To evaluate the safety and efficacy of cognitive targeting in multiparametric MRI-guided biopsy to obtain samples of the dominant nodule in prostate cancer.
Material and methodsWe performed cognitive-targeted biopsy after multiparametric MRI in 53 patients with progressive elevation of PSA. All patients provided written informed consent. Biopsies were done via a transperineal route under ultrasound guidance. The first three samples were obtained by cognitive targeting, with the target lesion determined by multiparametric MRI according to the PI-RADS (prostate imaging, reporting, and data system) criteria. Then nine cylinders were obtained from the remaining segments of the prostate (systematic biopsies). The pathologist evaluated the 12 cylinders without knowing which ones were obtained by cognitive targeting. In patients with multifocal lesions, we defined the dominant lesion as the one with the highest Gleason score and tumor volume; in patients with unifocal lesions, we defined the dominant lesion as the lesion identified.
ResultsWe diagnosed 29 prostate tumors. In 89.7% (26/29), the dominant nodule was diagnosed by the cognitive-targeted biopsy. If only cognitive-targeted biopsy had been done, the dominant nodule would not have been diagnosed in two (3.8%, 2/53) patients and only one (1.8%, 1/53) patient, in whom no sample was obtained from the lesion with the highest Gleason score, would have been understaged. The rate of positivity of cognitive-targeted biopsy was 50.9% (27/53) in the entire group of patients and 46.3% (19/41) in the group of patients with previous negative biopsies. No significant immediate or late complications were observed.
ConclusionCognitive targeting is safe and efficacious for detecting the dominant lesion in prostate cancer.
Evaluar la seguridad y eficacia de la biopsia guiada cognitivamente (BGC) con la resonancia magnética multiparamétrica (RMmp) para obtener muestras del nódulo dominante del cáncer de próstata.
Material y métodosRealizamos una BGC en 53 pacientes por elevación progresiva del PSA tras hacer una RMmp. Todos los pacientes firmaron el consentimiento informado. Las biopsias se realizaron por vía transperineal con control ecográfico. Las 3 primeras muestras se obtuvieron de la lesión diana determinada por la RMmp, utilizando criterios PIRADS (corresponden a las BGC). Posteriormente se obtuvieron 9 cilindros del resto de los segmentos de la próstata (biopsias sistemáticas). El anatomopatólogo valoró los 12 cilindros sin saber cuáles correspondían a las BGC y cuáles a las biopsias sistemáticas. Definimos como lesión dominante la de mayor valor Gleason y volumen tumoral en lesiones multifocales y a la única lesión detectada en tumores unifocales.
ResultadosDiagnosticamos 29 tumores de próstata. En el 89,7% (26/29), el nódulo dominante fue diagnosticado por las BGC. De haber realizado únicamente las BGC no se hubieran diagnosticado 2 pacientes (3,8%, 2/53) y se hubiera infraestadificado solo uno (1,8%, 1/53) en el que no se obtuvo muestra de la lesión con mayor valor Gleason. La tasa de positividad de la BGC fue del 50,9% (27/53), y en los pacientes con biopsias previas negativas del 46,3% (19/41). No observamos complicaciones significativas inmediatas ni tardías.
ConclusiónLa BGC es una técnica segura y eficaz para detectar la lesión dominante del cáncer de próstata.
Prostate cancer (PC) is the most frequent tumor in men.1 Most of them are diagnosed through an ultrasound-guided transrectal biopsy (UGTB). The percentage of positivity in the first UGTB ranges from 40 to 50%2 and from 18 to 32%3,4 in the second one. Managing a patient with progressive increase of prostate specific antigen (PSA) and repeatedly negative UGTB poses a challenge for both urologists and radiologists.
