Bovine respiratory syncytial virus (BRSV) affects both beef and dairy cattle, reaching morbidity and mortality rates of 60–80% and 20%, respectively. The aim of this study was to obtain a recombinant MVA expressing the BRSV F protein (MVA-F) as a vaccine against BRSV and to evaluate the immune response induced by MVA-F after systemic immunization in homologous and heterologous vaccination (MVA-F alone or combined with a subunit vaccine), and after intranasal immunization of mice. MVA-F administered by intraperitoneal route in a homologous scheme elicited levels of neutralizing antibodies similar to those obtained with inactivated BRSV as well as better levels of IFN-γ secretion. In addition, nasal administration of MVA-F elicited local and systemic immunity with a Th1 profile. This study suggests that MVA-F is a good candidate for further evaluations combining intranasal and intramuscular routes, in order to induce local and systemic immune responses, to improve the vaccine efficacy against BRSV infection.
El virus respiratorio sincicial bovino (BRSV) afecta al ganado bovino de cría y lechero, con niveles de morbilidad del 60-80% y de mortalidad del 20%. El objetivo de este trabajo fue obtener virus MVA recombinantes que expresen la proteína F de BRSV (MVA-F) como candidato vacunal contra BRSV y evaluar la respuesta inmune inducida por dicho virus luego de administrarse por vía sistémica, tanto en esquemas de vacunación homóloga como heteróloga (MVA-F solo o combinado con una vacuna a subunidad proteica), y luego de su inoculación intranasal en ratones. La administración de MVA-F por vía intraperitoneal en un esquema homólogo indujo niveles de anticuerpos neutralizantes similares a los obtenidos con una vacuna inactivada de BRSV, así como niveles superiores de secreción de IFN-γ. Además, la inoculación intranasal de MVA-F indujo inmunidad local y sistémica, con un perfil de respuesta de tipo Th1. Este estudio sugiere que MVA-F es un buen candidato para continuar su evaluación combinando las vías nasal e intramuscular para de este modo inducir respuesta local y sistémica y así mejorar la eficacia contra la infección por BRSV.
Bovine respiratory syncytial virus (BRSV) plays a predominant role in bovine respiratory disease (BRD), with a direct impact on animal welfare and cattle industry performance. BRSV affects both beef and dairy cattle16 and is responsible for morbidity rates of 60–80% and mortality rates of 20%, particularly in young calves36. In addition, BRSV infection predisposes to secondary bacterial infections causing pneumonia outbreaks35. The virus is transmitted by direct contact between infected and non-infected animals. Environmental stressors and intensification practices (such as crowding conditions, changes in the diet and transport) can increase infection rates13,29,33. BRSV is endemic worldwide. In North America it has been identified as the leading cause of respiratory disease in calves20. In addition, several European and South American countries reported high prevalence of BRSV infection13,36. Strategies to control infection should include both good management practices within herds and vaccination programs against the virus.
Several licensed vaccines against BRSV are available, both inactivated or live attenuated, in monovalent formulations or combined with other agents from the BRD complex. However, none of them are fully protective. In fact, on the one hand, anti-BRSV maternal antibodies interfere with vaccine response in calves10,11,18. On the other hand, inactivated and live vaccines may cause severe enhanced respiratory disease (ERD)2. Several studies in mice and calves suggested that disease exacerbation resulted from a Th2 biased immune response through increased IL-4 production, high levels of IgE and lack of anti-BRSV-specific CD8+ T cells, resulting in pulmonary eosinophilia23. In this sense, live vaccine MVA-vectors mainly induce Th1 biased immune responses17 and when rationally designed, they do not contain pathogenic components of BRSV eliminating the risk of ERD27. In this context, the development of recombinant (subunit and viral vectored) immunogens against BRSV can be efficacious to prevent the disease while eliminating the undesirable effects of conventional vaccines.
Most recombinant vaccines against BRSV are based on the expression of the fusion (F) protein. This protein is highly conserved among different strains of RSV, is the main target of neutralizing antibodies and induces protective responses in different animal models21.
Modified vaccinia Ankara virus (MVA) constitutes an excellent platform to develop rationally designed viral vectored vaccines due to its safety profile, its capability to induce humoral and cellular immune responses and its intrinsic adjuvant capacities1,38. It has been extensively used as a viral vector for human and veterinary vaccines28.
Thus, in this study we generated a recombinant MVA expressing the BRSV F protein (MVA-F) and characterized its immunogenicity after systemic and mucosal immunization of mice.
Materials and methodsCell lines and viral stocksMVA viral stocks were propagated in primary cultures of chicken embryo fibroblasts (CEFs), and titrated as described elsewhere14. Viral immunization stocks were purified by ultracentrifugation through a 25% v/v sucrose cushion.
