To determine the usefulness of various parameters based on T2-weighted fetal magnetic resonance (MR) imaging measurements of the uninvolved lung for the neonatal prognosis of congenital diaphragmatic hernia (CDH).
Materials and methodsWe used ultrasonography and MR imaging to study 28 fetuses with CDH. We retrospectively analyzed (a) on fetal ultrasonography, the observed-to-expected lung to head ratio (O/E LHR) and the position of the liver, and (b) on fetal MR imaging, the lung-liver signal ratio (LLSR) and the lungcerebrospinal fluid ratio (L/CSF SR). To determine the prognostic value of these parameters, we compared them with the following postnatal parameters: survival, pulmonary hypertension, need for oxygen supplementation, and need for extracorporeal membrane oxygenation.
ResultsWe found significant differences between O/E LHR and the need for postnatal extracorporeal membrane oxygenation (p=.033) and postnatal survival (p=.01). We also found significant differences in LLSR between fetuses that survived more than 45 days and those that died within 45 days (1.91 vs 2.56; p=.039).
ConclusionsIn fetuses with CDH, the LLSR correlates with postnatal survival and can potentially be used as a prognostic parameter in CDH.
Determinar el pronóstico neonatal de la hernia diafragmática congénita (HDC) partiendo de la señal relativa del pulmón contralateral en secuencias rápidas T2 de resonancia magnética (RM) fetal.
Material y métodosEstudiamos mediante ecografía y RM 28 fetos afectos de HDC y valoramos retrospectivamente en la ecografía fetal la relación entre el cociente observado y el esperado del diámetro axial máximo del pulmón dividido por el perímetro craneal (O/E LHR) y la posición hepática, y en la RM fetal el cociente de señal pulmón/hígado (LLSR) y el cociente de señal pulmón/líquido cefalorraquídeo (L/SF SR). Para determinar su valor pronóstico, los comparamos con los parámetros posnatales: supervivencia, hipertensión pulmonar, necesidad de oxígeno y la necesidad de oxigenación con membrana extracorpórea.
ResultadosEncontramos diferencias significativas entre O/E LHR y la necesidad de oxigenación con membrana extracorpórea posnatal (p=0,033) y la supervivencia posnatal (p=0,01), y entre el LLSR de los fetos que sobrevivieron más de 45 días y los que no, con unas medianas de 1,91 y 2,56 respectivamente (p=0,039).
ConclusionesEl LLSR se correlaciona con la supervivencia posnatal en fetos con HDC y puede potencialmente utilizarse como parámetro pronóstico de la HDC fetal.
The right growth and maturation of fetal lungs are key in the post-natal period. The processes compressing the chest cavity such as the congenital diaphragmatic hernia (CDH) and the oligohidramnios can affect the development of fetal lungs leading the pulmonary hypoplasis and secondary pulmonary hypertesions.1 The fetal pulmonary evaluation in fetuses with CDH is usually done through ultrasound even though recently the utility of magnetic resonance imaging (MRI)1 has been confirmed. The most valued ultrasound prognostic indicator for the assessment of pulmonary development is the ratio between the observed value and the observed-to-expected lung area to head circumference ratio O/E LHR.1 Also the Doppler echographic parameters of the pulmonary artery and pulmonary perfusion can be studied which will tell us, respectively, if there is pulmonary hypertension and how the intrapulmonary vascularization really is–indirect signs of pulmonary hypoplasia.2,3 The location of the liver in the thorax–inside the CDH is an independent sign of poor neonatal prognosis correlated with the degree of hypoplasia.1 These data can be assessed echographically yet it is recommended to study it through MRIs since the liver is better differentiated from other organs and adjacent structures that are similar echographically.4 There are other MRI parameters that can help us study pulmonary hyperplasia, while most of them are based on the volume like the homo and/or contralateral relative pulmonary volume, the percentage of herniated liver or the gastric location of the hernia–all of them indirectly reporting about pulmonary compression due to the mass effect of hernia.4–6 Recently other parameters of fetal pulmonary imaging providing qualitative data of pulmonary maturation have been reported like the relative pulmonary signal in T2 and T2-weighted MRIs, diffusion-weighted and spectroscopic MRIs or ultrasound-based images on pulmonary texture–useful to distinguish normal from hypoplasic lungs.7–9
The goal of this study is to determine the neonatal prognosis of CDH from the contralateral lung relative signal in T2-weighted fast sequences of fetal MRIs.
