To determine the diagnostic performance of ultrasound-guided core-needle biopsy in thyroid nodules after two inconclusive fine-needle aspiration biopsies. To assess the complications of core-needle biopsy. To analyze the reliability of diagnoses obtained with core-needle biopsy. To measure the economic impact of avoiding lobectomies in patients with benign core-needle biopsy findings.
Material and methodsThis retrospective study reviewed 195 core-needle biopsies in 178 patients. To determine the reliability of the core-needle biopsy findings, we compared the diagnosis from the core-needle specimen versus the histologic findings in the surgical specimens when core-needle biopsy findings indicated malignancy or follicular proliferation and versus the stability of the nodule on ultrasound follow-up for one year when core-biopsy findings indicated benignity.
ResultsCore-needle biopsy yielded a diagnosis for 179 (91.7%) nodules, of which 122 (62.5%) were classified as benign, 50 (25.6%) as follicular proliferation, and 7 (3.6%) as malignant. The findings were inconclusive for 16 (8.3%) nodules. Minor complications were observed in 4 (2%) patients; no major complications were observed. The sensitivity of core-needle biopsy for the diagnosis of thyroid cancer was low (42.8%) because the technique was unable to detect capsular or vascular invasion, although the specificity and positive predictive value (PPV) were 100%. However, when we considered histologic findings of malignancy and follicular proliferation positive because both require surgical resection, the sensitivity increased to 97.5% and the PPV decreased to 83.3%. There were 79 nodules with ultrasound follow-up for at least one year; 76 (96.2%) had negative core-needle biopsy findings, and 74 (97.3%) of these remained stable. The negative predictive value (NPV) for malignancy of the benign nodules was 98.6%, although no malignant transformation was observed. Nevertheless, the results of the statistical analysis do not allow us to recommend forgoing ultrasound follow-up in patients with benign core-biopsy findings. The cost savings of avoiding lobectomy in patients with benign nodules and stability of the nodule on ultrasound follow-up for at least one year was about 90%.
ConclusionsCore-needle biopsy of thyroid nodules is effective because it diagnoses more than 90% of nodules with inconclusive findings after fine-needle aspiration biopsy. It is safe if done by experienced professionals. It is reliable because it yields 100% specificity and 100% PPV for malignant nodule, 97.5% sensitivity for the detection of nodules that require surgery, and 98.6% NPV for benign nodules. It is efficient because it reduces the costs of diagnosis compared to lobectomy in benign nodules.
Conocer el rendimiento diagnóstico de la biopsia con aguja gruesa (BAG) ecoguiada en nódulos tiroideos con dos punciones aspirativas con aguja fina (PAAF) previas no diagnósticas. Evaluar complicaciones de la BAG. Analizar la fiabilidad de los diagnósticos obtenidos mediante BAG. Medir el impacto económico de evitar lobectomía tras BAG con resultado benigno.
Material y métodosRevisión retrospectiva de 195 BAG realizadas en 178 pacientes. Las referencias utilizadas para medir la fiabilidad de los resultados de la BAG fueron el análisis de la pieza quirúrgica tras una biopsia con malignidad o proliferación folicular (PF) y la estabilidad ecográfica superior a 1 año tras una BAG benigna. Se compararon costes directos de BAG más seguimiento ecográfico frente al que hubiera tenido realizar lobectomía sin complicaciones en los pacientes con estabilidad ecográfica superior a 1 año tras BAG benigna.
ResultadosDe los 195 nódulos sometidos a BAG, el resultado fue diagnóstico en 179 (91,7%), incluyendo 122 benignos (62,5%), 50 PF (25,6%) y 7 malignos (3,6%). No fue diagnóstico en 16 nódulos (8,3%). Hubo complicaciones menores en 4 pacientes (2%) y mayores en ninguno. La sensibilidad de la BAG para el diagnóstico de cáncer de tiroides fue baja (42,8%) por su incapacidad para detectar invasión capsular o vascular, aunque con especificidad y valor predictivo positivo (VPP) del 100%. Al considerar los diagnósticos de malignidad y PF como positivos, pues ambos obligan a resección quirúrgica, la sensibilidad ascendió al 97,5%, con descenso al 83,3% del VPP. Hubo 79 nódulos con seguimiento ecográfico superior a 1 año, 76 con BAG benigna (96,2%), de los cuales mostraron estabilidad 74 (97,3%). El valor predictivo negativo (VPN) para malignidad de los nódulos benignos fue del 98,6%, aunque no se detectó ninguna transformación maligna. Sin embargo, el análisis estadístico no permite recomendar la supresión del seguimiento ecográfico tras BAG benigna. La reducción del coste diagnóstico respecto a lobectomía en pacientes con nódulos benignos y estabilidad ecográfica superior a 1 año fue próxima al 90%.
ConclusionesLa BAG de tiroides es efectiva porque diagnostica más del 90% de los nódulos con PAAF no diagnósticas; segura si es realizada por personal experimentado; fiable porque presenta especificidad y VPP del 100% en nódulos malignos, sensibilidad del 97,5% para detectar que nódulos necesitan cirugía y VPN del 98,6% en nódulos benignos; y eficiente porque reduce los costes diagnósticos respecto a lobectomía en nódulos benignos.
Ultrasound-guided fine needle aspiration (FNA) is the technique of choice to assess thyroid nodular disease as it is easy to carry out and has diagnostic performance and minimal complications. The main drawback with FNA is the proportion of nondiagnostic specimens, due to insufficient or bloody material being obtained (category I of The Bethesda System For Reporting Thyroid Cytopathology, TBSRTC).1 This accounts for 10–33% of FNA,1–9 with an estimated risk of malignancy of 5–10%.1 Other causes of nondiagnostic FNA are those with atypia or follicular lesions of undetermined significance (Bethesda III), which account for 15–40% of those performed2,4–7 and have an estimated risk of malignancy of 6–18%.1 Other publications generically report 20–30% of FNA being nondiagnostic.10–12
Repeating the FNA achieves a diagnosis in 60–80% of cases.2,4,8,10–13 In nodules with repeatedly nondiagnostic FNA, lobectomy is recommended, particularly in those strongly suspected of malignancy on ultrasound or showing significant growth on repeat ultrasounds, or when there are clinical risk factors for thyroid cancer.1,2,4,8,11,14 However, this strategy means having to accept the risks and costs of surgery, which causes an overload on the healthcare system due to the high prevalence of thyroid nodules, especially in women over 50.2,3
Ultrasound-guided core needle biopsy (CNB) has shown promising results in terms of diagnostic performance and safety in these patients.3–9,12–15 The American Thyroid Association (ATA) guidelines do not yet include CNB among its recommendations due to the lack of sufficiently strong evidence,2 while the American Association of Clinical Endocrinologists, American College of Endocrinology and Associazione Medici Endocrinologi [Italian Medical Endocrinologists Association] (AACE/ACE/AME) suggest the use of CNB in nodules with repeatedly nondiagnostic FNA,16 and the Korean Society of Thyroid Radiology (KSThR) extends the recommendations to nodules with indeterminate FNA or with troublesome cytological diagnosis.4,6 Good results have also been reported with CNB as an initial diagnostic technique, without prior FNA.10,11 The main limitations of CNB are: the fact that the procedure has to be performed with great care due to the risk of neck complications and so has a longer learning curve than FNA4; the lack of standardisation in its diagnostic categories4,8–10; and the fact that the small size of the samples means it does not detect capsular or vascular invasion after a diagnosis of follicular cell proliferation, making lobectomy necessary to differentiate between adenoma and follicular carcinoma.3,4,6,9–12,15.
