To describe the most characteristic imaging findings for sclerosing encapsulating peritonitis, with an emphasis on the computed tomography findings.
ConclusionThe incidence of sclerosing encapsulating peritonitis is low. The pathophysiology of this condition is unclear. Two types are recognised: idiopathic and secondary; the secondary type is generally a complication of peritoneal dialysis. Its nonspecific clinical presentation and the absence of blood markers mean that sclerosing encapsulating peritonitis is usually diagnosed late. Thus, it is important to know the imaging signs; these include thickening and calcification of the peritoneum and dilation of bowel loops with thickening and calcification of bowel walls, whether in isolation or in association with loculated ascites. Although ultrasonography allows the complexity of the collections to be evaluated, computed tomography is the most useful technique for the general assessment of the signs mentioned above.
Describir los hallazgos radiológicos más característicos de la peritonitis esclerosante encapsulante en los diferentes métodos de imagen, con énfasis en la tomografía computarizada.
ConclusiónLa peritonitis esclerosante encapsulante es una enfermedad de baja incidencia, aunque desconocida, su fisiopatología no está clara y se reconocen dos tipos: idiopática y secundaria; esta última generalmente como complicación de la diálisis peritoneal. Su diagnóstico suele ser tardío debido a la presentación clínica inespecífica y a la ausencia de marcadores serológicos. Por este motivo es importante conocer los signos radiológicos, que incluyen engrosamiento y calcificación del peritoneo, dilatación de asas intestinales con engrosamiento y calcificación de sus paredes, ya sea aislados o asociados con ascitis loculada. Si bien la ecografía permite valorar la complejidad de las colecciones, la tomografía computarizada es el método de mayor valor para la delineación general de los signos radiológicos mencionados.
Sclerosing encapsulating peritonitis (SEP) is a rare disease characterised by inflammatory infiltrate and formation of fibrous tissue in the peritoneum that causes its thickening and rigidity. The affected small intestine loops lose motility, which can lead to bowel obstruction.
Owtschinnikow defined the entity for the first time in 1907. He described the encapsulation of the intestine by a fibrocollagen membrane and called it “chronic fibrous incapsulated peritonitis”.1 Due to its different causes and non-specific clinical manifestations, it is not usually initially considered among possible diagnoses, which delays its treatment and is associated with high morbidity and mortality.
It can be classified as idiopathic and secondary. The former affects mainly young women from tropical or subtropical countries2–4 or men from non-tropical countries.5,6 However, in 20157 Akbulut conducted a literature review on SEP of idiopathic origin and identified a 2:1 ration with predominance in men from tropical or subtropical regions. China, India, Turkey and Nigeria are the countries with the most reported cases.7
Its origin is linked to chronic irritation of the peritoneum, which generates an uncontrolled inflammatory response with the development of fibrosis. Multiple hypotheses about idiopathic aetiology have been proposed: retrograde menstruation with viral superinfection, tissue damage by immune cells secondary to gynaecological infections, and asymptomatic chronic peritonitis. Embryological disorders such as congenital greater omentum dysplasia and vascular anomalies have also been postulated, although none has been confirmed.7 The cases described in males and children do not support some of these hypotheses either.
It is important to differentiate SEP from peritoneal encapsulation, which corresponds to a rare congenital malformation characterised by an accessory peritoneal membrane that partially or completely covers the small intestine. It is usually asymptomatic and an incidental finding during surgery.8
The causes of secondary SEP are local or systemic irritative peritoneal factors such as continuous ambulatory peritoneal dialysis (CAPD), fungal infections, tuberculosis, luteinizing ovarian thecoma, ventriculoperitoneal and peritoneovenous shunts, terminal liver disease,9 orthotopic liver transplant,10 previous abdominal surgeries,11 sarcoidosis, systemic lupus erythematosus and familial Mediterranean fever. Other systemic causes include asbestosis, prolonged use of the beta-blocker practolol, methotrexate and protein S deficiency.7
CAPD is the most common cause, with an SEP prevalence of 0.4–8.9%.12 CAPD time is the main risk factor, presumably due to the exposure of the peritoneum to dialysis solutions. The risk of developing the disease after 5 years varies between 0.6 and 6.6%, it can even occur before the CAPD is discontinued.12 It has also been shown that exposure to glucose dialysis solutions not free of glucose degradation products is associated with increased risk.13 Other related factors are the use of conventional dialysis solutions, episodes of peritonitis, ultrafiltration failure, high membrane transport and early age of onset of CAPD.12,13
The pathophysiological mechanism is attributed to a chronic inflammatory peritoneal process triggered by dialytic solutions that activate a proinflammatory [transforming growth factor β1 (TGF-β1), interleukin 6 (IL-6)] and proangiogenic [vascular endothelial growth factor (VEGF)] cascade. This activity generates depletion of peritoneal mesothelial cells, production of extracellular matrix (type 1 collagen) with formation of fibrocollagenous tissue.14,15
The mechanism of action of beta-blockers is unknown, but probably related to the inhibition of surfactant release.14 In addition to practolol, other beta-blockers such as timolol, metoprolol, propranolol and atenolol have been associated with the development of this entity.7,14 In patients with terminal liver disease, it is believed to be linked to peritoneal irritation due to spontaneous bacterial peritonitis and ascites, and may occur despite liver transplantation.9,14
The clinical spectrum of the disease is variable. It can occur asymptomatically, with non-specific symptoms such as pain and abdominal distension, nausea and vomiting. In other cases, the clinical symptoms are more significant, with palpable mass, anorexia, weight loss and bowel obstruction. In the most severe cases it can lead to malnutrition, sepsis and death.
