Our objectives are: To describe the radiological semiology, clinical-analytical features and prognosis related to the target sign (TS) in COVID-19. To determine whether digital thoracic tomosynthesis (DTT) improves the diagnostic ability of radiography.
Material and methodsRetrospective, descriptive, single-centre, case series study, accepted by our ethical committee. Radiological, clinical, analytical and follow-up characteristics of patients with COVID-19 and TS on radiography and DTT between November 2020 and January 2021 were analysed.
ResultsEleven TS were collected in 7 patients, median age 35 years, 57% male. All TS presented with a central nodule and a peripheral ring, and in at least 82%, the lung in between was of normal density. All TS were located in peripheral, basal regions and 91% in posterior regions. TS were multiple in 43%. Contiguous TS shared the peripheral ring. Other findings related to pneumonia were associated in 86% of patients. DTT detected 82% more TS than radiography. Only one patient underwent a CT angiography of the pulmonary arteries, positive for acute pulmonary thromboembolism. Seventy-one per cent presented with pleuritic pain. No distinctive laboratory findings or prognostic worsening were detected.
ConclusionsTS in COVID-19 predominates in peripheral and declining regions and can be multiple. Pulmonary thromboembolism was detected in one case. It occurs in young people, frequently with pleuritic pain and does not worsen the prognosis. DTT detects more than 80 % of TS than radiography.
Nuestros objetivos son describir la semiología radiológica, los datos distintivos clínico-analíticos y el pronóstico relacionados con el signo de la diana (SD) de la COVID-19. Determinar si la tomosíntesis digital torácica (TDT) mejora la capacidad diagnóstica de la radiografía.
Material y métodosEstudio retrospectivo, descriptivo de una serie de casos, unicéntrico, aceptado por nuestro comité ético. Se analizaron las características radiológicas, clínicas, analíticas y evolutivas de los pacientes con COVID-19 y SD en radiografía y TDT entre Noviembre 2020 y Enero 2021.
ResultadosSe recogieron 11 SD en 7 pacientes, con edad mediana de 35 años, 57% varones. Todos presentaron un nódulo central y un anillo periférico y, en al menos el 82%, el pulmón entre ambos fue de densidad normal. Todos se situaron en regiones periféricas, basales y el 91% en regiones posteriores. Fueron múltiples en un 43%. Los SD contiguos compartieron el anillo periférico. El 86% asoció otras manifestaciones pulmonares de neumonía. La TDT detectó un 82% más de SD que la radiografía. Solo en un paciente se realizó una angio-TC de arterias pulmonares, positiva para tromboembolia pulmonar aguda. El 71% acudió con dolor pleurítico. No se detectaron hallazgos analíticos distintivos ni empeoramiento pronóstico.
ConclusionesEl SD en la COVID-19 predomina en regiones periféricas y declive y puede ser múltiple, encontrando tromboembolia pulmonar en un caso. Se da en jóvenes, frecuentemente con dolor pleurítico y no empeora el pronóstico. La TDT detecta más del 80% de SD que la radiografía.
The target sign (TS) in computed tomography (CT) images, less frequently known as the bull’s eye or rings of Saturn sign, has been described as a highly specific sign for COVID-19 patients with pulmonary involvement.1–3 It consists of a dense central nodule surrounded by a dense peripheral ring, with another ring of lower attenuation in between those, which often corresponds to normal lung parenchyma. Occasionally it comprises several interspersed rings of varying attenuation.4,5 In radiology, it has been described as a variant of the reversed (reverse) halo sign.3 The reversed halo sign, typical of organising pneumonia, consists of a dense ring surrounding an area of ground-glass density.1–6 On an anatomical-pathological level, the dense peripheral ring in the reversed halo sign corresponds to granulation tissue in the peripheral air spaces, and the central ground-glass opacity corresponds to inflammation of the alveolar septa.6,7 Although organising pneumonia is frequent in COVID-19 pneumonia,8 to say that it provides an explanation for TS images in CT is controversial, mainly but not exclusively as this explanation does not account for the central nodule.7,9 The combination of organising pneumonia and arterial thrombosis has been suggested as the underlying pathoanatomy of the TS,10 although this association is not fully understood.4 Likewise, the point at which the TS appears in the course of the disease or whether the TS has a significant impact on prognosis has not been investigated in depth. Finally, given its usefulness, it would make sense to improve our ability to diagnose the sign. In comparison to conventional imaging techniques, tomosynthesis has demonstrated several diagnostic advantages, for example, in the early detection of breast cancer or lung nodules.11 However, there have been no studies into whether it improves on the diagnostic capacity of chest radiography in cases of COVID-19 with lung involvement, and more specifically, if it has a greater capacity to detect the TS. If it does, it could reduce the number of CT scans performed.
Our objectives in this investigation are:
- 1.