Although PC is a multifocal tumor, there are studies that show that up to 90% of the tumor volume can be attributed to a dominant lesion5 that becomes progressively aggressive. This lesion is hard to detect through ultrasound and the UGTB does not guarantee that the sample comes from it, especially if it is located in the apical or anterior area. The result can be the understadification of the PC whose percentage published is 38%.6,7
Multiparametric magnetic resonance (mpMRI) is more accurate than other diagnostic modalities to detect and characterize PC.8,9 It is the most reliable modality to guide prostate biopsy toward the dominant lesion. During the last few years, several modalities have been developed to guide biopsy through mpMRI (cognitive, fusion software, biopsy in magnetic resonance).10,11 The biopsies within magnetic resonance require compatible robotic equipment, are expensive and increase the time of the study.11 Both the fusion computer software between the mpMRI and the ultrasound images require additional expenses in ultrasound equipment and they do not improve the results of cognitive-guided biopsy (CGB).12 In the CGB the operator makes a “mental” correlation between the mpMRI images and those of the ultrasound while performing the biopsy. To this end he can be assisted by printed images or by images from an additional screen.
The goal of this study is to assess the safety and effectiveness of the CGB through findings in the multiparametric MRI-guided biopsy to obtain samples of the PC dominant node.
Materials and methodsPatientsBetween January and December 2013, we included 53 patients in whom prostate biopsy was indicated due to progressive increase of PSA and who had undergone mpMRI 2 months earlier at the latest. Among them, 41 had previous negative UGTB and high PSA since the last biopsy, 9 did not have previous biopsies, the PSA was >4ng/ml, in 3 patients the PSA was >2ng/ml of the NADIR after being treated with external radiotherapy due to their PC. All patients were informed of the procedure and they signed a written informed consent. Authorization was not requested from our hospital ethics committee since it was a retrospective study that did not mean any changes in the management of patients.
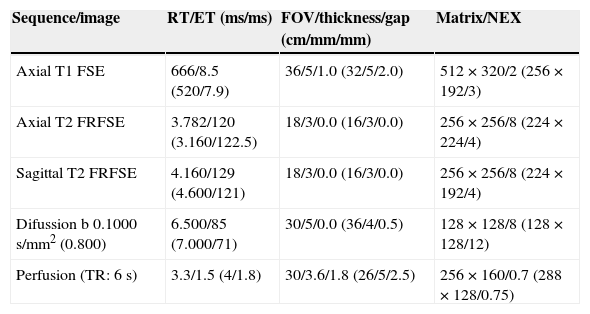
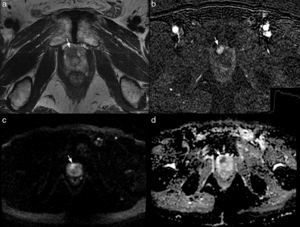
ModalitympMRI. The studies were carried out in a 1.5T resonance (Signa, GE, Milwaukee, WI, USA) in 28 patients and a 3T resonance (Signa, GE, Milwaukee, WI, USA) in 25. In all of them an 8-channel surface antenna without endorectal probe was used. The parameters of the sequences are described in Table 1. The mpMRI report described the target lesion as the largest and most aggressive following the PIRADS criteria13 (Fig. 1).
Technical parameters of the multiparametric MRI sequences.a
| Sequence/image | RT/ET (ms/ms) | FOV/thickness/gap (cm/mm/mm) | Matrix/NEX |
|---|---|---|---|
| Axial T1 FSE | 666/8.5 (520/7.9) | 36/5/1.0 (32/5/2.0) | 512×320/2 (256×192/3) |
| Axial T2 FRFSE | 3.782/120 (3.160/122.5) | 18/3/0.0 (16/3/0.0) | 256×256/8 (224×224/4) |
| Sagittal T2 FRFSE | 4.160/129 (4.600/121) | 18/3/0.0 (16/3/0.0) | 256×256/8 (224×192/4) |
| Difussion b 0.1000s/mm2 (0.800) | 6.500/85 (7.000/71) | 30/5/0.0 (36/4/0.5) | 128×128/8 (128×128/12) |
| Perfusion (TR: 6s) | 3.3/1.5 (4/1.8) | 30/3.6/1.8 (26/5/2.5) | 256×160/0.7 (288×128/0.75) |
FOV, field of vision; FRFSE, fast relaxation fast spin echo; FSE, fast spin echo; NEX, number of excitations; TR, temporal resolution; ET, echo time; RT, repetition time.