In dialysis patients a decrease in ultrafiltration and bloody dialytic effluent is observed.12,14 Although in the serum analysis it is possible to find an increase in C reactive protein, anaemia and hypoalbuminaemia, there are no specific blood markers or other reliable methods of screening.12
The histological substrate is characterised by the mesothelial cell layer partially replaced by fibrocollagen tissue composed of proliferation of fibroblasts and fibrin deposition.16,17 Sometimes lymphocyte and plasma cell infiltration can be found5,16 (Fig. 1H and I). These changes are non-specific and can also be observed in cases of ultrafiltration failure, long-term peritoneal dialysis and peritonitis of infectious aetiology.3
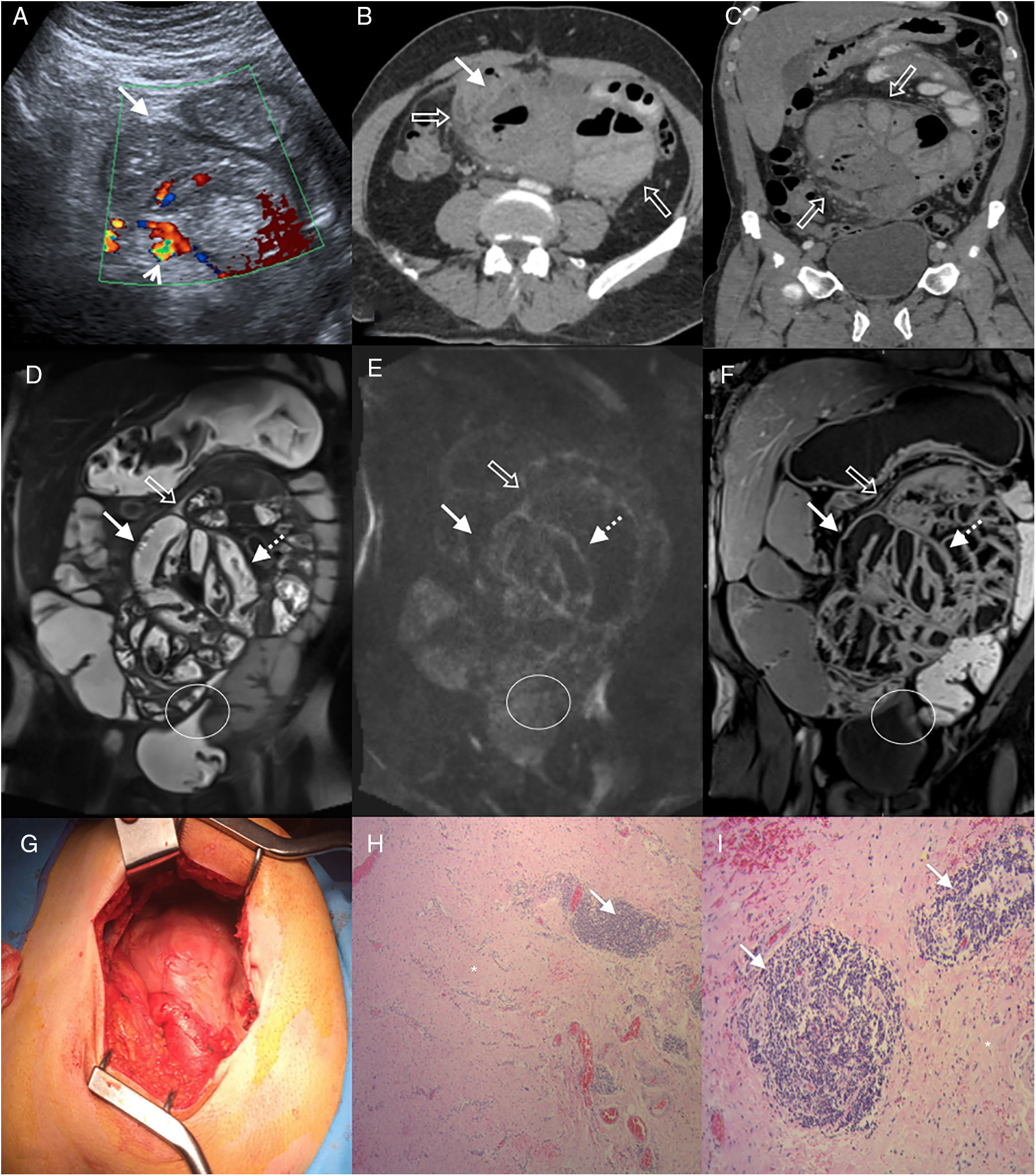
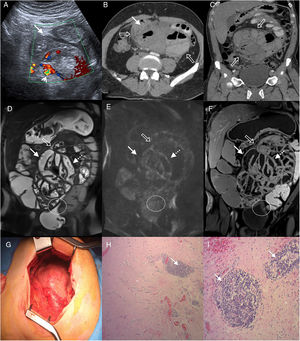
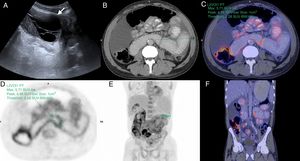
55-Year-old man examined due to intermittent abdominal pain and abdominal distension, with no relevant clinical history. (A) Doppler ultrasound, cross section of the abdomen showing grouped small intestine loops (white arrow) with mural thickening and signs of increased parietal flow (arrowhead). Multidetector computed tomography (MDCT) with intravenous contrast (B), cross section (C) and oblique coronal reconstruction, showing central abdominal conglomerate of dilated small intestine loops with moderately thickened walls and “encapsulated” by a thickened peritoneal membrane (hollow arrows). MR enterography with contrast, coronal sections (D) TSE T2 sequence (E), diffusion b 800 (F) and VIBE T1 sequence with fat saturation after administration