BRSV A51908 strain (ATCC, variant Atue51908, GenBank accession no. AF092942) was grown using Madin-Darby bovine kidney (MDBK) cells (ATCC) at a low multiplicity of infection13. Five days post infection (dpi), infected cells were collected, frozen, thawed, clarified and stored at −70°C until use.
Construction of recombinant MVA-FFirstly, the complete coding sequence of BRSV fusion protein (F) was amplified by RT-PCR from total BRSV RNA (strain A51908), using the primers 5′ GCTAGCATGGCGACAACAGCCATGAGGATG and F-r 5′ AGGCCTTTATATGGAGGTGTGTTGTTAG. These primers contain NheI and NcoI restriction enzymes sites, respectively (underlined sequence), to facilitate subcloning of the F gene into the transfer vector (TV) obtained elsewhere14. The TV contains the restriction enzyme sites downstream of the poxviral synthetic early-late promoter (pE/L, which will control the expression of the F gene) and codifies for the β-glucuronidase enzyme (GUS) under the regulation of the promoter of the vaccinia virus H6 gene. Both transcriptional units (pE/L-F and pH6-uid A) are flanked by viral genomic regions of the MVA086R gene [which codes for the thymidine kinase (TK) enzyme] to allow in vivo recombination with the poxviral genome.
MVA-F was generated by transfecting TV-F into CEFs previously infected with MVAD008-βgal (referred as MVA throughout the text). This recombinant virus, which was obtained in a previous study12, lacks the MVA008L gene that codes for the interleukin 18 binding protein. Screening and plaque lysis purification of MVA-F were performed using β-glucuronidase substrate.
Molecular characterization of recombinant MVA-F virusTotal DNA was extracted from uninfected or MVA and MVA-F infected CEFs using extraction buffer 2× (200mM Tris pH 8, 20mM EDTA, 200mM NaCl, 2% SDS and 20mM β-mercaptoethanol). Purity of the recombinant virus was achieved after 10 plaque-purification rounds and assessed by PCR using TK1 (5′-TCCCCGCGGTGAACGGCGGACATATTC-3′) and TK4 (5′-GGGGTACCTTATGAGCCGACGTAACA-3′) primers that specifically amplify the MVA086R sequence (the site of foreign gene insertion)17. The PCR cycling conditions (T° denaturation at 94°C for 1min, T° annealing at 58°C for 30s, T° elongation at 72°C for 30s, 30 cycles) favored the amplification of a 550bp fragment (MVA086R gene) from the wild-type genome rather than the 4500bp amplicon from the recombinant virus genome (comprising MVA086R+uidA+F gene).
The presence of the F gene was confirmed by PCR amplification of a 481bp (126–581 nt) fragment using B3 (5′-GTGCAGTTAGTAGAGGTTATCTTAGT-3′) and B4A (5′-TAGTTCTTTAGATCAAGTACTTTGCT-3′) primers37.
Transcription of the F gene was evaluated by RT-PCR. Total RNA from uninfected or infected CEFs was extracted using TRIzol® (Thermo Scientific™) and reverse-transcribed with the reverse transcriptase M-MLVRT (200U, Promega) using random hexamers. The cDNA obtained was used to amplify by PCR a fragment of 711bp corresponding to an internal region of the F gene (114–803 nt). The primers used were B1 5′-AATCAACATGCAGTGCAGTTAG-3′ and B2A 5′-TTTGGTCATTCGTTATAGGCAT-3′37.
Genetic stability of MVA-F was assayed after ten passages of the pure recombinant virus in cell culture. Then, PCR and RT-PCR analyses were performed to evaluate the absence of wild-type genomic DNA and to confirm the transcription of the F gene, respectively.
Recombinant Ft productionRecombinant baculovirus expressing a truncated version of the BRSV F protein that lacks the membrane anchorage region (Ft), were previously obtained in our laboratory (unpublished data). For protein production for immunization, Sf9 cells (0.5×106 cells in T175 cell culture flasks) were grown at 27°C in TNM-FH medium (SIGMA) supplemented with 10% fetal bovine serum (FBS) and antibiotic-antimycotic solution (GIBCO). Twenty-four hours later, the medium was removed, cells were washed and infected at 1.5 MOI with the recombinant baculovirus using a serum and protein free medium (Sf-900TM, Thermo Fisher Scientific). At 3 days post infection, the supernatant of infected cells was harvested, clarified (5min at 300g), aliquoted and conserved at −80°C until used. Total protein concentration was quantified by the Bradford protein assay (Thermo Fisher Scientific) and then, recombinant Ft concentration was estimated by Western blot using an anti-His antibody (GeneScript). The intensity of the band obtained with the recombinant Ft was compared with the intensity of the signal of known amounts of BVDV E2 recombinant protein carrying a Histidine tag (ImageJ software).