Our aim was to determine the diagnostic performance of CNB in nodules with two previous nondiagnostic FNA, assess the associated complications, analyse the reliability of the diagnoses obtained, and measure the economic impact of avoiding lobectomy after CNB with benign findings.
Material and methodsStudy designBecause of the high proportion of non-diagnostic FNA in our centre and the consequent problems of delay and additional care costs, in 2011 a multidisciplinary unit (MU) was set up with a radiologist, a pathologist, an internal medicine specialist and an endocrine surgeon, all with more than 8 years of experience in their respective areas of expertise on thyroid nodular disease, to discuss the management of these patients at periodic meetings. The fact that FNA was performed by various radiologists with different levels of experience, and the limited availability of sonographers and pathologists to assess the suitability of samples in situ due to their heavy workload and the time-consuming nature of this procedure, made it difficult to significantly improve the performance of FNA. On the basis of the results reported in several published studies,3,12 we decided to perform CNB on all nodules with two nondiagnostic FNAs which met the criteria for biopsy recommended by the ATA guidelines in force at that time.17
This study was a retrospective review of all CNB performed from December 2011 to January 2019. It was approved by our centre's ethics committee, which specified that the informed consent of the participants was not required because the data presented is aggregated and the research is on an activity previously established in the institution’s routine healthcare practice.
Information on the biopsy technique, complications and results, as well as the ultrasound follow-ups, was obtained from the local radiological system. The information on surgery, surgical-specimen pathology and number of follow-up consultations was obtained from each patient's digital medical records.
Biopsy procedureOnce the medical decision has been made to perform a CNB, before scheduling the procedure, the MU radiologist checks the patient’s medical records for laboratory abnormalities or medications that increase bleeding risk, although in fact a thyroid biopsy is considered to be a procedure with low risk of bleeding.18,19 If there are no results for platelet levels or international normalised ratio (INR) within the last year, a blood test is requested, to be performed a few days before the biopsy. If significant abnormalities are found (platelets < 50,000/μl, INR>2) or the dose of some medication needs to be changed before the CNB, the haematology department or the treating physician is notified of the date of the biopsy, so they can make arrangements for any necessary changes and reintroduction after the CNB.
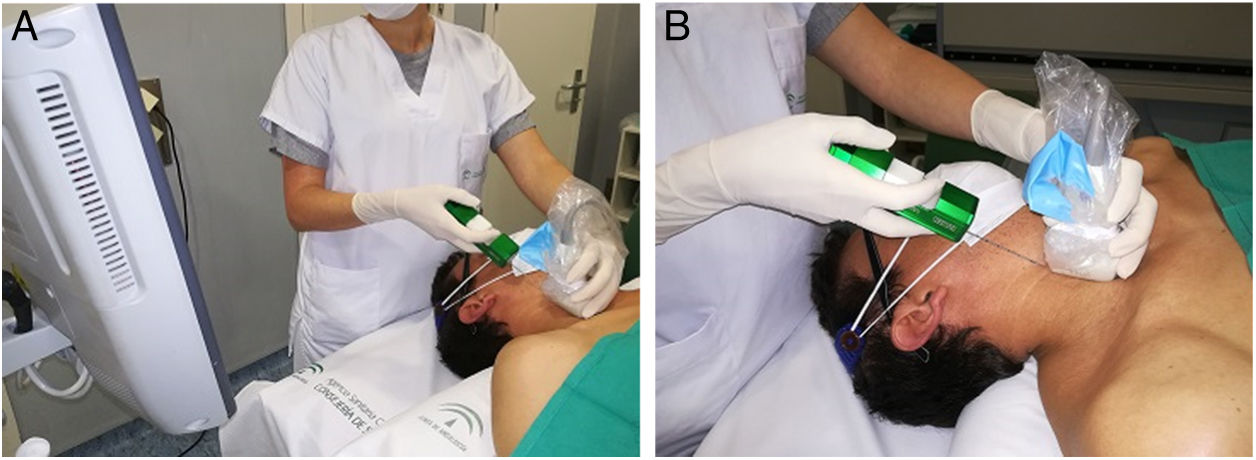
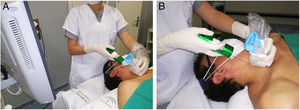
The MU radiologist performed all of the CNB. Written consent was obtained in all cases after informing patients of the benefits and risks of the test. With the patient's neck hyperextended, the radiologist is generally positioned behind the patient (Fig. 1) to allow a longitudinal approach in a craniocaudal direction. This allows the needle and the target to be visualised in the same plane. The skin is sterilised with povidone iodine or chlorhexidine, 10ml (200mg) of 2% subcutaneous mepivacaine is administered, and a small skin incision is then made to facilitate the entry of the needle into the subcutaneous fat on the neck. Positioning the tip of the needle on the edge of the nodule, a CNB is taken with an 18G needle and automatic biopsy gun (Magnum®, Bard Medical Division, Covington, Georgia, USA), which produces cylinders of 15 or 22mm in length, depending on the size and position of the nodule. Two hands-free passes are performed and, in exceptional circumstances, a third if visual inspection shows a lack of sufficient tissue. After applying compression for about five minutes, in the absence of bleeding or other complications, the skin incision is covered with skin adhesives plus a sterile dressing and the patient is released, with the recommendation that they return to the department the next day for a check-up. The cylinders are sent in to the laboratory formalin and are always examined by the MU pathologist. In the event of a malignant result, this information is communicated to the other members of the MU and a priority appointment is assigned in the surgery department. The rest of the results are presented at the next MU meeting, for medical discussion and decision making.