To describe the radiological features of the TS in COVID-19 patients with lung involvement and its similarities and differences to the reversed halo sign.
- 2.
To describe the clinical characteristics, laboratory values and prognosis of patients with the TS.
- 3.
To evaluate whether digital tomosynthesis (DTS) of the chest offers any advantage over radiography for detecting the TS.
This was a cross-sectional, observational, single-centre study, accepted by the Ethics Committee of our hospital (EST code 55/20). We noted all patients with the TS detected by chest radiography, DTS or both during the second (October–November 2020) and third (January 2021) waves of the SARS-CoV-2 pandemic. In our setting, all patients clinically suspected of pneumonia during the pandemic period who were given a chest radiography were simultaneously given a DTS, whether they were referred from the emergency unit or primary care. DTS involves a series of very low dose exposures during a single sweep with a stationary detector and a tube that moves within a sweep angle range limited to 30 °. The DTS system used was VolumeRAD Digital Tomosynthesis GE Healthcare system (3D radiography)™ (GE Healthcare. Milwaukee, WI, USA). After acquiring the images, the system reconstructs approximately 60 images that correspond to different coronal planes, parallel to the detector, and which show anatomical structures at different depths. The sweep duration is 11 s.
Imaging variablesWe gathered data for all patients with SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction (RT-PCR) and the presence of one or more TS on chest radiograph, DTS or both.
Based on the chest radiograph and DTS assessment, the following was determined:
- □
The density of the dense peripheral ring: pulmonary consolidation, when the opacity prevented pulmonary vessels from being seen, or ground glass, when vessels were identifiable.4
- □
Lung density between the dense peripheral ring and the central nodule of each TS. It was classified as ground-glass density when the density was higher than that of the normal lung but lower than that of the dense peripheral ring, and normal lung density, when it was similar to that of other parts of the lung not affected by other pathologies.
- □
TS number: single or multiple.
- □
Laterality of TS: unilateral or bilateral.
- □
TS dense peripheral ring: joined or not joined. In cases of multiple TS, the dense peripheral ring is considered joined when a portion of it forms part of another. This is seen in other TS-related published cases.4,12
- □
Size of the TS, in relation to the intercostal space and adjacent ribs.
- □
TS Location:
- o
Peripheral or central. Peripheral where the target sign was nearer to the costal pleura than the mediastinal pleura. Central otherwise.
- o
Inferior or superior. Inferior, when located in the inferior, middle or lingular lobes, or superior if located in the superior lobes.
- o
Anterior or posterior depending on whether it was closer to the anterior or posterior costal margins, respectively.
- o
If any patient had a CT scan during the period of COVID-19 lung involvement, we assessed the imaging features of the TS and associated findings.
In addition, we assessed the presence of consolidations and ground-glass opacities in other parts of the lung away from the TS in relation to COVID-19 pneumonia.13
Non-imaging variables- □
Age, sex, personal medical history.
- □
Time between the positive RT-PCR test and the chest radiograph/DTS in which the TS appeared.
- □
Respiratory symptoms and O2 saturation on arrival at hospital.
- □
Laboratory markers recorded in the emergency room or, where relevant, during admission for COVID-19, as close as possible to the time when the TS was detected. Specifically, age-adjusted D-dimer, LDH and CRP.
- □
Level of medical assistance required: if they were treated as an outpatient, required admission to hospital or into the Intensive Care Unit (ICU).
- □
Evolution, favourable, if they got better, or unfavourable if they died.
We represented our quantitative variables as median and interquartile range and qualitative variables as absolute and relative frequencies (percentages). Given the small sample size, no statistical analyses were performed.
ResultsWe recorded 11 TS in seven patients, with a median age of 35 years (interquartile range 26–6 years), four of whom were male (57%). Two patients had comorbidities, one was undergoing treatment for colorectal cancer and the other had previously suffered a past myocardial infarction. A CT pulmonary angiogram was performed on one patient.