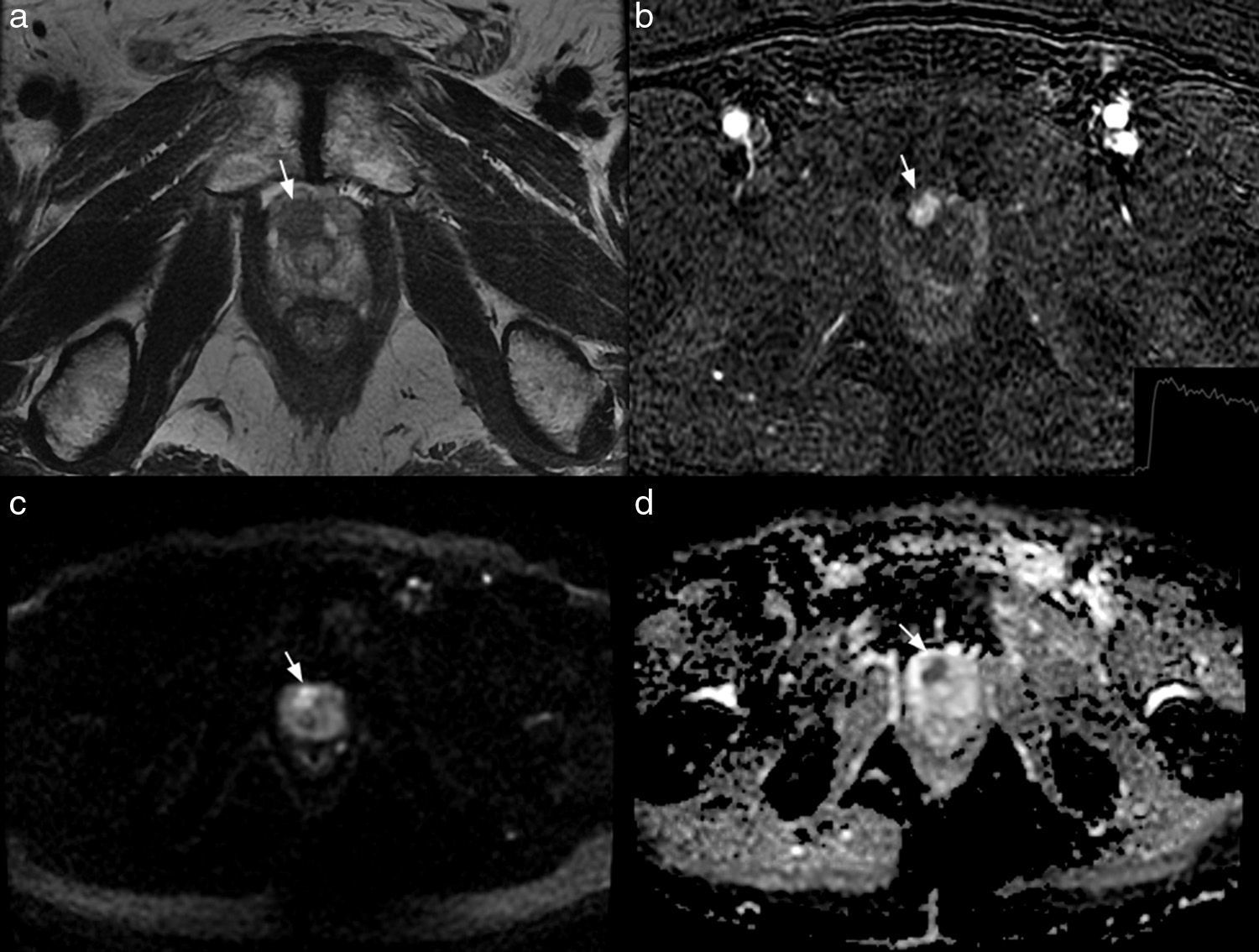
Multiparameter 3T MRI in a patient of 70 years with PSA of 6.3ng/ml and a transrectal ultrasound-guided biopsy previanegativa. The axial T2-weighted image shows a focal hypointense lesion (arrow in a) located on the right apicalanterior segment enhances PIRADS 4. The injury early in the subtraction image (arrow in b) and shows a delayed washing (graph b), PIRADS 5. the lesion is hyperintense on diffusion sequence, (b) value 1000 (arrow in c) and has a low coefficient dedifusión (arrow in d), PIRADS 5.