Approach for core needle biopsy. A) The patient is in the supine position. His neck is hyperextended and rotated in the opposite direction to the location of the nodule. Hyperextension is aided by placing a pillow under the shoulders. The radiologist stands at the head of the patient, standing or sitting, depending on the height of the trolley, with the ultrasound at their side and the screen turned towards them to continuously monitor the progression of the needle inside the neck, longitudinally and in a craniocaudal direction, normally keeping the biopsy plane away from the airway and neck vessels. If the nodule is located in the lower pole of the thyroid lobe and measures less than 15mm in diameter in the longitudinal axis, the transisthmic approach is considered, to avoid penetrating the confluences of the neck and subclavian veins and the pulmonary vertices, and the risk of mediastinal haematoma or pneumothorax, when using a biopsy gun with a minimum needle advance of 15mm. B) The transducer and needle are shown in the same plane, on the craniocaudal axis. The transducer is covered with a sterile sleeve, to maintain the asepsis of the biopsy site.gr1
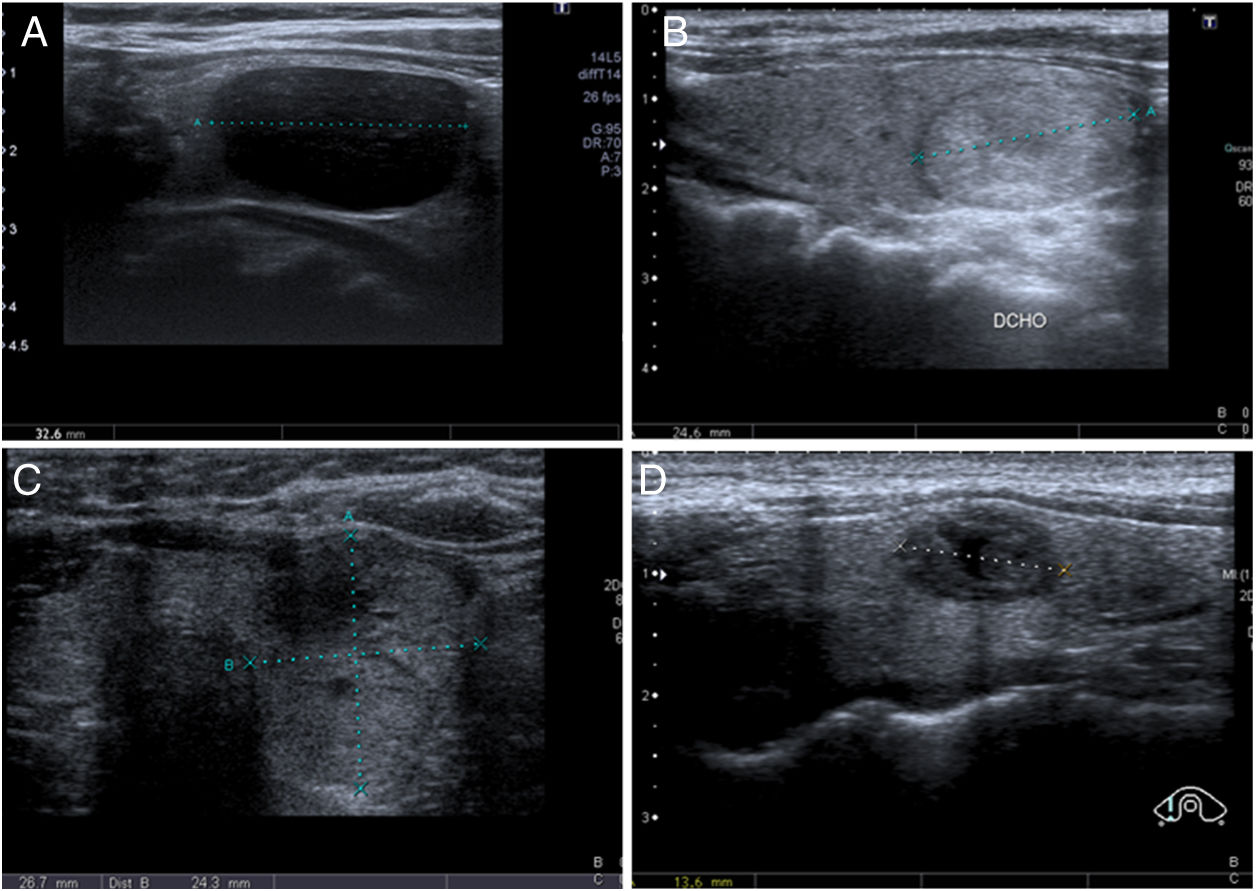
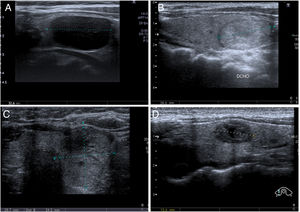
Unlike in FNA, the diagnoses obtained by thyroid CNB are not standardised. At our institution we use a classification system similar to that described by Paja et al. in their articles10,11 with four main diagnostic categories: insufficient (I); benign (B), including adenomatous hyperplasia and thyroiditis; follicular or Hürthle cell proliferation, including follicular/Hürthle cell adenoma and carcinoma; and malignant, which includes papillary carcinoma, medullary carcinoma and other less common types (lymphoma, metastasis, etc). Examples of each category are shown in Fig. 2. In some studies they use the same TBSRTC as for FNA,8,9,20 while in others they propose an adapted version with slight modifications.4,6
Diagnostic categories for core needle biopsy (CNB) of the thyroid. A) Insufficient (I): 58-year-old woman. Nodule with intermediate ultrasound suspicion of malignancy (solid and hypoechoic nodule, oval in shape and without microcalcifications) and a diameter greater than 1cm. CNB with insufficient result. In the multidisciplinary unit (MU) it was decided to perform diagnostic lobectomy, resulting in papillary carcinoma with free margins, in the context of colloid nodule and goitre. B) Benign (B): 56-year-old woman. Nodule with low ultrasound suspicion of malignancy (solid and isoechoic, oval in shape and without microcalcifications) and a diameter greater than 1.5cm. CNB with result of adenomatous hyperplasia. Subsequent ultrasound stability for 18 months. C) Follicular proliferation (FP): 55-year-old woman. Nodule with high ultrasound suspicion of malignancy (solid and isoechoic, without microcalcifications, but "thicker than it is wide in the axial plane) and a diameter greater than 1cm. CNB with result of follicular cell proliferation. Follicular carcinoma was diagnosed in surgery. D) Malignancy (M): 34-year-old woman. Nodule with high ultrasound suspicion of malignancy (solid and hypoechoic, with a small central cystic area and microlobulated margins) and a diameter greater than 1cm. A fine needle aspiration biopsy was performed, it was classified as Bethesda III, and at the MU it was decided to perform CNB, the result of which was medullary carcinoma, later confirmed by surgery.gr2
Lobectomy is required for the diagnoses of malignancy and follicular cell proliferation. With a benign diagnosis (except in very symptomatic nodules, which would be candidates for surgery) ultrasound and clinical follow-up are necessary to rule out changes in the ultrasound characteristics or significant growth which might suggest a false negative for thyroid cancer, in which case the biopsy should be repeated. Both suspicious ultrasound signs and significant growth are defined in various different guidelines.2,16,21 As after FNA with benign results, the recommendation is for the first ultrasound 12 months after the biopsy and two subsequent scans at intervals of 18 months. Follow-up should not be continued for more than five years if there are no changes.2
There are other results (normal thyroid tissue, fibrosis/fibromuscular tissue, sclerosis), which usually indicate a failed aspiration of the nodule and the sample being of the surrounding tissue, either due to the small size of the nodule, its posterior or inferior location, making access difficult and increasing the risk of injury to surrounding structures, or both.4,10–12 Both in the above cases, which we define as an inadequate sample, and in cases of insufficient diagnosis (I), the decision to repeat CNB, perform surgery or start follow-up was made in the MU based on the ultrasound characteristics and risk factors for malignancy.