Animal experiments and sampling procedureFemale BALB/c (H-2d) mice (6–8 weeks old), certified as specific pathogen-free, were purchased from Fundación Facultad de Ciencias Veterinarias (UNLP, La Plata, Argentina) and maintained in animal facilities at the IABiMo INTA-CONICET. All experiments were performed following international welfare guidelines with the approval of the Comité Institucional para el Cuidado y Uso de Animales de Experimentación, CICUAE-CNIA, INTA, Argentina (CICUAE-INTA 52-2014 and 01-2017). Isoflurane and CO2 inhalation were used as anesthetic and sacrifice method respectively to minimize mice suffering.
Intraperitoneal (IP) immunizationGroups of 5 BALB/c mice were immunized via the IP route with three doses containing 1ml of the corresponding immunogen on days 0, 21 and 44. For the homologous approach, animals were inoculated with 3×106 PFU/dose of viral vectors or 300ng/dose of Ft protein. For the heterologous scheme, mice were sequentially vaccinated with MVA-F (day 0), recombinant Ft protein (day 21) and MVA-F (day 44). Control groups were inoculated with sterile PBS or BEI inactivated BRSV (4.7×104TCID50/dose). Inactivated BRSV and the Ft protein were formulated in oil adjuvant (MONTANIDE ISA 50 V2, Seppic, in a ratio of 40% antigen/60% adjuvant). Blood samples were collected at 0, 19, 35 and 53 days post immunization (dpi) to determine specific anti-BRSV antibodies. At day 53, mice were sacrificed, and spleen cells were obtained to evaluate IFN-gamma (IFN-γ) secretion. Two independent experiments were performed.
Intranasal (IN) immunizationGroups of 5 BALB/c mice (H-2d) were immunized on days 0 and 21 via the IN route with 5.6×105 PFU of purified MVA or MVA-F. A control group inoculated with PBS was included in the study. Blood samples were collected on days 14 and 35 dpi to determine serum anti-BRSV antibodies. At 35 dpi, animals were sacrificed and nasal washes were taken as described previously14. Two independent experiments were performed.
Detection of anti-BRSV antibodies by ELISASpecific anti-BRSV serum antibodies were determined using the INgezim BRSV Compac kit (INGENASA, Spain) following the manufacturer's instructions. Briefly, serum samples were incubated in a mix with a monoclonal antibody anti-BRSV HRP-conjugated in a microplate coated with inactivated BRSV. Then, TMB (3,3,5,5-tetramethylbenzidine) was added and the reaction was stopped after 10min with the stop solution; the absorbance was determined at 450nm in a spectrophotometer (Multiskan, Labsystems, Basingstoke Hants, U.K.). Since this is a competition ELISA, the lower absorbance detected indicates the higher level of specific anti BRSV antibodies in the sample. Positive and negative controls (PC and NC, respectively) provided by the manufacturer were included in each assay. The cut-off value was calculated using the following formula: NC−[(NC−PC)×0.40].
Nasal IgA and serum isotypes (IgG1 and IgG2a) anti-BRSV were evaluated using an indirect ELISA adapted from Kovarcik et al.19 Briefly, BRSV was concentrated by ultracentrifugation, purified with a discontinuous sucrose gradient (30–55%) and stored at −70°C until used8. Then, microplates were coated with purified BRSV and samples were added in the appropriate dilution. The following antibodies were used in this study: (i) anti-IgA (Bethyl) and a secondary antibody HRP-conjugated; (ii) anti-IgG1 and anti-IgG2a biotin conjugated (CALTAG) and HRP-conjugated streptavidin. Reactions were developed by adding 0.4mg/ml ABTS (2-20-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid; ICNBiochemicals) and 0.0015% H2O2 in 50mM citric acid buffer (pH 5). The reactions were read at 405nm in a Multiskan spectrophotometer.
Determination of BRSV neutralizing antibodiesThe serum neutralization assay (SNA) was conducted as described previously by Ferella et al.13 Briefly, inactivated serum samples were 4-fold diluted and then incubated with 100 TCID50 of A51908 BRSV strain for 1h at 37°C in a 5% CO2 atmosphere. This mixture was inoculated in duplicate onto MDBK cell monolayers in 96-well plates and the cytopathic effect (CPE) was observed 5 days post inoculation (dpi). The neutralizing antibody (NA) titer was expressed as the reciprocal of the maximal dilution in which no CPE was observed. The samples with titers below 4 were considered negative.