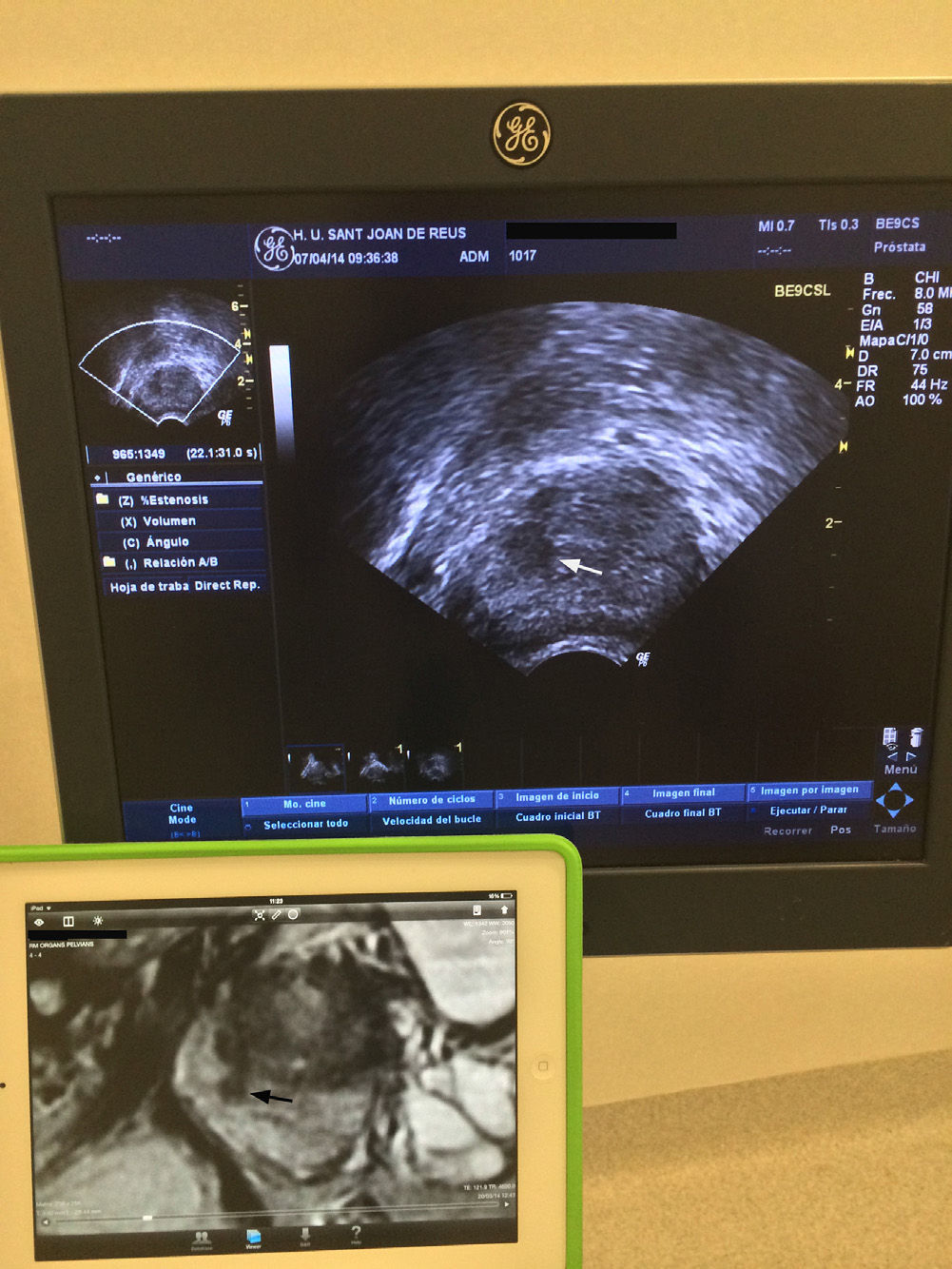
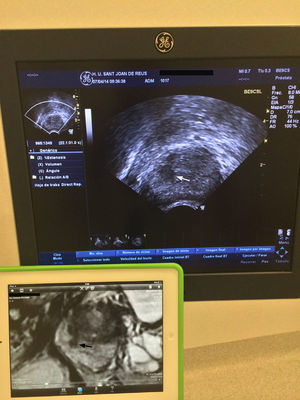
Transperineal biopsy. The patients received prophylactic antibiotic treatment (1g IV ceftriaxone before the biopsy and oral 500mg ciprofloxacin every 12h during the following 5 days). Biopsies were performed with the patient in lithotomy position and a biplane transrectal probe was used (BE9CS, GE Healthcare, Milwaukee, WI, USA) to obtain sagittal images of the prostate. These images were correlated with the T2 weighted mpMRI sagittal images, available on an adjacent screen (Fig. 2). After applying iodine on the perineum skin, the prostate was blocked with 10ml of 1% mepivacaine for each lobe, using a 22G×15cm Chiba needle. From two dots marked on the skin, a mean of six cylinders per lobe, using a 18Gx20cm needle and an automatic biopsy gun (Bard, Magnum Biopsy instrument, AZ, USA) were obtained. The first three cylinders were obtained from the target lesion and they corresponded to the CGB. The remaining cylinders were obtained from the other segments of the prostate (systematic biopsies) (Fig. 3). There was a total of 12 cylinders separated into 4 jars, one with the CGB.
Relationship in the time of biopsy guiadacognitivamente between the image of the transrectal ultrasound. Convision longitudinal and sagittal T2-weighted image of multiparameter. Larm in an accessory screen. Focal hipointensa injured right middle peripheral gland (flechanegra) that is hypoechoic on ultrasound (white arrows).