Cost analysis after core needle biopsy with benign resultThe total cost of the diagnosis in each patient with a benign CNB includes a prior blood test if there is no recent one, the CNB, histopathological analysis of the samples, an appointment at the internal medicine clinic to communicate the result and prescribe the follow-up, a follow-up ultrasound in 12 months, and another appointment with internal medicine and discharge from hospital follow-up if there are no changes, with referral to primary care for subsequent check-ups. If diagnostic lobectomy had been performed in these patients, the total cost would have included a full preoperative assessment, surgery, hospital stay, histopathological analysis of the surgical specimen, a surgical wound check-up in a nursing clinic and a check-up in a surgical clinic, with discharge from hospital follow-up and referral to primary care. We considered the standard hospital stay after an uncomplicated lobectomy to be one day, as this was the case in 86.2% of the 503 lobectomies performed in our institution from 2011 onwards. The information on these costs is shown in Table 1.
Cost comparison between ultrasound-guided core needle biopsy of the thyroid gland and uncomplicated lobectomy.
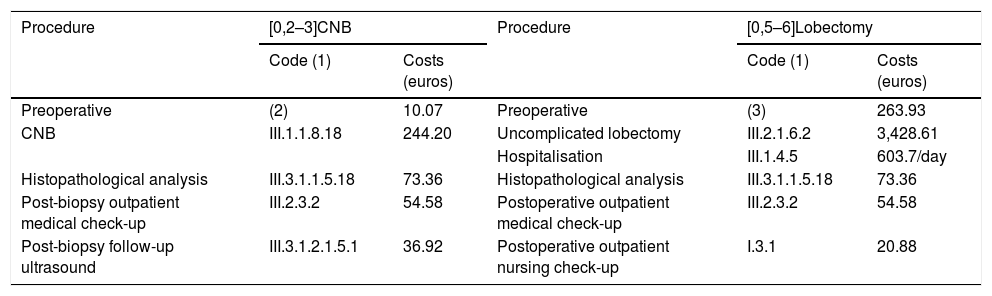
| Procedure | [0,2–3]CNB | Procedure | [0,5–6]Lobectomy | ||
|---|---|---|---|---|---|
| Code (1) | Costs (euros) | Code (1) | Costs (euros) | ||
| Preoperative | (2) | 10.07 | Preoperative | (3) | 263.93 |
| CNB | III.1.1.8.18 | 244.20 | Uncomplicated lobectomy | III.2.1.6.2 | 3,428.61 |
| Hospitalisation | III.1.4.5 | 603.7/day | |||
| Histopathological analysis | III.3.1.1.5.18 | 73.36 | Histopathological analysis | III.3.1.1.5.18 | 73.36 |
| Post-biopsy outpatient medical check-up | III.2.3.2 | 54.58 | Postoperative outpatient medical check-up | III.2.3.2 | 54.58 |
| Post-biopsy follow-up ultrasound | III.3.1.2.1.5.1 | 36.92 | Postoperative outpatient nursing check-up | I.3.1 | 20.88 |
CNB: core-needle biopsy.
1. Codes for each procedure. Available at: https://www.juntadeandalucia.es/servicioandaluzdesalud/profesionales/recursos-para-profesionales/precios-publicos.
2. Includes blood analysis with complete blood count (III.3.1.3.1.309, 5.30 euros) and haemostasis test (III.3.1.3.1.444, 4.77 euros).
3. Includes: preoperative assessment by anaesthetics and surgery (III.2.3.1; 114, 12 euros each consultation), chest X-ray (III.3.1.2.1.1.1, 9.23 euros), electrocardiogram (III.3.4.11, 16.23 euros), blood analysis with complete blood count (III.3.1.3.1.309, 5.30 euros) and haemostasis test (III.3.1.3.1.444, 4.77 euros).
We did not include the costs of previous FNA (238.91 euros each FNA, code III.3.1.1.2.14) or out-of-hospital follow-up or indirect costs, such as the number of working hours lost.
Statistical analysisWe carried out a descriptive analysis of frequency distribution for qualitative variables.
In patients who had surgery, the reference used to measure the reliability of the CNB was the surgical specimen pathology result. Initially, we only considered CNBs with a diagnosis of malignancy as positive. The detection of carcinoma in the biopsied area of the surgical specimen was considered true positive, while non-detection was considered false positive. We considered CNBs without a diagnosis of malignancy as negative, and the detection of carcinoma in the biopsied area of the surgical specimen was considered false negative, while non-detection was considered true negative.
Subsequently, in line with previous publications,10,11 we started to consider the diagnoses of malignancy and follicular cell proliferation as positive, given that lobectomy is required in both cases. The detection of carcinoma or follicular adenoma in the biopsied area of the surgical specimen was considered true positive, while non-detection was considered false positive. We considered CNB without a diagnosis of malignancy or follicular cell proliferation as negative, and the detection of carcinoma or follicular adenoma in the biopsied area of the surgical specimen was considered false negative, while non-detection was considered true negative.