of gadolinium (portal phase), which reveal parietal thickening with decreased folds (white arrows) of the loops encapsulated by the thickened peritoneal membrane (hollow arrows) and surrounding laminar fluid. Signs of restriction in diffusion and enhancement with contrast in the walls and the peritoneal membrane (dotted arrows), findings related to inflammatory changes and fibrosis. Note the retraction of the bladder roof in the area of contact with the peritoneal surface (circle). (G) Intraoperative photograph showing a thick membrane that encapsulates the small intestine. Histology of the peritoneum with haematoxylin–eosin 40× (H) and 100× (I) showing fibrosis with dense collagen fibres (asterisk) and chronic inflammation by lymphocyte infiltration and perivascular plasma cells (arrows).
Three phenotypes can be distinguished due to its macroscopic appearance17: type I, characterised by a thickened peritoneum, covered by fibrin and interloop membranes; type II, where the cover over the loops acquires a reticulated aspect; and type III, in which the capsule that encloses the intestine acquires rigidity with the tendency for shrinking and convergence of the loops involved, which gives it the appearance of an “intestinal cocoon” (Fig. 1G). This subtype is characterised by lower fibrin deposition.17
Diagnosis of SEP is based on clinical suspicion and confirmation by imaging techniques or a surgical approach. The alterations observed in the images can be grouped into presence of fluid collections, peritoneal affectation and small intestine disorders.