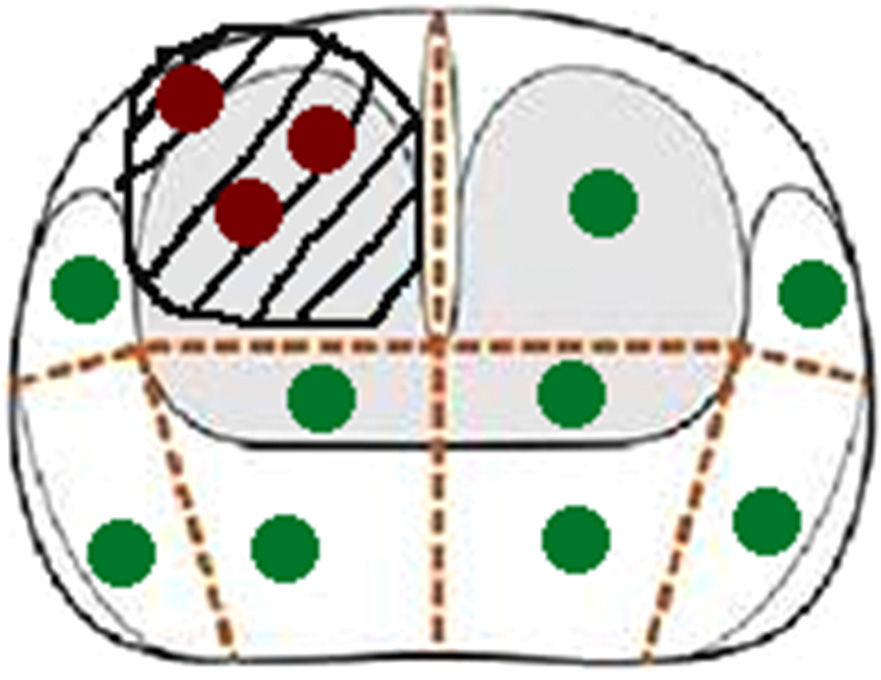
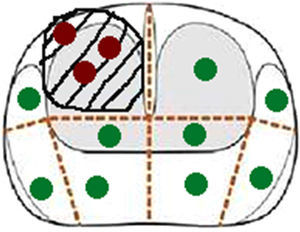
Scheduling scheme transperinealdel biopsy same patient in Fig. 1. The red dots corresponds to cylinders cognitively guided biopsy, and cylinders verdesa systematic biopsy.
The pathologist analyzed the samples not knowing the mpMRI results or which jar contained the CGB. The tumor was defined as multifocal when both the CGB and the systematic biopsies were positive, excluding the systematic positives due to the spreading of the target lesion to adjacent segments. The lesions with the highest Gleason score results and tumor volume in multifocal lesions, and the single lesion in unifocal lesions were defined as dominant lesions. Tumor volume was calculated by adding the volume of the positive cylinders multiplied by its percentage of affectation.
At the moment of the biopsy, immediate complications were reported such as bleeding through the perineum puncture points and urethrorrhagia. The patient was kept under observation for 5h and his body temperature and blood pressure were evaluated. The patients were followed for the next 3 months through our own hospital information system to detect any possible late complications (visits to the ER or hospital admissions).