In patients who did not have surgery, the reference to measure the reliability of the CNB was ultrasound stability for more than one year, which is the one most used.7,9,13,15 We considered CNBs with a non-benign result as positive, and the detection of growth or significant ultrasound changes was considered as a true positive, while ultrasound stability was considered as a false positive. We considered CNBs with a benign result as negative, and the detection of growth or significant ultrasound changes was considered as a false negative, while ultrasound stability was considered as a true negative.
Sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) for thyroid cancer were measured in both groups of patients. In patients who did not have surgery, we also assessed the relationship between CNBs with a benign result and ultrasound stability for more than one year using Fisher's exact test, considering p <0.05 as significant.
The statistical analysis was performed with EPIDAT software, version 3.1 (Department of Epidemiology, Dirección Xeral de Sáude Pública de la Xunta de Galicia, Santiago de Compostela, Spain).
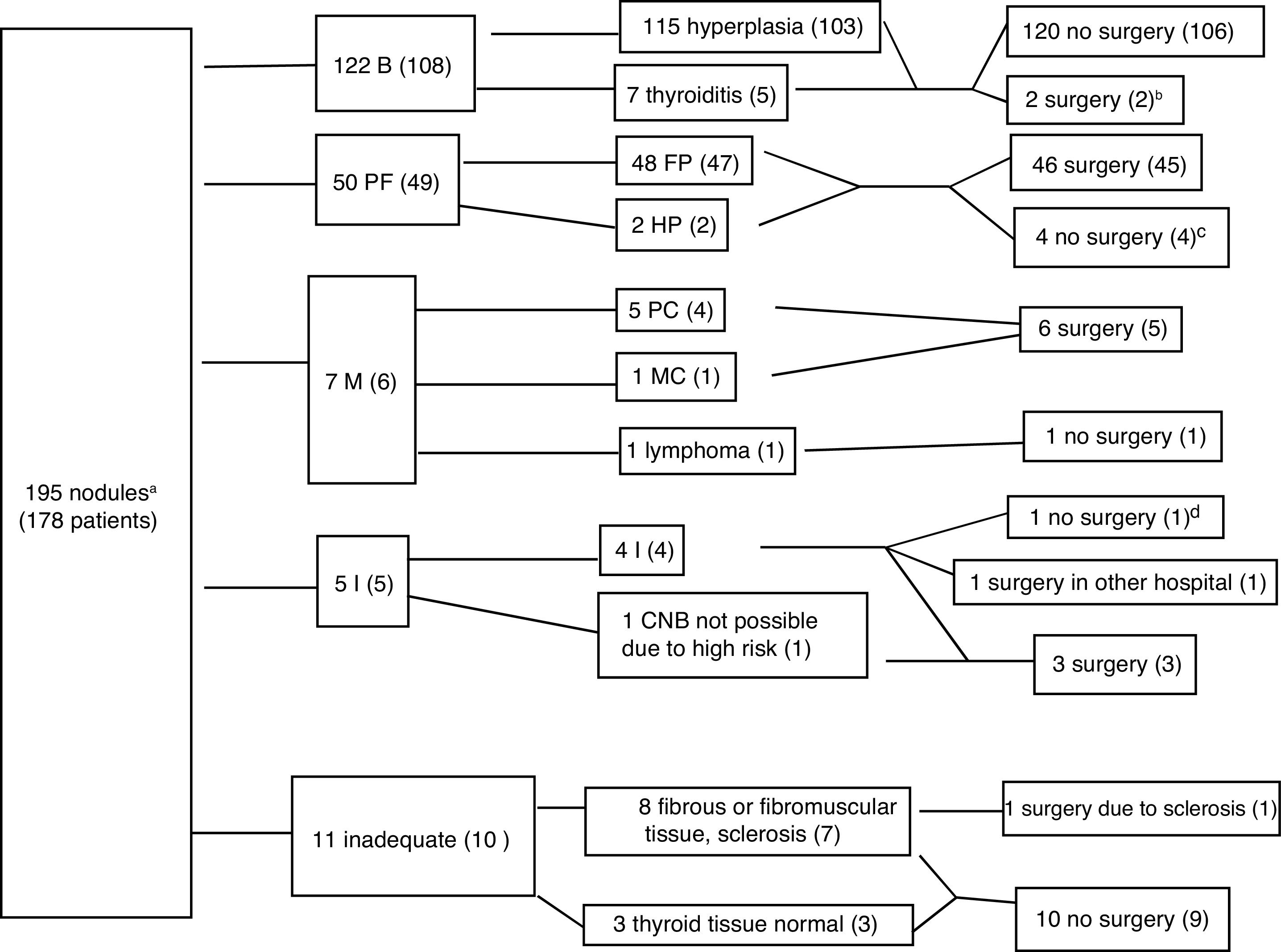
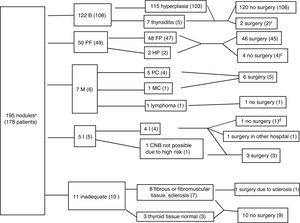
ResultsCNB was performed on 195 nodules from 178 patients (83.1% women, mean age 55.2 with standard deviation of 12.56 years), with a prior TBSRTC category I diagnosis in 98.4% of the nodules. Fig. 3 shows the diagnoses and the distribution between patients who had and did not have surgery for each outcome. The CNB was diagnostic in 179 nodules (91.7%), including 122 benign nodules (62.5%), 50 follicular cell proliferation (25.6%), and seven malignant (3.6%). CNB was not diagnostic in 16 nodules due to insufficient or inappropriate material (8.2%), and it was insufficient in five patients (2.5% of all the biopsies), four of whom had surgery, with cancer detected in two cases (one operated on in another hospital) and adenomatous hyperplasia in another two, while the remaining case was followed up by ultrasound (legend to Fig. 3). In the remaining 11 nodules, the MU recommended ultrasound follow-up in all except one with highly suspicious ultrasound features, and analysis of the surgical specimen confirmed the result of the CNB (sclerosis and necrosis, without evidence of malignancy).
Algorithm with the core needle biopsy (CNB) results. The data are the number of nodules, with number of patients in brackets. Benign (B), follicular proliferation (FP), Hürthle cell proliferation (HP), malignant (M), papillary carcinoma (PC), medullary carcinoma (MC), insufficient (I). Four patients had two nodules with different histology: one had insufficient and hyperplastic nodules, one had fibromuscular and hyperplastic nodules, and two had nodules with follicular proliferation and hyperplastic nodules.gr3
a192 nodules with Bethesda I cytology and 3 nodules with Bethesda III cytology, which in the CNB were diagnosed respectively as goitre, medullary carcinoma (confirmed by surgery), and follicular cell proliferation, which in surgery was found to be follicular variant papillary carcinoma.
bThere were two benign (hyperplastic) nodules which were subject to surgery because both patients had other nodules with follicular cell proliferation.
cOne patient was ruled out for surgery by the anaesthetics department due to suffering a serious cardiac injury, one patient was not operated on because of advanced-stage breast carcinoma, and two refused surgery.
dThe patient also had a benign nodule. No cold areas were detected on scintigraphy. Conservative management was decided on, and the patient had ultrasound stability for more than one year.