Lymphocyte isolation and IFN-γ detection by ELISASpleen cells were recovered from vaccinated BALB/c mice, resuspended in RPMI containing 10% FCS (Natocor, Argentina) and cultured in triplicate (106cells/well) into 96-well microtiter flat-bottom plates. Stimulation of splenocytes was performed with 2×104 TCID of UV inactivated BRSV (specific stimulus). Because BRSV was produced in MDBK cells, stimulation with uninfected MDBK cells (mock stimulus) was included to detect a possible non-specific reaction with cellular antigens. Concanavalin A (0.25μg/ml) and RPMI culture medium stimulation were included as positive and negative controls respectively. Plates were incubated for 72h and the level of secreted IFN-γ was determined by a capture ELISA following the manufacturer's recommendations (OptEIA™ kit, BD Labware).
Statistical analysisStatistical analysis was performed using GraphPad Prism 5 for Windows (version 5.01 2007, La Jolla, CA). Total and NA levels in serum were analyzed using two-way ANOVA (repeated measures) and Bonferroni's post-test. Analysis of data obtained from IFN-γ secretion was performed using one-way ANOVA and Bonferroni's multiple comparison test. For IgA antibodies in nasal washes, the Kruskal–Wallis test and the Dunn's multiple comparison test were used. Values of p<0.05 were considered significant.
ResultsConstruction and in vitro characterization of recombinant MVA-FTo obtain a vaccine candidate against BRSV, we constructed a recombinant MVA containing the complete coding sequence of the fusion (F) protein of BRSV, interrupting the MVA086R viral gene (TK) in the backbone of the MVAD008 vector (Fig. 1A).
(A) Schematic representation of the MVA genome. MVA-F contains the F gene and a marker gene uid A (GUS) interrupting the viral TK gene (the right – r and left – l regions are shown). Primers used for the molecular characterization are indicated with arrows of different colors. (B and C) PCR amplification using specific primers for TK [B, primers used shown in red in (A)] and F [C, primers used shown in blue in (A)] genes using DNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs; TK+: transfer vector containing TK gene; F+: transfer vector containing F gene; M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®); (D) corroboration of expression of F RNA by RT-PCR [primers used shown in green in (A)] using total RNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs. M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®).
First, we isolated the recombinant virus by plaque-purification based on its ability to produce blue lysis plaques in the presence of X-Gluc. Then, the presence of the F sequence in MVA-F was confirmed by PCR amplification of a 481bp internal fragment of the F gene (Figs. 1A and C). The purity of the recombinant viral stock was confirmed using TK primers, flanking the region of the homologous recombination between the transfer vector and the viral genome. The amplification of a 550bp product was detected only in MVA samples (parental MVA genome) (Fig. 1B). A RT-PCR assay confirmed the expression of the F gene at the transcriptional level, as evidenced by the amplification of a 711bp fragment only in the sample from CEFs infected with MVA-F (Fig. 1D).
In order to detect the presence of the F protein in MVA-F infected cells, several monoclonal antibodies against the F protein and specific sera against BRSV were tested in Western blot assays. Antibodies neither recognized the F protein from the cell extract nor showed unspecific interactions that inhibited visualization of a differential band corresponding to the F protein. Since we were unable to detect the presence of F protein, we confirmed the expression of the F gene at the transcriptional level by a RT-PCR assay. Indeed, we observed the amplification of a 711bp fragment only in the sample from CEFs infected with MVA-F (Fig. 1D).
Furthermore, the stability of the inserted F gene was confirmed by PCR and RT-PCR analyses of samples recovered after 10 blind passages in CEFs (Supplementary Fig. 1).