ResultsThe patients’ average age was 65 (48–71) years and the PSA was 12.6 (2.3–90)ng/ml. The average number of previous biopsies in the group of patients with negative biopsies was 2.1–5 Two patients whose target lesions were located next to the prostatic urethra suffered from a self-limited post-puncture urethrorrhagia. None of the patient had fever, low blood pressure or required transfusions or admission after keeping them under control for 5h. During the 3 following months, only one patient went to the ER, 1 month after the biopsy due to perineum pain, without inflammatory signs in the examination or the analysis, which subsided with analgesia.
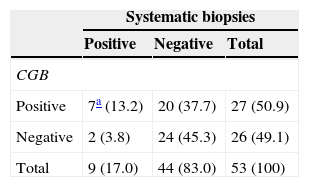
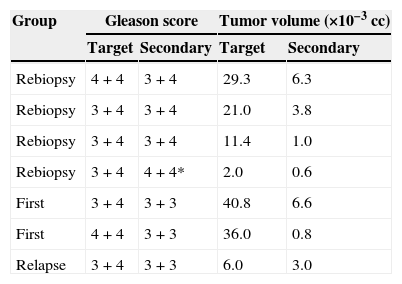
The result of the CGB and their systematic biopsies is summarized in Table 2. Among the 53 patients biopsied, 29 had PC. In 20, the CGBs were positive and the systematic CGBs were negative; in 7, both the CGB and the systematic biopsies were positive (multifocal tumor); and in 2, the CGBs were negative and the systematic biopsies were positive (1 patient with clinical suspicion of relapse and the other 1 without previous biopsies). Both the Gleason score results and the volume tumor results of the target lesion and the secondary foci in the seven multifocal tumors are summarized in Table 3. In six of the seven lesions the Gleason score of the target lesion was greater than or equal to that of the secondary foci, and in one lesion it was lower (one patient with former negative biopsies).