There were complications in four procedures (2%). Using the Society of Interventional Radiology definitions of complications,22 there were four minor complications (three superficial haematomas, which resolved spontaneously, and one vasovagal syncope mid-procedure which required temporary interruption, with recovery after fluid therapy and postural measures) and no major complications (those which require hospital treatment, or cause permanent adverse sequelae or death).
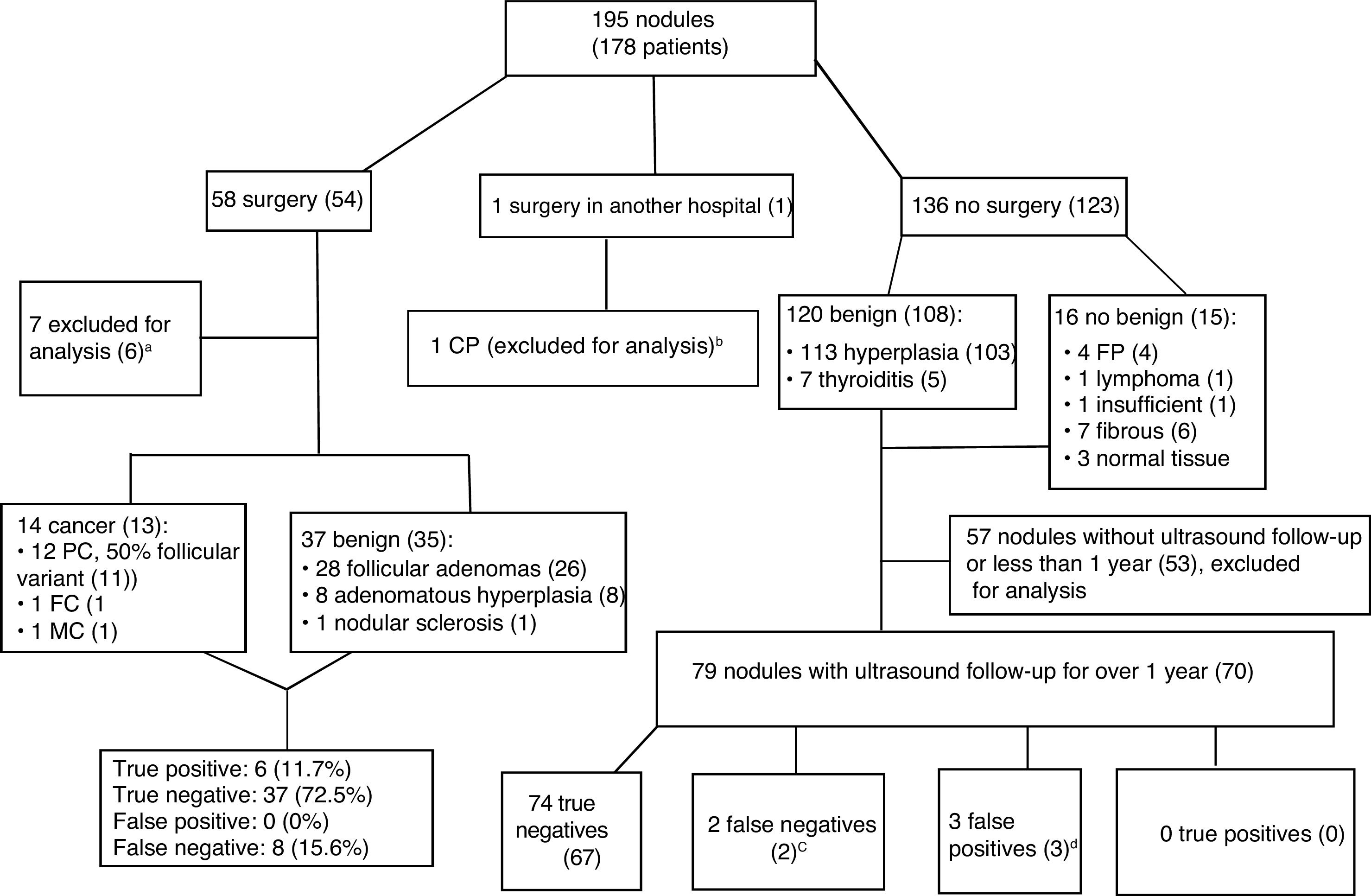
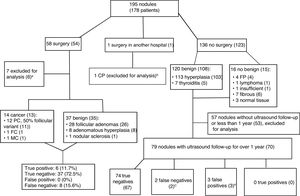
Fig. 4 shows the management of patients after CNB and the final diagnoses. Only 130 of the 195 nodules in the sample (66.6%) were able to be used for measuring the reliability of the CNB results: 51 nodules in 48 patients who had surgery; and 79 nodules with ultrasound follow-up longer than one year in 70 patients who did not have surgery. The 13 patients with a final diagnosis of cancer had a similar distribution by gender (84.6% women) and age (52.69+7.69 years) with respect to the sample overall.
Algorithm for patient management after core needle biopsy and final diagnoses. The data are the number of nodules, with number of patients in brackets. Papillary carcinoma (PC), follicular carcinoma (FC), medullary carcinoma (MC).gr4
aFive cancers detected in the surgical sample in non-biopsied areas and two biopsies insufficient for diagnosis (one not performed after several attempts as high risk due to location and size of the nodule), with benign results in surgery, so they are not true negatives or false positives.
bCNB insufficient for diagnosis. Surgery and histopathology analysis performed in another hospital.
cIn one patient, the biopsy was repeated, with benign results once again. The other patient was lost to follow-up after growth was detected.
dTwo nodules with fibromuscular and insufficient results in two patients with additional benign nodules, and one patient with a nodule with follicular proliferation rejected for surgery by the anaesthetics department due to associated cardiac lesion.