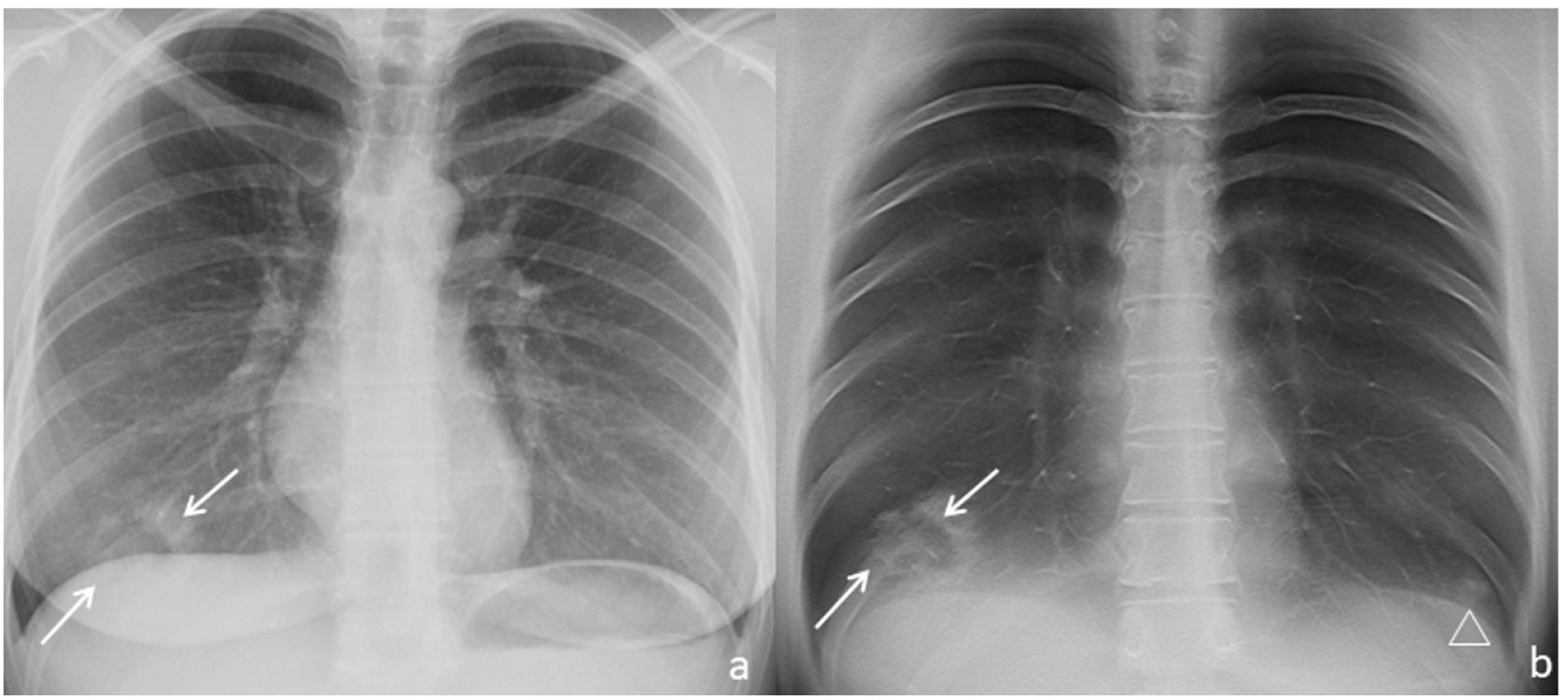
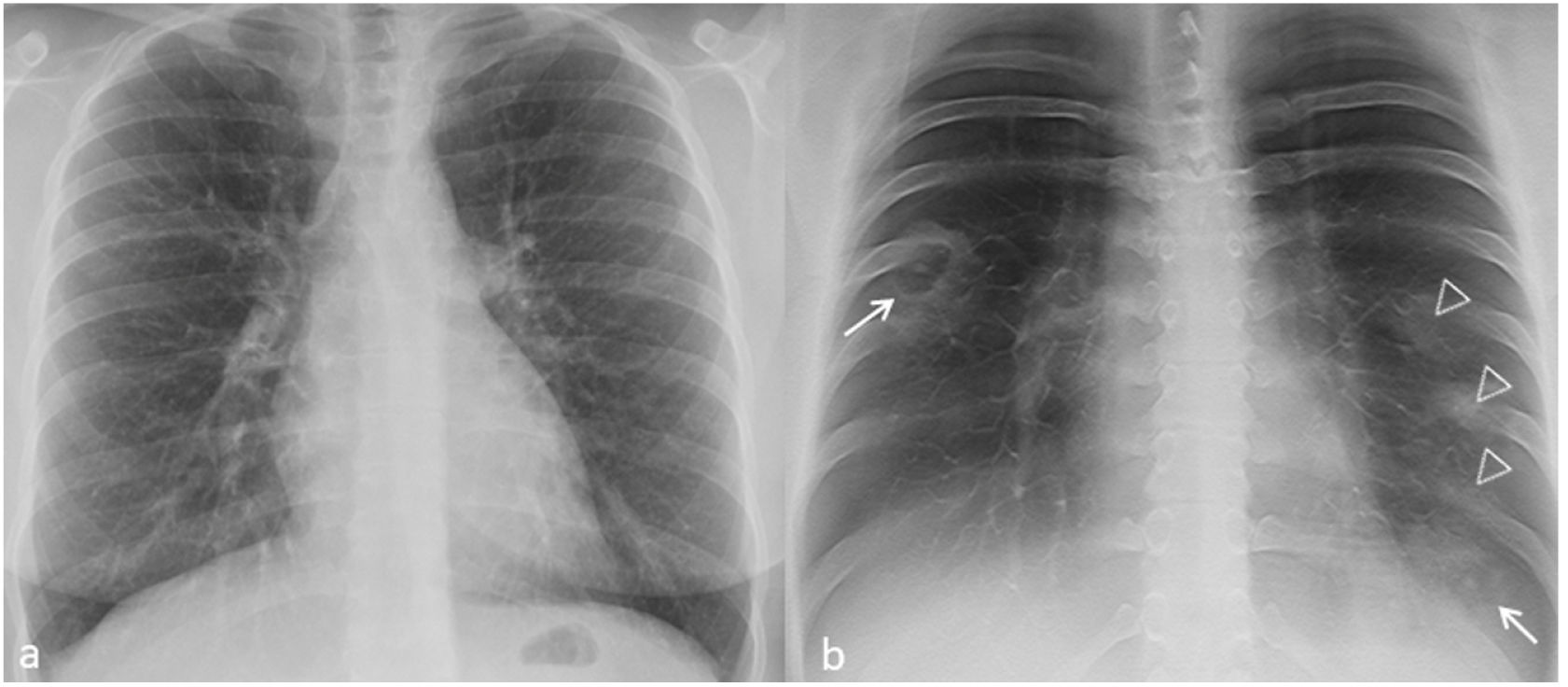
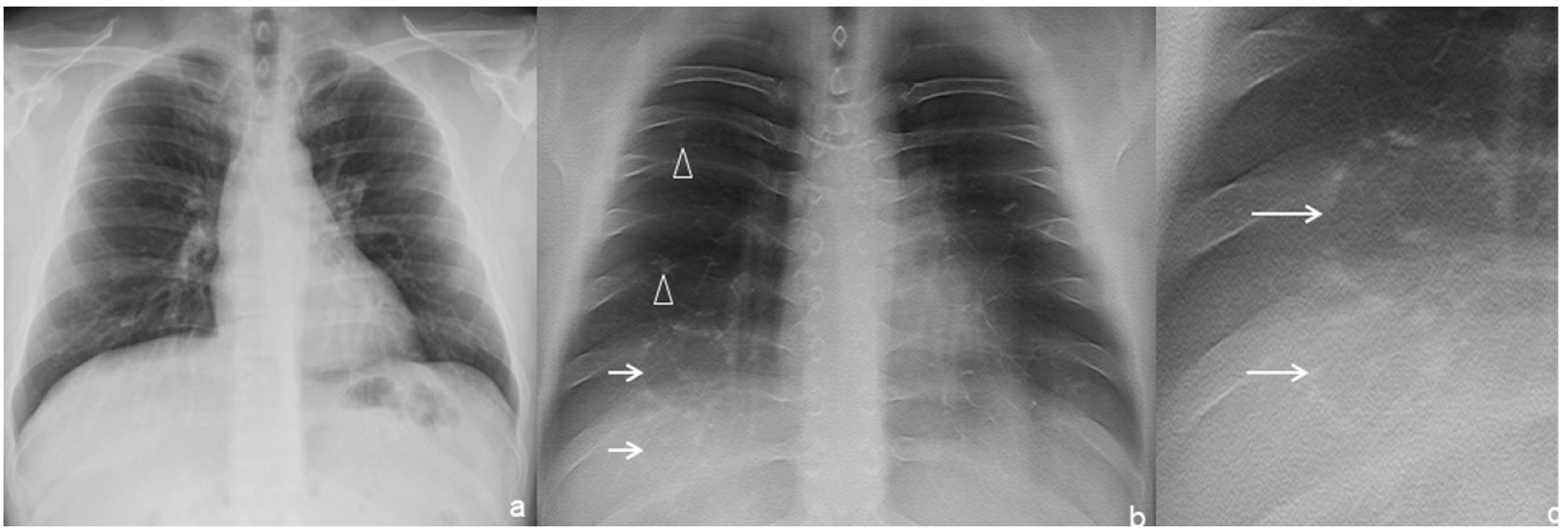
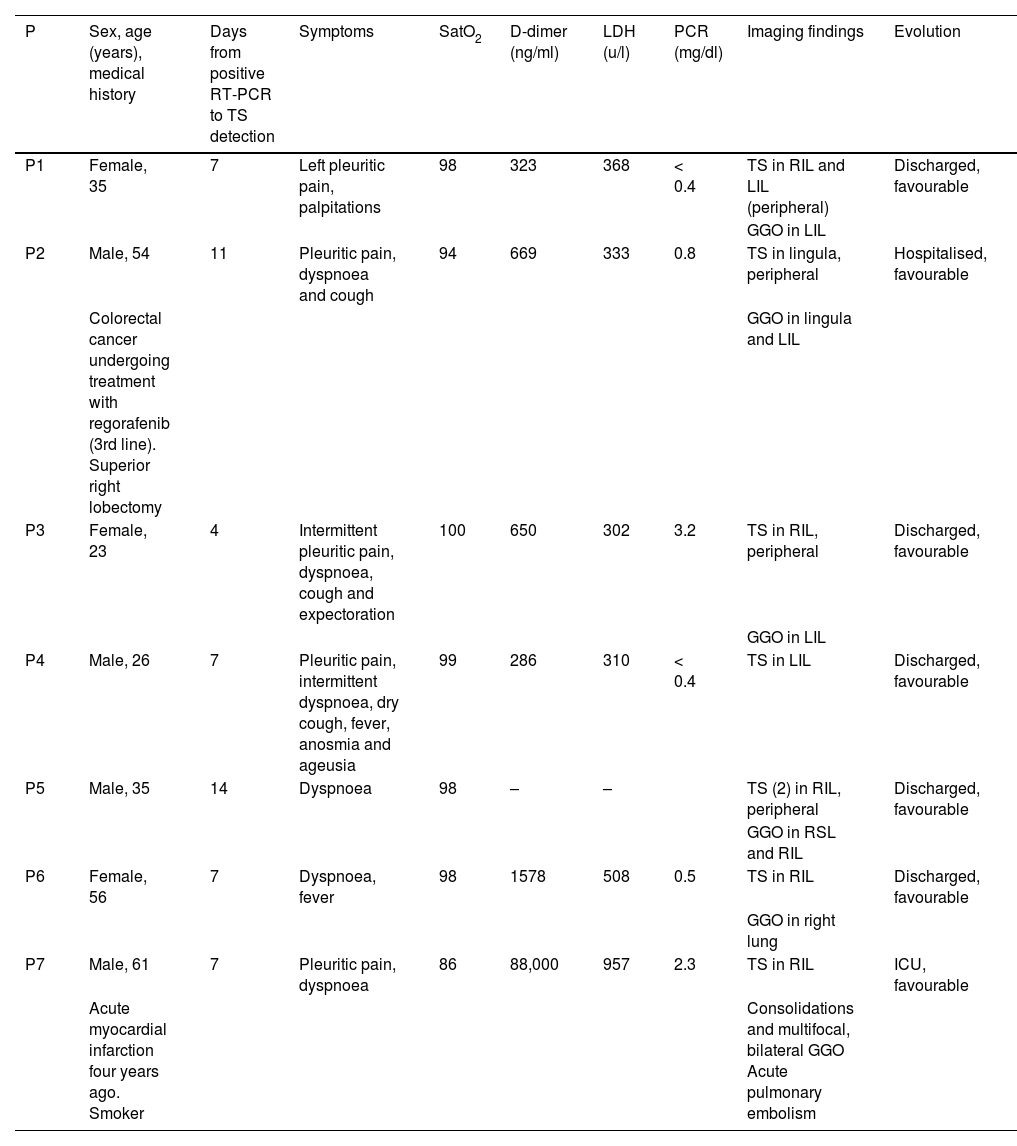
Imaging features of TSThe dense peripheral ring was classified as consolidation density in 2 of the 11 TS (Fig. 1) and ground-glass density in 2 of the 11 TS (18%, Fig. 2) In 9 of the 11 TS (82%), the density of the lung surrounded by the dense peripheral ring was similar to that of the normal lung (Figs. 1–4), and in the rest (2/11, 18%) it was unclear whether this density reached ground-glass density. In all cases the density was lower than that of the dense peripheral ring (11/11, 100%).
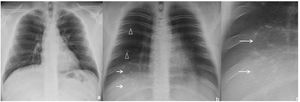
Chest radiograph (a) and digital tomosynthesis (DTS) (b and c) in a patient with target sign (white arrows) visible only on DTS. Two adjacent TS observed with joined peripheral ring opacities (b and c). Ill-defined opacities, patchy, peripheral, ground-glass (arrowheads) due to COVID-19 pneumonia.