Contingency chart of CGB results and the corresponding systematic biopsies.
| Systematic biopsies | |||
|---|---|---|---|
| Positive | Negative | Total | |
| CGB | |||
| Positive | 7a (13.2) | 20 (37.7) | 27 (50.9) |
| Negative | 2 (3.8) | 24 (45.3) | 26 (49.1) |
| Total | 9 (17.0) | 44 (83.0) | 53 (100) |
Percentages are shown between brackets.
Comparison of Gleason scores and tumor volumes between the target lesion and the secondary foci.
| Group | Gleason score | Tumor volume (×10−3cc) | ||
|---|---|---|---|---|
| Target | Secondary | Target | Secondary | |
| Rebiopsy | 4+4 | 3+4 | 29.3 | 6.3 |
| Rebiopsy | 3+4 | 3+4 | 21.0 | 3.8 |
| Rebiopsy | 3+4 | 3+4 | 11.4 | 1.0 |
| Rebiopsy | 3+4 | 4+4* | 2.0 | 0.6 |
| First | 3+4 | 3+3 | 40.8 | 6.6 |
| First | 4+4 | 3+3 | 36.0 | 0.8 |
| Relapse | 3+4 | 3+3 | 6.0 | 3.0 |
In one patient (*) the secondary lesion showed a Gleason score higher than that of the target lesion.
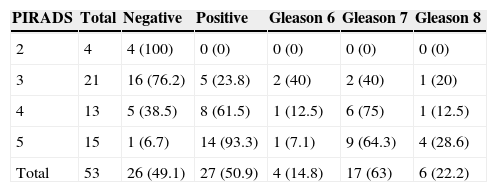
Adding the total number of tumors detected in the biopsies, in 89.7% (26/29) the dominant node was diagnosed through the CGB. If only the CGB had been performed in the 53 patients, 2 (3.8%, 2/53) would not have been diagnosed and only one (1.8%, 1/53) would have been understratified. The CGB percentage of positivity was 50.9% (27/53) and in the subgroups with previous biopsies, without previous biopsies and with biochemical relapses it was 46.3 (19/41), 66.7 (6/9) and 66.7% (2/3), respectively. The percentage of target lesions in the anterior and posterior segments was 64 (34/19) and 36% (19/53), respectively. The correlation between the target lesions PIRADS and the results of their CGB is summarized in Table 4. The percentage of positive lesions in the PIRADS lesions 2, 3, 4 and 5 was 0, 23.8, 61.5 and 93.3%, respectively. The percentage of Gleason lesions >6 in the PIRADS lesions 3, 4 and 5 was 60, 87.5 and 92.9%, respectively.
Correlation between the PIRADS score of target lesions and the CGB results.
| PIRADS | Total | Negative | Positive | Gleason 6 | Gleason 7 | Gleason 8 |
|---|---|---|---|---|---|---|
| 2 | 4 | 4 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 21 | 16 (76.2) | 5 (23.8) | 2 (40) | 2 (40) | 1 (20) |
| 4 | 13 | 5 (38.5) | 8 (61.5) | 1 (12.5) | 6 (75) | 1 (12.5) |
| 5 | 15 | 1 (6.7) | 14 (93.3) | 1 (7.1) | 9 (64.3) | 4 (28.6) |
| Total | 53 | 26 (49.1) | 27 (50.9) | 4 (14.8) | 17 (63) | 6 (22.2) |
Between brackets the percentage of positive and negative lesions and the percentage of lesions Gleason 6, 7 and 8 for every PIRADS value is shown.
In this study, the dominant node was diagnosed through the CGB in almost 90% of the cases, without any significant immediate or late complications being the detection rates of the dominant node and the positivity rates of the CGB high.