They can be single or multiple and occur in up to 90% of cases.18 Ultrasound is useful in its initial study and in the evaluation of the content or presence of septa (Fig. 2A), although limited when the collections are large. Multidetector computed tomography (MDCT) facilitates the general delineation of the loculations with a panoramic evaluation of the abdomen and pelvis (Fig. 3). It is important to remember that these collections increase in size as the disease progresses.
Remember that: thickening of the peritoneum is the most characteristic sign of the disease, evidenced in all cases.18,19
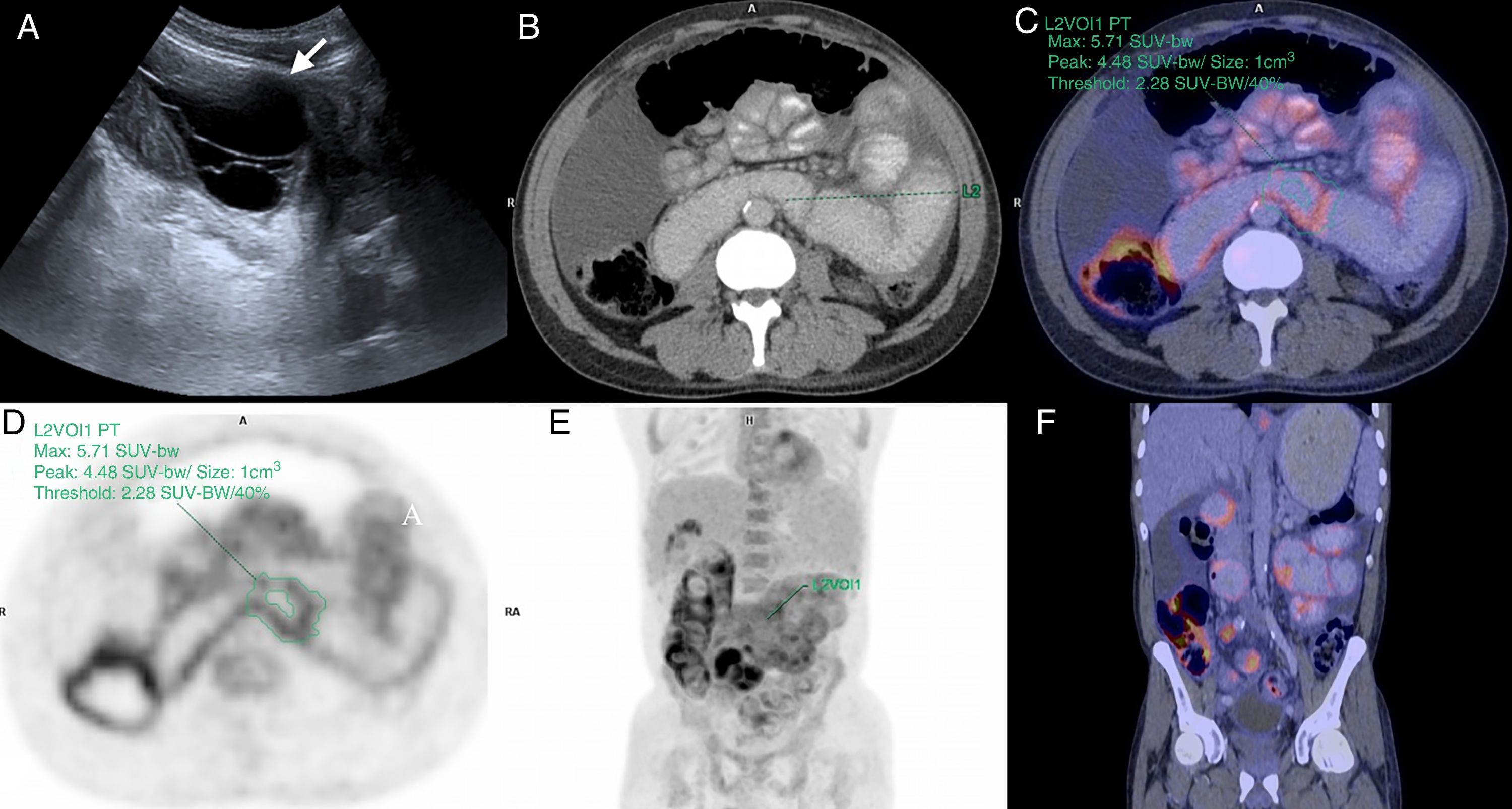
A 49-year-old man was examined due to abdominal pain, with a history of chronic kidney disease in stage V, in peritoneal dialysis for 5 years. (A) Abdominal B-Mode ultrasound, cross section of the left flank where a loculated fluid collection is observed (arrow). Positron emission tomography–computed tomography, cross (B–D) and coronal (E and F) sections showing diffusely increased radiotracer uptake (18F-FDG) in the walls of the small intestine. Maximum SUV: 5.71.
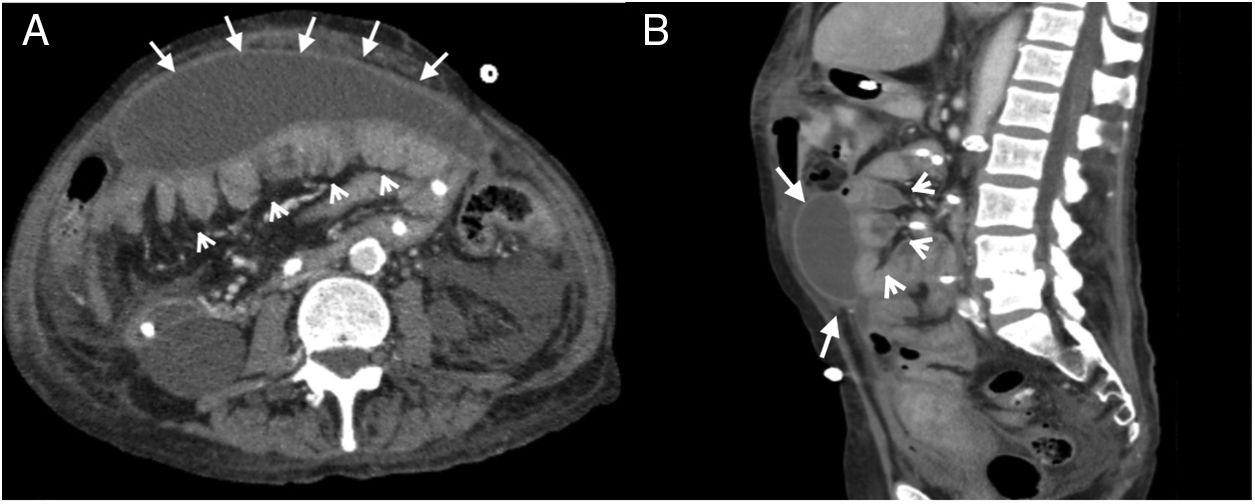
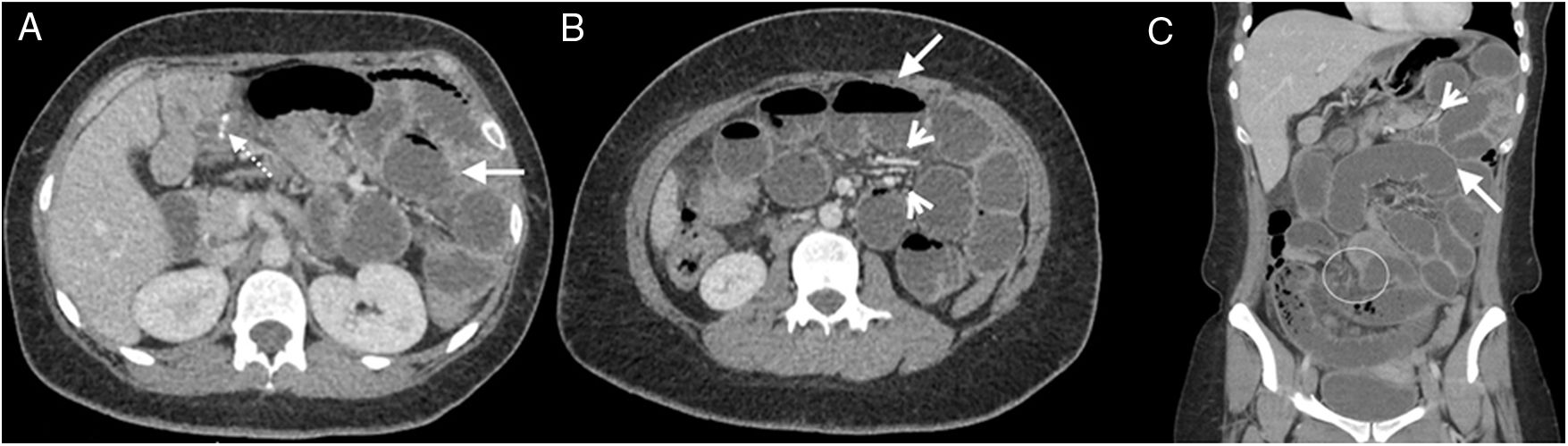
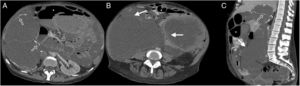
A 57-year-old woman was examined due to repeated intestinal obstruction, with a history of chronic kidney disease and peritoneal dialysis. Abdominal multidetector computed tomography with intravenous contrast, portal phase, cross section (A) and sagittal reconstruction (B), where a loculated fluid collection (arrows) with a defined wall that enhances with contrast is observed. Produces a mass effect posteriorly rejecting the small intestine loops (arrow heads).