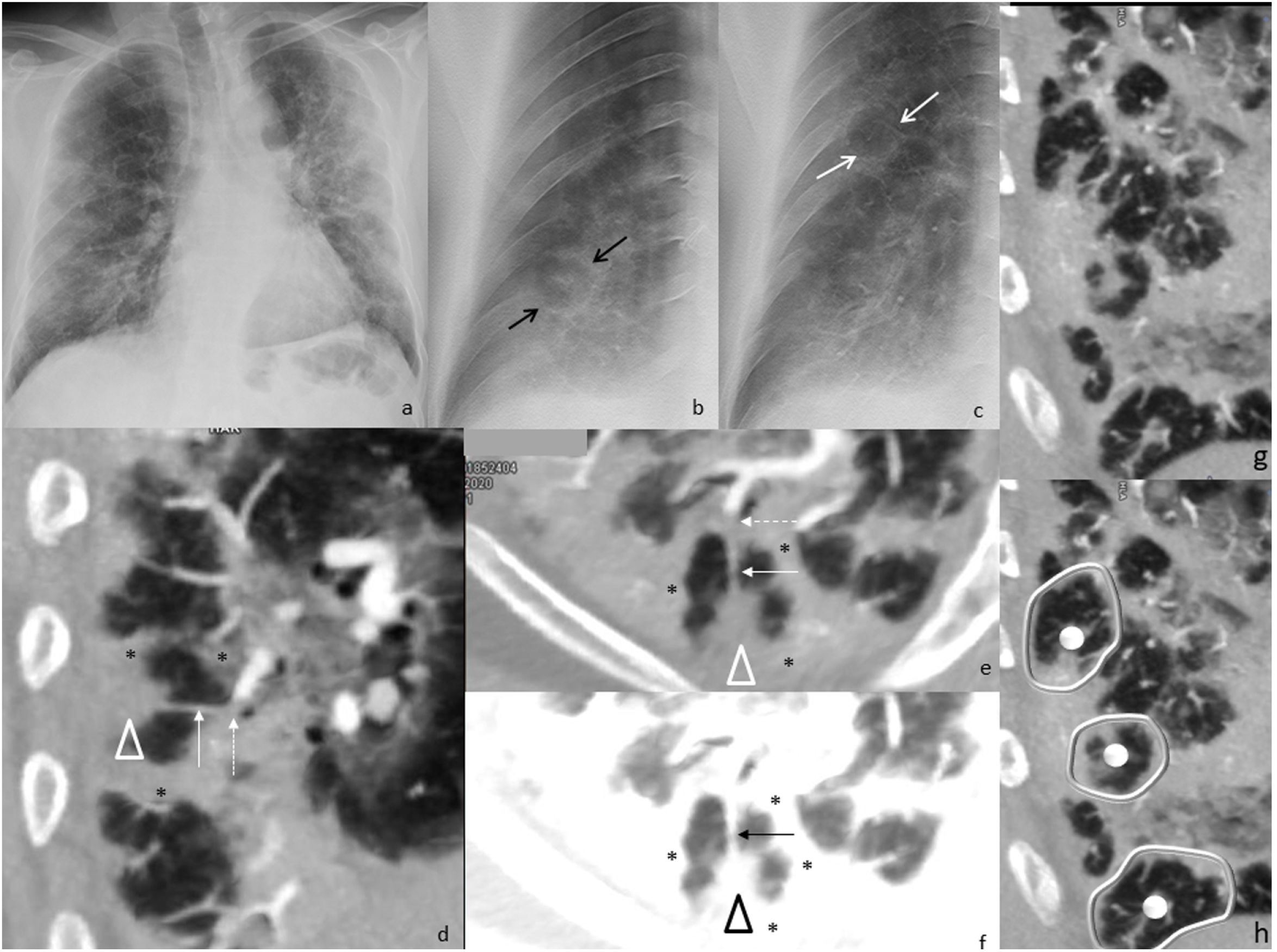
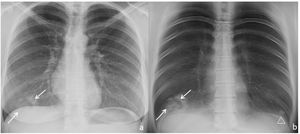
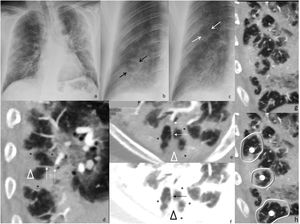
Chest radiograph (a) and digital tomosynthesis (DTS) (b) in a patient with multiple target signs (white arrows) visible only on DTS. Angio-CT curved reconstruction of pulmonary arteries with angiographic (d and e) and lung (f, same plane as e) window, in which peripheral arteries are observed with endoluminal thrombotic material (dashed arrows), whose distal portion (continuous line arrow) reaches the central nodule of the target sign (arrowheads); asterisks indicate the dense peripheral ring. In images (g and h) multiple joined dense peripheral rings (white lines) surround normal lung tissue and show the central nodule (white circles) as seen in a patient with target sign during COVID-19 pneumonia associated with pulmonary embolism.
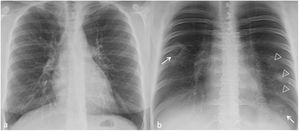
Multiple TS were found in three of the seven (43%) patients. Of these, one patient had bilateral TS—one in each of the inferior lobes (1/7, 14%, Fig. 2)—, another had two adjacent TS in the right inferior lobe (RIL) (1/7, 14%, Fig. 3) and the third had numerous TS in the RIL (1/7, 14%, Fig. 4).
In the two patients with multiple contiguous TS (Figs. 3b, c and 4g, h), the TS presented with joined dense peripheral rings.
The approximate maximum size of all the TS was the width of one intercostal space plus the thickness of one or both adjacent ribs (Figs. 1–4).
All the TS were peripheral (11/11, 100%), all were located in inferior (inferior lobes or lingula, 11/11, 100%) and posterior regions, except for the lingular TS (10/11, 91%) (Table 1).