Our results are similar to and confirm the results already published. In a study including 95 patients with and without previous biopsies, the guided biopsies diagnosed the most aggressive lesion in 63 (88%) of the 72 tumors diagnosed.12 If only the guided biopsies had been performed, 6 (6.3%) of the 95 patients studied would not have been diagnosed and the PC would have been misdiagnosed in 3 (3.2%). In our study, the CGB did not diagnose two patients (3.8%, 2/53) and only one was understratified (1.8%, 1/53). In another study comparing the results of the CGB with the systematic biopsies in 555 patients without previous biopsies, the CGB did not detect 13 (2.3%) clinically significant tumors being the percentage of positivity 43%.14
The complications of transperineal biopsies have been described before.15 Guided biopsies require a further manipulation of the biopsy needle when its trajectory is adjusted toward the target lesion. Some of the lesions are adjacent to the prostatic urethra with a risk of causing urethrorrhagia appearing in 1.5–3% of the unguided transperineal biopsies and increasing with the number of shots attempted.15 In our study, two patients presented with urethrorrhagia (3.8%) and they both had a periurethral lesion. In 6–14% of the unguided transperineal biopsies, the patient visited the ER during the following 20 days.15 Only one of our patients (1.9%) did 1 month later due to perineal pain. None of the patients required transfusions or hospital admission after the 5-h observation period.
The mpMRI-guided biopsies allow us to diagnose lesions unaccessible through conventional biopsies and their use in on the rise.10 One of the questions that come up when guided biopsies are performed is whether it is necessary to obtain samples from the remaining segments of the prostate. The benefit of not doing so would entail fewer complications15 and it would avoid indolent cancers from being detected.14,16 Our results show that in most tumors detected the CGB targeted the most aggressive lesion which in turn defines the prognosis of the PC without misdiagnosing tumors in the group of patients with previous biopsies and one tumor being understratified only.
Our study has several limitations. It is a descriptive, retrospective study and the number of patients is small in the group without previous biopsy and in the one with suspicion of relapse. On the other hand, we were unable to compare the Gleason scores of the biopsies with those of the prostatectomy since most of our patients were treated through external radiotherapy. Another limitation is the low percentage of multifocal tumors – 13.2% (7/53) only. Most of our patients had previous negative UGTB so there is a certain selection bias toward unifocal tumors in anterior segments that may have improved the performance of the CGB. So it would be interesting to repeat this study only evaluating patients without previous biopsies with a more homogeneous sample of posterior and anterior lesions and a greater number of multifocal tumors.
In sum the CGB performed through the mpMRI findings is a safe procedure, one capable of obtaining a sample of the PC dominant lesion especially in patients with former negative biopsies.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Confidentiality of dataThe authors declare that the protocols of their institution on the publishing of data from patients have been followed.
Right to privacy and informed consentThe authors declare that in this article there are no data from patients.
Authors- 1.
Manager of the integrity of the study: J.G.B., A.C.O., D.P., J.A.A.C.
- 2.
Study idea: J.G.B., A.C.O., E.R., D.P.
- 3.
Study design: J.G.B., A.C.O., E.R., J.A.A.C.
- 4.
Data mining: C.H.S., E.S.A., A.C.O.
- 5.
Data analysis and interpretation: J.G.B., A.C.O., D.P., E.R., J.A.A.C.
- 6.
Statistical analysis: N/A.
- 7.
Reference search: J.G.B., C.H.S., E.S.A.
- 8.
Writing: J.G.B., A.C.O., C.H.S., E.S.A., J.A.A.C.
- 9.
Critical review of the manuscript with intellectually relevant remarks: J.G.B., A.C.O., E.R., D.P., J.A.A.C.
- 10.
Approval of final version: J.G.B., A.C.O., C.H.S., E.S.A., E.R., D.P., J.A.A.C.
The authors declare no conflict of interests.
Please cite this article as: Garcia Bennett J, Conejero Olesti A, Hurtado Salom C, Rebenaque E, Parada D, Serrano Alcalá E, et al. Utilidad de la biopsia guiada cognitivamente por resonancia magnética multiparamétrica para diagnosticar la lesión dominante del cáncer de próstata. Radiología. 2015;57:428–33.