Peritoneal calcifications can be seen in advanced stages.20 MDCT is the most useful method for demonstrating the involvement of the parietal and visceral peritoneum. It appears thickened and with intravenous contrast uptake due to inflammatory infiltration and diffuse fibrotic changes (Fig. 4). At first the thickening occurs in the form of isolated plaques, and then it is diffuse.21 Contrast enhancement is most evident when the peritoneum is surrounded by low attenuation structures such as fluid or fat.18 It also facilitates the evaluation of peritoneal thickening in studies without contrast. However, it must be considered that these findings are nonspecific, and similar in peritoneal infectious diseases.18
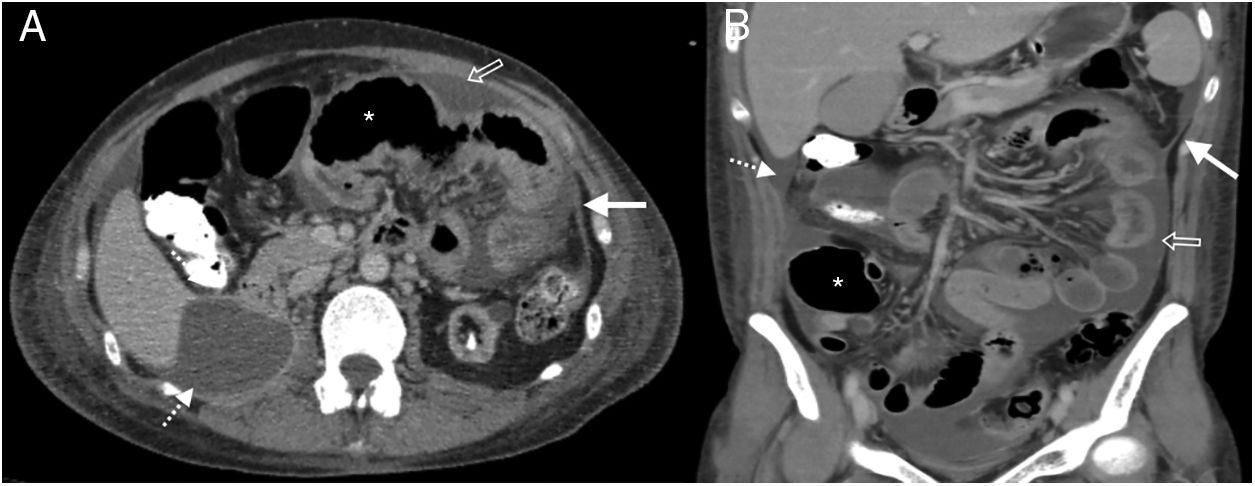
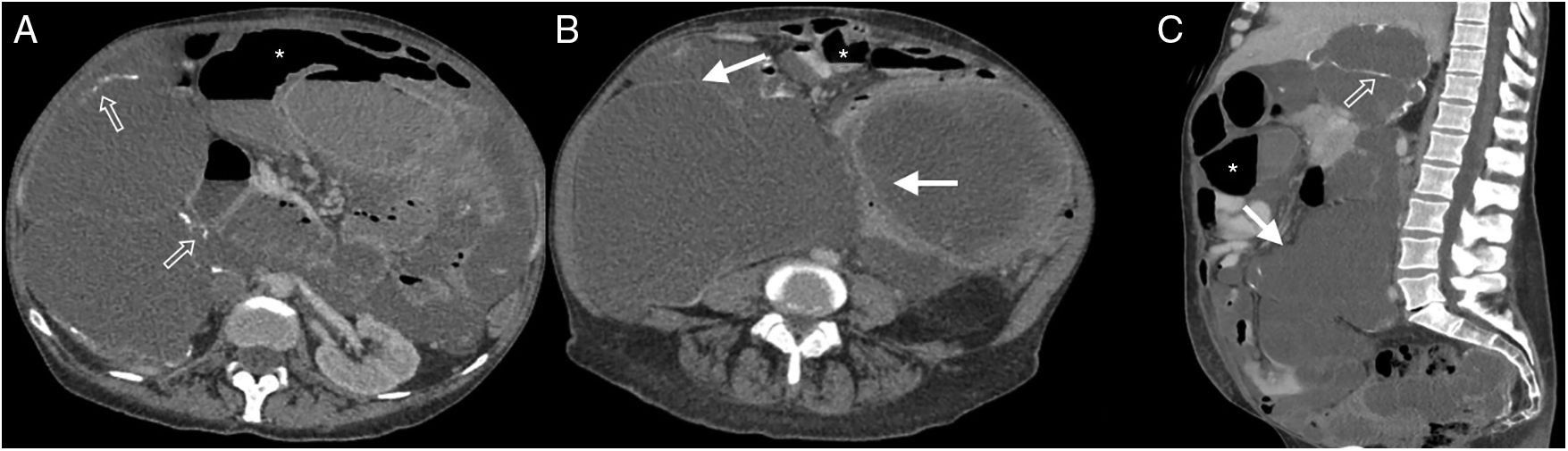
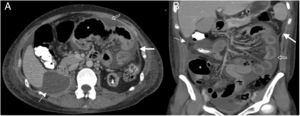
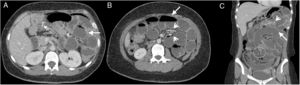
38-Year-old woman admitted for abdominal pain. History of systemic lupus erythematosus and chronic renal failure, in peritoneal dialysis. Multidetector computed tomography of the abdomen and pelvis with oral and intravenous contrast, cross section of the upper abdomen (A) and coronal reconstruction (B) where thickening and enhancement of the peritoneal surface on the left flank (white arrows), subhepatic loculated ascites (dotted arrow), free fluid between intestinal loops (hollow arrow) and distension of small intestine loops (asterisk) is observed. Bad evolution with death six months after the study.