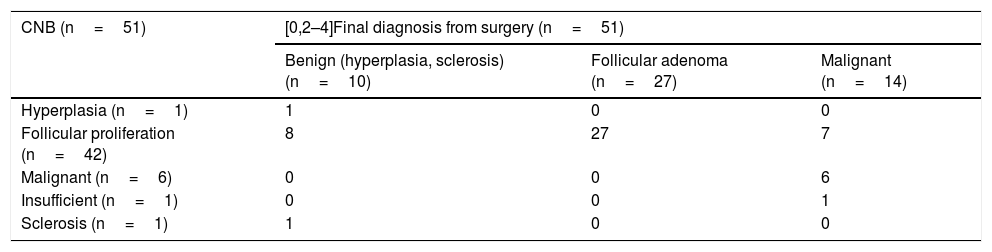
Table 2 shows the correlation between the CNB results and those of the surgery. The Se, Sp, PPV and NPV values for the CNB for thyroid cancer were 42.8%, 100%, 100% and 82.2% respectively. There was a high percentage of false negatives (8 of the 14 nodules with a final diagnosis of carcinoma, 57%) which explain the low sensitivity. However, in seven nodules the diagnoses obtained by CNB were false positives (87.5%). Of these, the final diagnosis after surgery was of lesions with a follicular pattern in four nodules (1 follicular carcinoma and 3 papillary carcinomas, follicular variant, with invasion of the surgical margin in one case); and in the remaining three nodules, small foci of papillary carcinoma with free surgical margins in thyroids with follicular adenomas. As malignancy and follicular cell proliferation were considered to be positive, there was only one false negative, a nodule with insufficient CNB and papillary carcinoma with free margins after surgery. In this case, surgery was decided on at the MU due to the moderate ultrasound suspicion of malignancy (Fig. 2A). There were 40 true positives (34 follicular cell proliferation plus 6 malignancies), eight false positives due to a diagnosis of follicular cell proliferation in CNB and adenomatous hyperplasia in surgery, and two true negatives with concordance between CNB and surgery (adenomatous hyperplasia and nodular sclerosis). The values obtained for Se, Sp, PPV and NPV were 97.5%, 20%, 83.3% and 66.6% respectively.
Correlation between core-needle biopsy and surgery.
| CNB (n=51) | [0,2–4]Final diagnosis from surgery (n=51) | ||
|---|---|---|---|
| Benign (hyperplasia, sclerosis) (n=10) | Follicular adenoma (n=27) | Malignant (n=14) | |
| Hyperplasia (n=1) | 1 | 0 | 0 |
| Follicular proliferation (n=42) | 8 | 27 | 7 |
| Malignant (n=6) | 0 | 0 | 6 |
| Insufficient (n=1) | 0 | 0 | 1 |
| Sclerosis (n=1) | 1 | 0 | 0 |
Of the 79 nodules which had ultrasound follow-up for more than one year, two, due to enlargement, ended up being false negatives (Fig. 4), but there were no diagnoses of malignant transformation. In one case. CNB was repeated, again obtaining a benign result, so it really was a true negative (Fig. 4 legend). The values obtained for Se, Sp, PPV and NPV were 0%, 96.1%, 0% and 98.6% respectively. There was no statistically significant relationship between a benign result and ultrasound stability for more than one year (p >0.05).
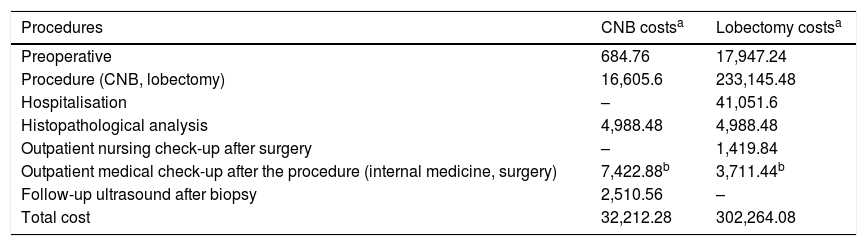
Table 3 compares the diagnostic costs in the 68 patients with benign CNB and ultrasound stability for more than one year with the costs involved in performing 68 uncomplicated lobectomies. CNB reduced the costs by 89.4%.
Cost comparison between benign diagnosis with core-needle biopsy plus ultrasound stability for more than one year versus uncomplicated lobectomy.
| Procedures | CNB costsa | Lobectomy costsa |
|---|---|---|
| Preoperative | 684.76 | 17,947.24 |
| Procedure (CNB, lobectomy) | 16,605.6 | 233,145.48 |
| Hospitalisation | – | 41,051.6 |
| Histopathological analysis | 4,988.48 | 4,988.48 |
| Outpatient nursing check-up after surgery | – | 1,419.84 |
| Outpatient medical check-up after the procedure (internal medicine, surgery) | 7,422.88b | 3,711.44b |
| Follow-up ultrasound after biopsy | 2,510.56 | – |
| Total cost | 32,212.28 | 302,264.08 |
In our series of 195 thyroid nodules with two previous non-diagnostic FNAs, the CNB achieved a diagnostic performance of 91.7%, with minor complications in 2% of cases and no major complications. CNB showed 100% specificity and PPV for detection of malignancy, and sensitivity of 97.5% for detecting nodules that require surgery, either as treatment (malignant) or to complete the diagnosis (follicular proliferation). In benign nodules, CNB had a NPV of 98.4% and there were no cases with proven malignant transformation. This suggests that it can replace lobectomy, with a large reduction in diagnostic costs, although ultrasound follow-up is required after the CNB to confirm the benign result. These results are consistent with those described in other publications.