Characteristics of seven patients with COVID-19 and target signs.
| P | Sex, age (years), medical history | Days from positive RT-PCR to TS detection | Symptoms | SatO2 | D-dimer (ng/ml) | LDH (u/l) | PCR (mg/dl) | Imaging findings | Evolution |
|---|---|---|---|---|---|---|---|---|---|
| P1 | Female, 35 | 7 | Left pleuritic pain, palpitations | 98 | 323 | 368 | < 0.4 | TS in RIL and LIL (peripheral) | Discharged, favourable |
| GGO in LIL | |||||||||
| P2 | Male, 54 | 11 | Pleuritic pain, dyspnoea and cough | 94 | 669 | 333 | 0.8 | TS in lingula, peripheral | Hospitalised, favourable |
| Colorectal cancer undergoing treatment with regorafenib (3rd line). Superior right lobectomy | GGO in lingula and LIL | ||||||||
| P3 | Female, 23 | 4 | Intermittent pleuritic pain, dyspnoea, cough and expectoration | 100 | 650 | 302 | 3.2 | TS in RIL, peripheral | Discharged, favourable |
| GGO in LIL | |||||||||
| P4 | Male, 26 | 7 | Pleuritic pain, intermittent dyspnoea, dry cough, fever, anosmia and ageusia | 99 | 286 | 310 | < 0.4 | TS in LIL | Discharged, favourable |
| P5 | Male, 35 | 14 | Dyspnoea | 98 | – | – | TS (2) in RIL, peripheral | Discharged, favourable | |
| GGO in RSL and RIL | |||||||||
| P6 | Female, 56 | 7 | Dyspnoea, fever | 98 | 1578 | 508 | 0.5 | TS in RIL | Discharged, favourable |
| GGO in right lung | |||||||||
| P7 | Male, 61 | 7 | Pleuritic pain, dyspnoea | 86 | 88,000 | 957 | 2.3 | TS in RIL | ICU, favourable |
| Acute myocardial infarction four years ago. Smoker | Consolidations and multifocal, bilateral GGO Acute pulmonary embolism |
RIL: right inferior lobe; LIL: left inferior lobe; RSL: right superior lobe; GGO: ground-glass opacities; P: patient; SatO2: oxygen saturation; TS: target sign; ICU: intensive care unit.
Angio-CT, performed on one patient, revealed multiple peripherally located TS in the right inferior lobe, whose dense peripheral ring surrounded lung of normal density, many of which had a dense central or subpleural nodule, as well as several TS with joined contiguous dense peripheral rings (Fig. 4). A thrombosed peripheral artery reached the central nodule in at least one of the TS (Fig. 4d, e).
All but one patient (6/7, 86%) showed other pulmonary manifestations typical of COVID-19 pneumonia (Table 1).
Clinical characteristics and laboratory values of patients with the TSFive of seven patients (71.4%) presented with pleuritic pain.
The age-adjusted D-dimer was elevated in four of the six (67%) patients and LDH in two of the six patients (33%). CRP was elevated in all patients, but the highest level was 3.2 mg/dl. This data was not available for one of the patients because blood biochemistry tests were not requested.
Five patients (5/7, 41%) were treated as outpatients. Only two of the seven (28%) required hospital admission, including one to the ICU (14%). The O2 saturation levels were > 94% for all patients except the one who required admission to the ICU, who had a saturation of 86%. All patients progressed favourably.
The TS appeared between 4 and 14 days after the positive RT-PCR test (Table 1).
DTS contribution to TS detectionAll TS were detected by DTS of the chest (11/11, 100%), whereas only 2 out of 11 (18%, Fig. 1–4) were detected by chest radiography.
DiscussionIn our case series of patients with COVID-19, the TS appeared predominantly in lower peripheral regions, some were multiple and were not always accompanied other signs of pneumonia in the lungs. They presented with a central nodule and peripheral ring. The peripheral rings in contiguous TS are joined. In more than 80% of cases, the lung between the central nodule and the peripheral ring was of normal density. These findings differ from those of the reversed halo sign in organising pneumonia,1,4,9 which suggests a different aetiopathogenesis. Detection of the TS is 80% higher when using DTS in comparison with radiography. The TS is detected between 4 and 14 days following diagnosis of the infection, in young patients (median of 35 years), accompanied by pleuritic pain in more than 70% of cases, with no specific findings from laboratory testing. It does not indicate a worse prognosis for COVID-19 infections.
There is evidence of underlying vascular involvement in COVID-19: histologically (thrombosis, endotheliitis, haemorrhagic infarction and angiogenesis, probably mediated by an endothelial thrombo-inflammatory syndrome and a procoagulant state8,14,15); molecular (affinity of SARS-CoV-2 surface proteins for endothelial ACE-2 receptors16) and radiological (dilated peripheral pulmonary vessels on CT scan,5 mosaic pattern probably due to perfusion disturbances17). Arterial thrombosis with associated infarction could explain the TS in COVID-19. We have based this hypothesis on radiological and clinical evidence, which should be confirmed in future studies.
