, hemostasis was obtained by manual compression. The procedure time (clip time) was 70minutes. Iodinated contrast was not used in this procedure. The patient was extubated and taken to the intensive care unit. She had a slow, but progressive recovery of spontaneous diuresis and stabilization of nitrogenous waste. Weaning of vasoactive amines was carried out, as well as of dialytic ultrafiltration.
The TEE performed 12 days after the procedure disclosed the presence of mild mitral regurgitation (1+/4+). She was discharged 15 days after the mitral clip implantation, walking with assistance and receiving oral medications: amiodarone, bisoprolol, furosemide, spironolactone, rivaroxaban, and pregabalin. At the 6 month follow-up, the patient was in functional class II.
DiscussionThe MitraClipTM system was recently approved for clinical use in Brazil for the percutaneous treatment of functional or degenerative mitral valve regurgitation. This device is based on the Alfieri procedure, which creates a double orifice by placing a suture between the A2 and P2 segments of the mitral valve cusps.5 The randomized clinical trial EVEREST II (Endovascular Valve Edge-to-Edge Repair Study) demonstrated the safety and the efficacy of MitraClipTM in selected cases, with results maintained up to the 4 year follow-up.6,7 In that study, the percutaneous repair was less effective to reduce the mitral regurgitation intensity than conventional surgical treatment. However, it was at least as safe as the surgical approach, with equivalent rates of death, infarction, and stroke, in addition to less need for blood transfusions. Additionally, the percutaneous treatment determined an equivalent improvement in heart failure symptoms and quality of life.6,7 Therefore, it has become an excellent alternative for the treatment of mitral valve regurgitation in high surgical risk patients, as in the two cases reported here, representing a pioneering experience in Brazil. These two patients had degenerative mitral regurgitation, due to prolapse/flail, associated with chordae tendineae rupture. In both cases, a significant reduction in mitral regurgitation intensity was obtained with the use of a single clip. The control echocardiograms performed within the first days after the procedure confirmed treatment efficacy, with mild residual mitral regurgitation in the two patients. The first patient died on the fourth day after the procedure due to massive pulmonary embolism, probably due to prolonged immobility in the hospital bed, despite the anticoagulation scheme started 2 days after the intervention. There is no report in the literature of this complication associated with the mitral clip use. The second patient showed significant clinical improvement and was in functional class II at the 8 month clinical follow-up.
It is noteworthy that knowledge of mitral valve anatomy and the interaction between the interventional cardiologist and the echocardiographist are crucial to procedural success, as this intervention is guided, almost entirely, by the images generated by the two- and three-dimensional TEE. There is no need to use iodinated contrast with the MitraClipTM system. Hemorrhagic (cardiac tamponade) and embolic (stroke) complications, although rare, can occur due to transseptal puncture and left atrial manipulation with thick-caliber catheters.8 Constant checking of anticoagulation levels (target activated clotting time between 300 and 350seconds) during the procedure is essential to prevent embolic complications.
Although in both cases reported here the MitraClipTM was used for the treatment of degenerative mitral regurgitation, its use for the treatment of functional mitral regurgitation is currently more frequent.9,10 Therefore, the MitraClipTM should be considered as an alternative to conventional surgical treatment in selected patients with degenerative or functional mitral regurgitation, especially when the surgical risk is high due to advanced age and the presence of comorbidities or significant left ventricular dysfunction.11 Some anatomical conditions, previously considered contraindications to the procedure in the EVEREST study, have recently become more acceptable for the MitraClipTM approach with the increasing experience of surgeons. However, some restrictions remain, among which are: significant calcification in the target location for clipping (cusp edge); degenerative lesions caused by rheumatic disease and endocarditis, due to severe deformity and cusp lesion; posterior cusp length ≤ 7mm; coaptation gap > 5mm between the cusp borders; gap ≥ 10mm between the borders of the anterior and posterior cusps caused by flail (flail gap).
In conclusion, the two cases reported in this article have shown the great potential of this innovative technology for percutaneous treatment of mitral valve regurgitation, which should be increasingly used in Brazil. The near future will bring the introduction of other technologies, including annuloplasty devices and prostheses for transcatheter prosthesis implantation, expanding the horizons of interventional cardiology and benefiting an even greater number of patients.
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the technical support provided by Mr. Flavio Toledo (Abbott). His extensive knowledge of the MitraClipTM system significantly contributed to the success of these procedures.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.