The rate of CNB with insufficient or inadequate material was 8.3%, similar to that described in other articles (5%,3 8%4 and 12.9%8). It is slightly higher, however, than that described by Paja et al. (3.4–5.8%), who use CNB as the first diagnostic tool.10,11 In our centre, we perform CNB after two non-diagnostic FNAs, generally in nodules whose size, location or composition make it difficult to obtain useful samples, and this bias may explain our higher rate. Comparing CNB to FNA (for example, of the 314 FNAs performed at our institution in 2018, 42.4% were non-diagnostic, the vast majority Bethesda category I, and 10.7% were nodules biopsied twice), we observed a difference similar to that reported by Suh et al. in their meta-analysis,20 although they used the Bethesda system to communicate the diagnoses obtained by both techniques. These results, alongside the similarity in the costs of FNA and CNB in our setting (238.91 vs 244.20 euros respectively) could be an argument in favour of using CNB as the first diagnostic tool. However, despite positive results in series with large samples,10 there is still not enough evidence to recommend its general use.4,6 Moreover, it would require several radiologists in a department to train in the technique in order to respond to the great demand and perhaps the use of more restrictive criteria than those of the ATA2 in the indication for biopsies, such as TI-RADS.21
Our percentage of complications was similar to that of other studies.3–6,10 Those that compare the complications of CNB and FNA do not report significant differences.5,20 In addition to the need for operator experience, to minimise risks, it is important to check the patient’s medical records for abnormal laboratory results or medications that might increase the risk of bleeding, and to plan the biopsy based on the ultrasound images. We have generally used the longitudinal approach, which means the needle path is well away from the trachea (more medial) and the neck vessels (more lateral) and the entire length of the needle can be visualised as it accesses the nodule. Although the specialised literature generally recommends the transisthmic approach to avoid recurrent laryngeal nerve injury and subsequent vocal cord paralysis,4,10,11 we had no such complications in our series. We apply compression to the skin wound for a brief period (approximately 5min) after completing the CNB, similar to that recommended by Paja et al.,10 and shorter than the 20−30min recommended by the KSThR.4,6
If we consider malignancy only as positive for thyroid cancer, our results are comparable with the Suh et al. meta-analysis,13 in which, after analysing four studies with 496 nodules, they obtained Se of 44.7%–85%, Sp and a PPV of 100% and a NPV of 65%–94%. When like Paja et al.,10,11 we consider malignancy and follicular cell proliferation as positive results, we obtain similar Se (97.5%) and PPV (83.3%) to theirs: 95% and 88.7% respectively.11 The high sensitivity of CNB for diagnosing nodules with a follicular pattern is clinically important, as surgery is required in order to distinguish between carcinoma and adenoma by detecting capsular or vascular invasion. Follicular carcinoma (approximately 12% of thyroid cancers, including Hürthle cell; one case in our series [7.14%], Fig. 2C), particularly its variants, is an aggressive, poorly differentiated tumour with extensive vascular invasion and 10-year survival of approximately 50%. Meanwhile, follicular adenoma is a common benign lesion.2,12,17 The decrease in PPV from 100% to 83.3% is possibly due to the biopsy being taken from the central area, which often has scarred or sclerosed areas with associated microfollicles in hyperplastic nodules, simulating a follicular adenoma in the CNB samples.12 If technically possible, it is preferable to obtain samples from the periphery of the nodules where they are less prone to necrosis or sclerosis.12
In the nodules with benign CNB, the NPV was 98.6%, with one false negative (1.4%), although this diagnosis was only based on growth during ultrasound follow-up, without histopathological confirmation of cancer. Paja et al et al. obtained a NPV of with 0.4% false negatives after ultrasound follow-up of 3,223 benign nodules for more than two years. This would suggest that the CNB is sufficient to confirm benign findings, making it unnecessary to perform surgery, confirmatory biopsy or even ultrasound follow-up.10 The lack of a statistically significant relationship between benign findings and ultrasound stability for more than one year in our series (p>0.05), possibly due to the small number of cases available (Fig. 4), does not allow us to suggest dispensing with ultrasound follow-up. Recommendations on utility and duration of follow-up are essentially defined for the cytological diagnosis. According to AACE/ACE/AME,16 the risk of false negatives in nodules with benign cytology is 1–2%, and like the ATA2 they recommend ultrasound follow-up with a first check-up at 12 months. There is no evidence to justify continuing follow-up for more than five years,2 but this remains an open debate, as thyroid cancer can grow and its ultrasound features can change very slowly.2,3,7,9,15
In nodules with benign CNB plus ultrasound stability for more than one year, we estimated a reduction in the diagnostic costs of close to 90% compared to lobectomy, only counting direct costs. Trimboli et al.14 estimated a reduction of one third, although in their analysis they also included malignant and indeterminate nodules, which require subsequent surgery, and did not include ultrasound follow-up in benign nodules. Improving diagnostic efficiency allows limited resources to be diverted to other priorities, which is a clear organisational need and an ethical imperative. Joint medical decision-making in the MU is essential if we are to achieve this objective.12
Our study has several limitations. Firstly, it was a retrospective review of a biased sample as we only included nodules with two previous non-diagnostic FNAs, which may have influenced the fact that our malignancy rate was lower than in series without this bias.10,11 Secondly, as it was a purely descriptive review, we only found ultrasound follow-up after biopsy in 63.3% of benign nodules (76 of 120, Fig. 4), which may have affected the statistical relationship between benign findings and ultrasound stability and cost reduction, which could be much higher. Thirdly, ultrasound stability for more than one year after a benign biopsy may be an insufficient time interval to rule out false negatives.3,7,9,15 Lastly, there is no consensus on the diagnostic categories for CNB, which may affect the reproducibility of the study.
In conclusion, thyroid CNB is effective because it enables diagnosis of the majority of nodules with repeatedly non-diagnostic FNA; safe if performed by experienced staff; reliable for diagnosing malignancy and benign lesions, and for identifying which nodules need surgery to complete the diagnosis because of their follicular histology; and efficient because it reduces costs compared to diagnostic lobectomy. Therefore, CNB is a promising tool for nodules difficult to diagnose by FNA, and could avoid many unnecessary surgical interventions.
Main points- •
Thyroid CNB is very effective in nodules with several non-diagnostic FNAs.
- •
Performed by experienced staff, it has minimal complications.
- •
It is reliable for detecting malignancy and identifying which nodules require diagnostic lobectomy.
- •
A benign result plus ultrasound stability reasonably rule out malignancy.
- •
CNB dramatically reduces the cost of diagnosis compared to lobectomy in benign nodules.
- 1
Person responsible for the integrity of the study: RCG.
- 2
Study conception: RCG.
- 3
Study design: RCG and MDME.
- 4
Data acquisition: RCG, MDME, LRC and CMS.
- 5
Analysis and interpretation of the data: RCG, MDME, LRC and CMS.
- 6
Statistical processing: RCG, MDME, LRC and CMS.
- 7
Literature search: RCG, MDME, LRC and CMS.
- 8
Drafting of the manuscript: RCG, MDME, LRC and CMS.
- 9
Critical review of the manuscript with relevant intellectual contributions: RCG, MDME, LRC and CMS.
- 10
Approval of the final version: RCG, MDME, LRC and CMS.
This research has not received funding from any sector, whether public, commercial or non-profit.
Conflicts of interestThe authors declare that they have no financial interests or personal relationships which might have influenced the work reported in this article.
To Francisco Rivas-Ruiz and the rest of the Research Unit staff for their help and advice in preparing the manuscript. To Alberto Jiménez-Puente (Assessment Unit) for obtaining and analysing the data on length of hospital stay for lobectomy since 2011.
Please cite this article as: Cortázar-García R, Martín-Escalante MD, Robles-Cabeza L, Martínez-Santos C. Utilidad de la biopsia con aguja gruesa ecoguiada en nódulos tiroideos con punción aspirativa con aguja fina no diagnóstica. Radiología. 2022;64:195–205.