- a)
Radiological:
- I)
Features similar to those of pulmonary infarction processes. The imaging features of light bulb sign and the rosette sign on CT, which consist of one or more dense peripheral rings, with a dense central nodule and lung parenchyma of variable density between and outside these rings18 are similar to those of the TS and have been described in the immediate period after performing lung radiofrequency ablation procedures. The underlying pathoanatomy is coagulative necrosis with secondary haemorrhage.18
- II)
Features of the TS. Both the size of the dense peripheral ring, similar to that of the secondary pulmonary lobule, and the perilobular distribution could be explained by a bronchogenic or angiogenic process, such as pulmonary infarction. When the dense peripheral ring surrounds lung of normal density, as occurred in at least 80% of the TS in our sample, the histological basis considered most likely is infarction, and when the ring surrounds lung of ground-glass density, organising pneumonia is most likely.9 Arteriole thrombosis in the secondary pulmonary lobule provides an explanation for the central nodule.5,10,19
- III)
Distribution of the TS. According to our work, and that of others,1 the TS can be multiple, bilateral, predominantly located in basal and peripheral regions and in the vicinity of bronchovascular bundles in 87% of cases.1 Although this distribution occurs in organising pneumonia, it also occurs in hematogenous dissemination processes, which tend towards posterior regions, probably because gravity encourages a greater flow in that region. This feature is shared by the TS in our series.
- IV)
Association with pulmonary embolism. In patients with COVID-19 and pulmonary embolism, present in the only angio-CT performed in our sample, the TS is more frequent (16–18% vs 2–5%).9
- I)
- b)
Clinical. Pleuritic pain is much more frequent in COVID-19 patients with lung involvement and the TS (71%) than in those without the TS (17%, 95% CI 11–25%, in a cohort of 133 admitted patients) in our setting.20 In turn, it is a rare symptom in organising pneumonia.6
The laboratory markers analysed did not provide relevant data, possibly because they overlapped with the systemic inflammatory context of COVID-19.
Regarding prognosis, the favourable evolution of all our patients, similar ICU admission requirements (14%) to a full set of patients with COVID-19 (10–15%)21 and low CRP figures (≤ 3.2 mg/dl) suggest that the presence of the TS does not increase the severity of COVID-19. Therefore, despite the relatively late manifestation of the TS, which according to our data appears between 4 and 14 days after the diagnosis of infection, and its low frequency (5%1), the specificity of the TS to COVID-19 suggests it will be primarily useful in diagnosis. It may be useful outside pandemic peaks and mass microbiological screening to detect the onset of new waves. Detection of the TS is 82% higher with DTS than chest radiography without the need for CT, suggesting that it could potentially be used to detect hidden pathologies or assess equivocal findings on chest radiography, whether related to COVID-19 pneumonia or not.13
Our study has various limitations. Our sample size is small. Patients were not all recruited consecutively, so the TS may have been excluded. We have not compared our sample with a sample of patients without the TS, so we cannot compare clinical characteristics, laboratory results or imaging findings. Nor can we know how prevalent it is. We can only describe the characteristics of the available sample. A CT pulmonary angiogram was available for only one patient. Although this was positive for peripheral arterial thrombosis, we cannot confirm the vascular nature of the TS. Finally, we do not have data to show imaging evolution, nor do we have CT images or histological samples for all patients, because it would not have been ethically acceptable to perform these tests for the sole objective of this study.
In conclusion, we have described 11 target signs in 7 patients with COVID-19. On imaging, the sign is characteristically multiple, and predominates in peripheral, lower regions. In one case it was associated with pulmonary embolism. In our case series, patients presented with a high incidence of pleuritic pain and prognosis was good. Detection of the TS is 80% higher with DTS than conventional radiography.
Author contributions- 1.
Research coordinators: JMPM, IGT, CBP, ABB.
- 2.
Development of study concept: JMPM, IGT, CBP, ABB.
- 3.
Study design: JMPM, IGT, CBP, ABB.
- 4.
Data collection: JMPM, IGT, CBP, ABB.
- 5.
Data analysis and interpretation: JMPM, IGT, CBP, ABB.
- 6.
Statistical analysis: not applicable.
- 7.
Literature search: JMPM, IGT.
- 8.
Writing of article: JMPM, IGT.
- 9.
Critical review of the manuscript with intellectually relevant contributions: JMPM, IGT, CBP, ABB.
- 10.
Approval of the final version: JMPM, IGT, CBP, ABB.
No funding was received to develop this publication.
Conflict of interestsThe authors declare that they have no conflicts of interest.