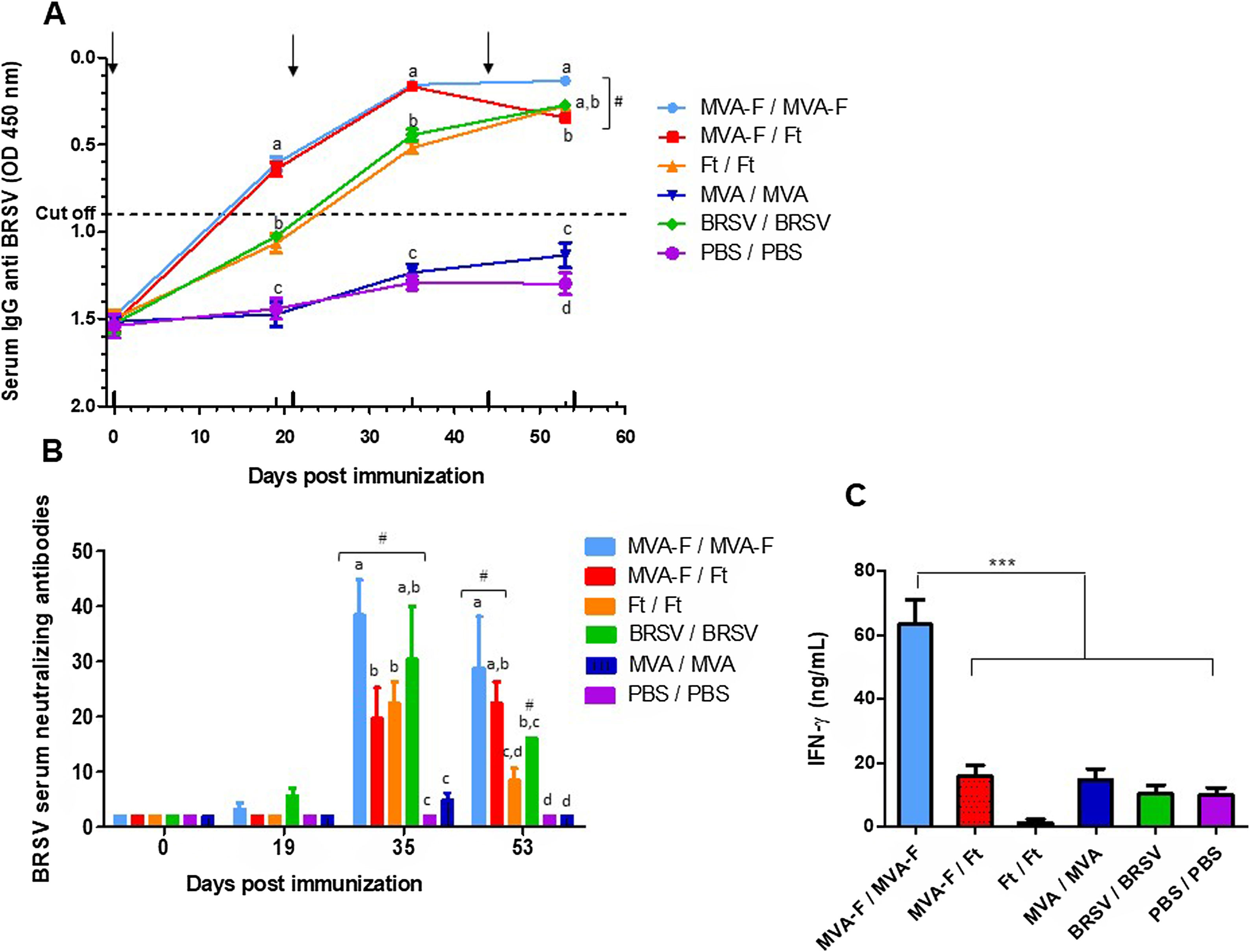
Systemic immunization with MVA-F induces a high and specific response against BRSV characterized by the presence of neutralizing antibodies and secretion of IFN-γWe initially analyzed the immunogenicity of MVA-F administered by the IP route alone or in combination with a subunit vaccine (homologous and heterologous schemes, respectively). After the first immunization (19 dpi), only mice receiving MVA-F exhibited specific antibodies against BRSV in serum (Fig. 2A). When the booster was administered, total antibodies were detected in all experimental groups. In addition, all groups had detectable BRSV NA after the second dose (Fig. 2B). Remarkably, homologous MVA-F immunization elicited the highest levels of both total and NA. Moreover, heterologous vaccination using MVA-F combined with Ft, induced the same levels of total antibodies as the homologous scheme, while NA levels were significantly lower throughout the studied period. The control animals (inoculated with MVA or PBS) remained seronegative throughout the experiment.
Evaluation of humoral and cellular immune response by IP administration of MVA-F in homologous and heterologous vaccination schemes. (A) Kinetics of total anti-BRSV antibodies determined by competition ELISA (Ingezim, INGENASA). Sera were evaluated in a 1/4 dilution. The dotted line indicates the cut-off value of the assay. (B) Levels of BRSV neutralizing antibodies induced by vaccination. (C) Quantification of IFN-γ content in supernatants of spleen cells from immunized mice determined by ELISA (OptiEIA, BD). Spleen cells were recovered and cultured for 72h in the presence of BRSV or mock infected cells (background). Supernatants were tested in 1/50 dilution and background subtracted results are depicted. The arrows indicate date of vaccination and revaccination. In (A) and (B), groups that statistically differ from PBS and MVA/MVA immunized animals are indicated with the hash symbol (#). In addition, at each time point, statistical differences between groups are indicated by using different letters, i.e., groups with the same letter do not differ significantly in the response induced while if different letters are shown, the response is significantly different. The results are representative of two independent experiments and are expressed as mean±SEM (n=5). For IFN-γ ***p<0.01.