MDCT is the most sensitive imaging method for the detection of calcifications,22 present in 70% of cases.19 Initially they are linear and can affect both peritoneal sheets. With the progression of the disease “sheet-like” plaques and conglomerates are formed18,21 (Fig. 5). An increase in number or size is associated with symptomatic worsening.18
Remember that: diffuse peritoneal calcification is associated with advanced disease; however, severe encapsulation can occur in the absence of calcifications.20
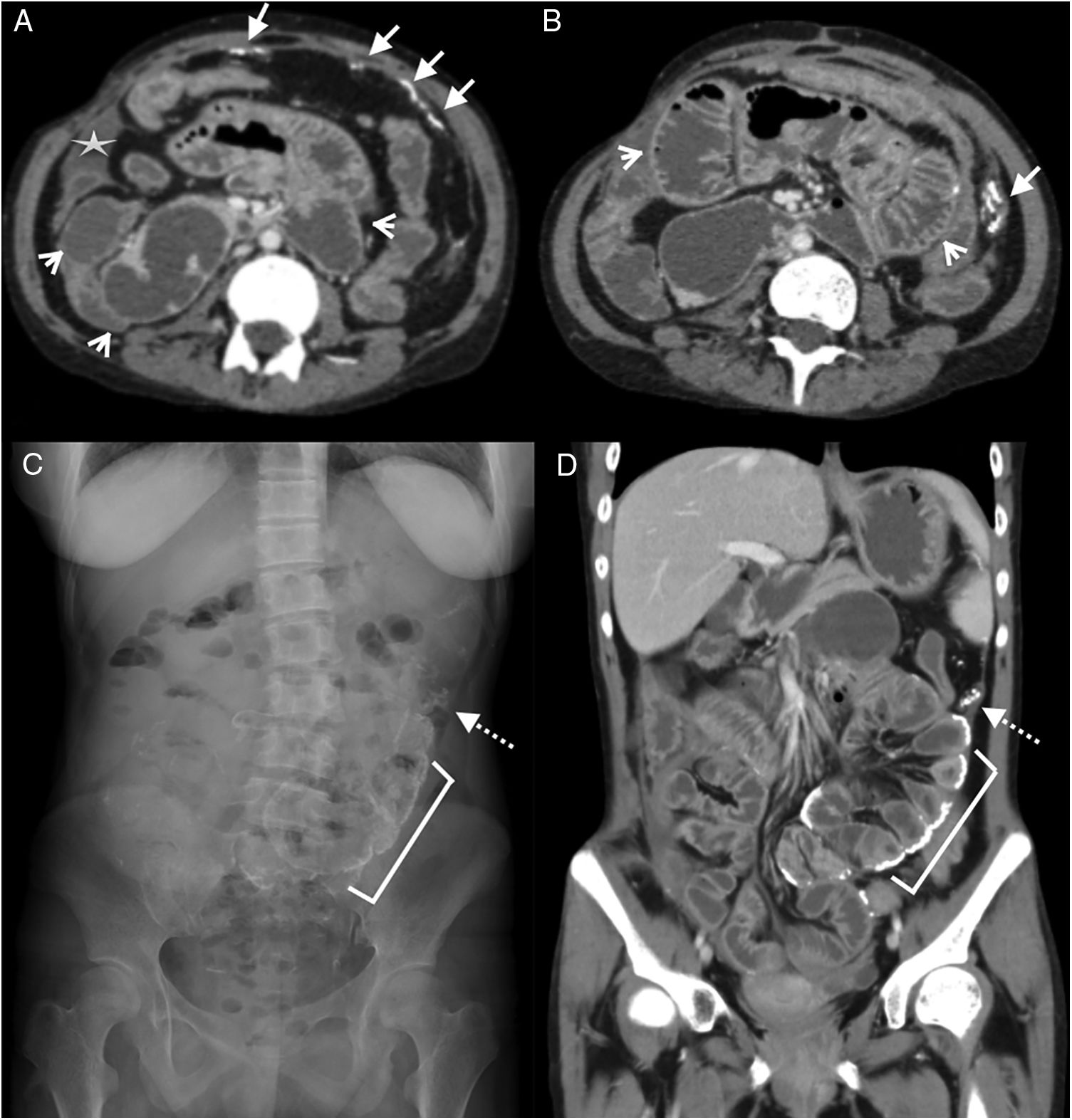
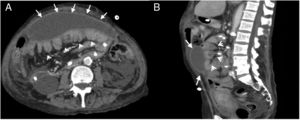
25-Year-old woman with diffuse abdominal pain. History of chronic kidney disease due to congenital nephrotic syndrome (diffuse mesangial sclerosis), in peritoneal dialysis for 10 years. Multidetector computed tomography (MDCT) with intravenous contrast, portal phase: (A) Cross section showing linear peritoneal calcifications (white arrows) and dilated sections of the small intestine (arrowheads), as well as a small subhepatic collection (star). (B) Cross section, caudal to A, showing calcifications in the form of a conglomerate in the parietal peritoneum of the left flank (white arrow) and dilation of small intestine loops (arrowheads). (C) Standing radiograph of the abdomen (anteroposterior) showing multiple linear calcifications corresponding to the intestinal wall (bracket) and peritoneum (dotted arrow). (D) MDCT, coronal reconstruction with findings similar to radiography.
On radiographs, the central abdominal distribution and the curvilinear appearance of the calcifications indicate their dependence on the intestinal wall or the visceral peritoneum. Linear peripheral calcifications tend to solidify in the parietal peritoneum (Fig. 5C).
The involvement of the small intestine is secondary to visceral peritoneal involvement, with decreased motility and progressive distension (Fig. 5). Sometimes it can form conglomerates at the expense of peritoneal encapsulation and/or due to the mass effect generated by the fluid collections.23 The peritoneal fibrotic process can progress and cause mural fibrosis with calcifications (Fig. 5D). In advanced stages, the mural thickening and the adhesions of the loops generate obstruction.23 Although these findings can be evaluated with ultrasound, they are more evident in the MDCT.
Another method that allows for evaluation of the peritoneal cavity is CT peritoneography using intraperitoneal iodinated contrast. Although it does not provide more information for the diagnosis of SEP, it is useful for the detection of complications of CAPD such as leaks, hernias and abscesses. In addition, it allows for the evaluation of the dynamics of the peritoneal fluid.18
There are few reports regarding the use of magnetic resonance imaging (MRI), although the findings are similar to those observed in MDCT24 (Fig. 1D–F).
In a preliminary study in which “cine” dynamic sequences were used, disturbance in the pattern of bowel movements was observed, which could help the initial diagnosis.25 Another alternative is the use of MRI without gadolinium, taking advantage of the peritoneal fluid as a contrast medium, but there is no experience with this technique for evaluating patients with SEP.
The absence of exposure to ionising radiation is an advantage of MRI; however, its higher cost and the potential risk of nephrogenic systemic fibrosis are disadvantages that restrict its use.22,26 It is important to remember this technique's limitation in the detection of calcifications.
Positron-emission tomography (PET) can be useful during the acute inflammatory phase where there is evidence of increased uptake of the radiotracer by the peritoneum.26 In our case (Fig. 2) increased fluorodeoxyglucose uptake was evidenced in the intestinal wall, although we have not found reports on this behaviour. The non-specificity of the signs, which are also observed for other causes of acute peritonitis, limits the use of this method. Studies of asymptomatic patients on CAPD have also not shown valuable findings.27 There is very little experience in the use of this method.14,26
Among the differential diagnoses, internal hernia in patients with signs of occlusion or intestinal sub-occlusion should be considered20,28 (Fig. 6). The main findings in MDCT are localisation and/or bundling with traction of small bowel loops, signs of obstruction, swelling and engorgement of mesenteric vessels and displacement by mass effect of adjacent organs.28 Another differential diagnosis is pseudomyxoma peritonei. This manifests low attenuation collections in the peritoneum, omentum and mesentery, including calcifications (Fig. 7). The absence of intestinal involvement helps radiological diagnosis. Pathologies that can cause peritoneal calcifications, such as tuberculosis, amyloidosis, hyperparathyroidism, and peritoneal carcinomatosis, should also be considered.20 In these cases, the history, clinical picture and blood markers will facilitate the diagnostic orientation.
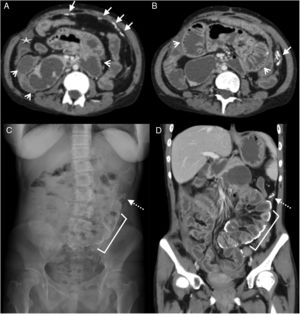
28-Year-old woman with a history of: gastric bypass one year prior, and recent appendectomy, admitted for abdominal pain in the epigastrium with irradiation to the left flank of 24h of evolution, without other associated symptoms. Multidetector computed tomography of the abdomen with intravenous contrast, portal phase, cross section (A and B) and coronal reconstruction (C) showing surgical traces of the jejuno-jejunal anastomosis in the right flank (dotted arrow), slight engorgement of the mesenteric vessels (arrowheads) and dilation of the small intestine (white arrow). At the central abdominal level, an obstruction site with a fine loop-thick loop (circle) transition is evident. Exploratory laparoscopy revealed a hernia through Petersen's space and central abdominal adhesions.
51-Year-old woman with multiple surgical interventions for pseudomyxoma peritonei. Multidetector computed tomography of the abdomen with intravenous contrast, portal phase, cross section (A and B) and sagittal reconstruction (C) where loculated low attenuation collections without enhancement (white arrows) and with calcified walls (hollow arrows) are observed. The intestinal loops are compressed and with oral contrast material inside (asterisk).
There is no consensus for the treatment of this disease.7 The majority agree on an initial approach with intestinal rest and enteral or parenteral nutritional support.7,12,13 In patients with SEP in CAPD, anti-inflammatory and anti-fibrogenic medication is recommended as well as corticosteroids13,29 or tamoxifen.12,13 Other drugs such as colchicine, azathioprine and mycophenolate mofetil can also be used, although experience with their use is limited.7 The CAPD must be discontinued and the patient transferred to haemodialysis.12
In cases of intestinal occlusion, when peritoneal thickening is confirmed by MDCT, the surgical approach for excision of the fibrotic membrane and adhesiolysis is indicated.7 Intestinal resection is only performed in the presence of necrosis.30 Surgery is not indicated in asymptomatic patients.30
In conclusion, SEP is a rare disease that represents a serious complication of CAPD and an important cause of bowel obstruction. Knowing of its existence and radiological signs allow for its suspicion and diagnosis so that the appropriate treatment can be implemented.
Authorship- 1.
Responsible for the integrity of the study: RLG and JAO.
- 2.
Study conception: RLG, AHM and JAO.
- 3.
Study design: RLG, AHM, MA and JAO.
- 4.
Data collection: RLG, AHM, MA, JADP and JAO.
- 5.
Data analysis and interpretation: RLG, MA and JAO.
- 6.
Statistical processing: RLG and JAO.
- 7.
Literature search: RLG, AHM, MA, JADP and JAO.
- 8.
Drafting of the paper: RLG, MA and JAO.
- 9.
Critical review of the manuscript with intellectually relevant contributions: AHM, JADP and JAO.
- 10.
Approval of the final version: RLG, AHM, MA, JADP and JAO.
The authors declare that they have no conflicts of interest.
Please cite this article as: López Grove R, Heredia Martínez A, Aineseder M, de Paula JA, Ocantos JA. Peritonitis esclerosante encapsulante: hallazgos en imágenes de una entidad poco frecuente. Radiología. 2019. https://doi.org/10.1016/j.rx.2019.02.005