The induction of cellular immune response was evaluated by measuring in vitro IFN-γ secretion of splenocytes stimulated with BRSV. Only the animals that received MVA-F in a homologous vaccination scheme displayed a specific and powerful response (Fig. 2C).
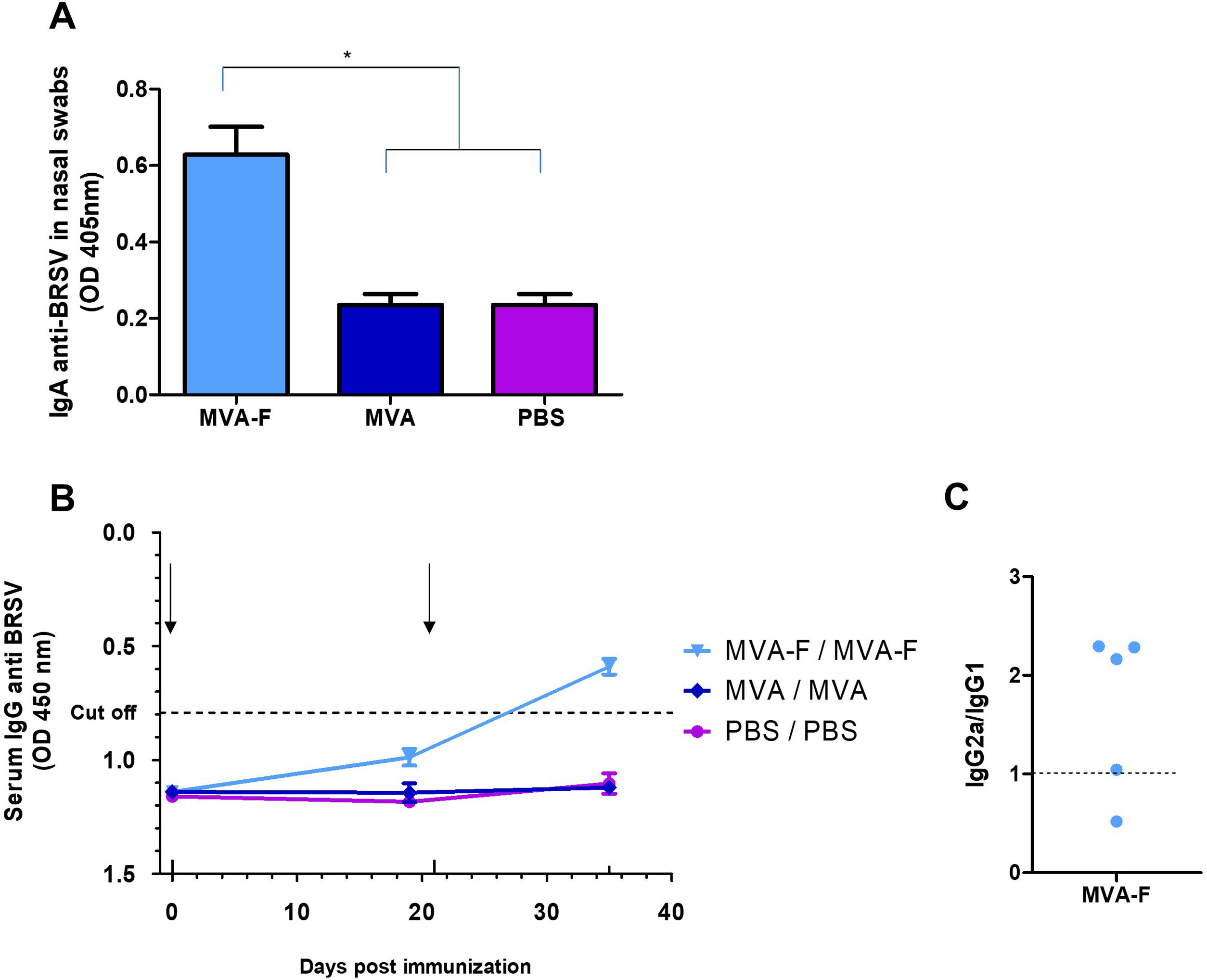
MVA-F induces a specific humoral response against BRSV in serum and nasal washes when administered by a mucosal routeGiven that BRSV enters the host via the respiratory tract, it is interesting to induce a local response capable of restraining the virus at the entry site. Therefore, we evaluated the induction of specific anti-BRSV IgA antibodies in nasal washes after IN administration of MVA-F. None of the animals immunized with MVA or PBS (control groups) showed specific antibodies in nasal washes or serum. Moreover, two doses of MVA-F elicited significantly higher levels of anti-BRSV IgA in nasal washes (*p<0.05, Fig. 3A). Furthermore, IN immunization induced anti-BRSV systemic immune response since specific serum antibodies were detected in all the animals immunized twice with MVA-F (Fig. 3B). Additionally, we tested serum isotypes in the MVA-F group, and calculated the ratio of isotype subclasses IgG1 and IgG2a as an indirect indicator of Th2 or Th1 bias of the immune response. Most of the MVA-F immunized animals showed a IgG2a/IgG1 ratio higher than 1, which indicates a Th1 biased response (Fig. 3C).
Mucosal and systemic anti-BRSV humoral responses induced by intranasal administration of MVA-F. (A) Presence of specific IgA against BRSV in nasal washes 35 days post vaccination. Samples were evaluated undiluted using an indirect ELISA (*p<0.05). (B) Kinetics of specific anti-BRSV serum antibodies analyzed by a competition ELISA (Ingezim, INGENASA). Sera were evaluated in a ¼ dilution. The dotted line indicates the cut-off value. (C) Serum isotypes (IgG1 and IgG2a) were evaluated in MVA-F immunized animals at 35 dpi. Dots represents IgG2a/IgG1 ratio for each animal. The results are representative of two independent experiments and are expressed as mean±SEM (n=5). The arrows indicate date of vaccination and revaccination.
BRSV infection causes respiratory disease in cattle and predisposes to secondary bacterial infections that lead to high morbidities and mortality rates worldwide. Infection occurrence could be diminished by improving biosecurity measures and vaccination. In this regard, live attenuated or inactivated vaccines are commonly used in countries with BRSV circulation. However, as reviewed by Theurer et al.34 and Ellis11, none of the experimental or commercially available vaccines are fully protective because they are unable to prevent clinical signs and virus shedding simultaneously.
Taking into account some characteristics of BRSV infection and animal susceptibility, a successful vaccine for BRSV should induce (a) strong mucosal immunity (because BRSV infection occurs in the respiratory tract); (b) a Th1 biased response (to avoid exacerbation of the disease after infection); and (c) an immune response that surpasses the inhibitory effect of maternal antibodies. In addition, vaccine effectiveness could be enhanced by applying different vaccination strategies, such as vaccinating pregnant cows in the last third of gestation, using mucosal delivery of vaccines or applying prime-boost schemes using different vaccine formulations (conventional and/or recombinant).
Given that MVA has been widely used for human and veterinary vaccines1,6,38, in this study we obtained a BRSV vaccine candidate based on MVA expressing the F protein of BRSV. We used the coding sequence of the F protein because it is highly conserved between different strains of RSV, even from different species24,26. The selection of the viral vector and the BRSV antigen was based on the profile of the immune response desired (Th1 biased) and the ability to induce NA against BRSV, respectively.
Our results showed that systemic immunization (IP route) with MVA-F (homologous scheme) triggered both branches of the adaptive immune response against BRSV with a Th1 bias. Remarkably, levels of neutralizing antibodies were similar after two doses of MVA-F and those induced with the inactivated BRSV vaccine. Furthermore, immunization with MVA-F combined with a subunit vaccine (heterologous scheme) did not improve the NA titers obtained in the homologous scheme. Our study concurs with other reports in which using MVA in homologous schemes induced better levels of immune response than heterologous Schemes5,14,22.
MVA has been previously evaluated as RSV vaccine in mice using recombinant MVAs expressing RSV F and/or G protein (glycoprotein)25,40. In accordance with our results, both studies reported the induction of specific (anti-RSV) humoral and cellular immune responses by recombinant MVAs. However, the immunization strategies and the doses used were different from the ones described in the present work. Wyatt et al.40 inoculated MVA-F simultaneously by the intramuscular (IM) and IN routes, while Olszewska et al.25 vaccinated animals with MVA via IP route and analyzed the immune response after RSV challenge. In addition, the MVA dose used in both studies was approximately 108 PFU, which is approximately 1.5-fold higher than the dose used in our study.
Local antibody responses in the mucosa play an important role in protection against respiratory viruses such as RSV15. In addition, mucosal routes of immunization have been suggested to be more resistant to the immunosuppressive effect of maternal antibodies10. In this regard, MVA-based vaccines administered intranasally induced a protective response against respiratory viruses such as influenza4, parainfluenza9 and bovine herpesvirus7,15.
In this study, we demonstrated that MVA-F administered via the IN route elicited systemic (IgG) and nasal (IgA) anti-BRSV antibodies. Moreover, an IgG2a/IgG1 ratio higher than 1 indicated a Th1 biased response. Indeed, the ratio of isotype subclasses IgG1 and IgG2a could be an indirect indicator of Th2 or Th1 bias of the immune response elicited by the recombinant immunogen administered by this route. To our knowledge, this is the first study that evaluates anti-BRSV responses using MVA in a homologous scheme by a mucosal route. By using RSV-viral vectored vaccines administered by the IN route, other researchers observed the presence of IgA in the upper and lower respiratory tract and a Th1 bias of the immune response27,39. In addition, animals were protected against RSV challenge, which suggested that local immunity may act as the first barrier against infection in the upper respiratory tract by RSV.
Only a few reports have described the protection against BRSV in calves using different strategies based on viral vector vaccines. Antonis et al. evaluated recombinant MVAs expressing the F protein alone or combined with the G protein and observed in both cases partial protection against BRSV challenge without presenting ERD3. On the other hand, Taylor et al. tested two recombinant viral vectors through different prime-boost schemes. The best results after challenge were observed when using an adenovirus-based vaccine (ChAd expressing RSV F, N and M2-1 proteins) as prime and MVA-F as boost32.
In a recent review, Ellis et al. described the clinical efficacy of intranasal vaccination of cattle with BRSV conventional vaccines, and points out that this route is not fully protective in the presence of maternal antibodies11. To improve the protective efficacy of BRSV vaccines, the author suggests the implementation of the strategy used by Stoltenow et al.31 and Stokka et al.30, in which neonatal intranasal vaccination and parenteral boost at weaning induced high anamnestic immune responses after the second dose. Taking into account that MVA induced partial protection against BRSV in calves vaccinated by a parenteral route3, and considering the results of this study, we believe that MVA-F is a good candidate to be tested in calves combining two different administration routes (intranasal in the prime and intramuscular in the boost) in order to improve the vaccine efficacy against BRSV infection.
ConclusionsIn this study we have demonstrated that MVA-F, when administered via the IP route in a homologous vaccination scheme is capable of inducing BRSV-neutralizing antibodies and a specific cellular immune response, both reported as relevant in preventing and controlling infection with BRSV. In addition, nasal administration elicited mucosal and systemic immunity with a Th1 profile.
In conclusion, our results are encouraging, and this study is to be taken as a starting point to explore BRSV vaccine strategies, considering systemic and mucosal MVA-F administration in homologous schemes.
FundingThis research was funded by the Instituto Nacional de Tecnología Agropecuaria (INTA) (grant number PNBIO 1131032). The institution provided only financial support of the research.
Conflict of interestsNone declared.
The authors would like to thank Mrs. M.J. Mónaco for technical assistance, Mr. Silvio Diaz for his support in handling and care of animals and Dr. Osvaldo Zabal and his team (IVIT, INTA) for preparing the primary chicken embryo fibroblasts.







![(A) Schematic representation of the MVA genome. MVA-F contains the F gene and a marker gene uid A (GUS) interrupting the viral TK gene (the right – r and left – l regions are shown). Primers used for the molecular characterization are indicated with arrows of different colors. (B and C) PCR amplification using specific primers for TK [B, primers used shown in red in (A)] and F [C, primers used shown in blue in (A)] genes using DNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs; TK+: transfer vector containing TK gene; F+: transfer vector containing F gene; M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®); (D) corroboration of expression of F RNA by RT-PCR [primers used shown in green in (A)] using total RNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs. M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®). (A) Schematic representation of the MVA genome. MVA-F contains the F gene and a marker gene uid A (GUS) interrupting the viral TK gene (the right – r and left – l regions are shown). Primers used for the molecular characterization are indicated with arrows of different colors. (B and C) PCR amplification using specific primers for TK [B, primers used shown in red in (A)] and F [C, primers used shown in blue in (A)] genes using DNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs; TK+: transfer vector containing TK gene; F+: transfer vector containing F gene; M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®); (D) corroboration of expression of F RNA by RT-PCR [primers used shown in green in (A)] using total RNA extracted from MVA (line 1), MVA-F (line 2) or non-infected (line 3) CEFs. M: 1 Kb Plus DNA Ladder (PB-L Productos Bio-Lógicos®).](https://static.elsevier.es/multimedia/03257541/unassign/S0325754123000834/v2_202312271007/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