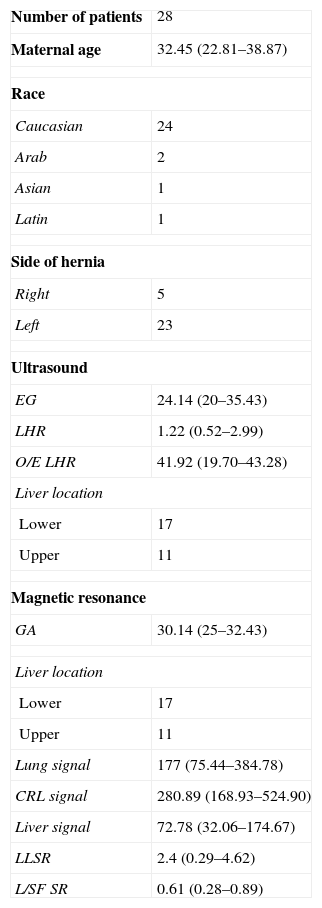
Materials and methodsPatientsFrom January 2006 to December 2011, 79 pregnant women with fetuses echographically diagnosed with CDH were seen in our center. All of them were studied through fetal ultrasound and MRI as part of the study protocol for CDH. The sample consisted of patients from the very hospital care units and patients from other hospitals sent to our center. Among them, 50 met the criteria for fetal endoscopic tracheal occlusion of which 43 underwent surgery and were excluded from the study while in the remaining seven the pregnancy was interrupted. The 29 patients who did not receive any therapies went on with their pregnancies and they made up the study sample. In all patients delivery took place in our center except for four cases that due to their good prenatal prognosis were referred to their corresponding centers of reference. One case was eliminated from the study due to lack of postnatal data. The final number of patients included in the study was therefore 28 whose characteristics can be seen in Table 1. No fetus showed other congenital or chromosomic malformations. This work is a part of a wider investigational protocol approved by our hospital ethical committee.
Characteristics of the sample.
| Number of patients | 28 |
| Maternal age | 32.45 (22.81–38.87) |
| Race | |
| Caucasian | 24 |
| Arab | 2 |
| Asian | 1 |
| Latin | 1 |
| Side of hernia | |
| Right | 5 |
| Left | 23 |
| Ultrasound | |
| EG | 24.14 (20–35.43) |
| LHR | 1.22 (0.52–2.99) |
| O/E LHR | 41.92 (19.70–43.28) |
| Liver location | |
| Lower | 17 |
| Upper | 11 |
| Magnetic resonance | |
| GA | 30.14 (25–32.43) |
| Liver location | |
| Lower | 17 |
| Upper | 11 |
| Lung signal | 177 (75.44–384.78) |
| CRL signal | 280.89 (168.93–524.90) |
| Liver signal | 72.78 (32.06–174.67) |
| LLSR | 2.4 (0.29–4.62) |
| L/SF SR | 0.61 (0.28–0.89) |
GA: gestational age measured in weeks, maternal age measured in years; L/SF SR: lung to spinal fluid signal ratio; CRL: cephalo-rachidian liquid; LLSR: lung to liver signal ratio; O/E LHR: observed-to-expected lung area to head circumference ratio.
The ultrasounds were obtained through the Siemens Sonoline Antares (Siemens Medical Systems, Malvern, PA, USA) or Voluson 730 Expert ultrasound scanners (GE Medical Systems, Milwaukee, WI, USA) following the usual technique.1
The MRIs were acquired through a 1.5 Tesla machine (Magneton Symphony, Siemens Medical Systems, Erlangen, Germany) using a 6-channel coil. The patient was placed in the decubitus supine position always under sedation. The patient never received corticoids before the MRI. The T2-weighted sequences were obtained through a half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence in the axial, coronal and sagittal planes orthogonal to the fetus of 5mm thickness without overlapping, FOV 380mm×380mm, matrix 173×256, TR/TE=1.000/88ms, a partial Fourier factor of 5/8 for a pixel resolution of 2.2mm×1.5mm×4mm, and a 475Hz/pixel bandwidth.
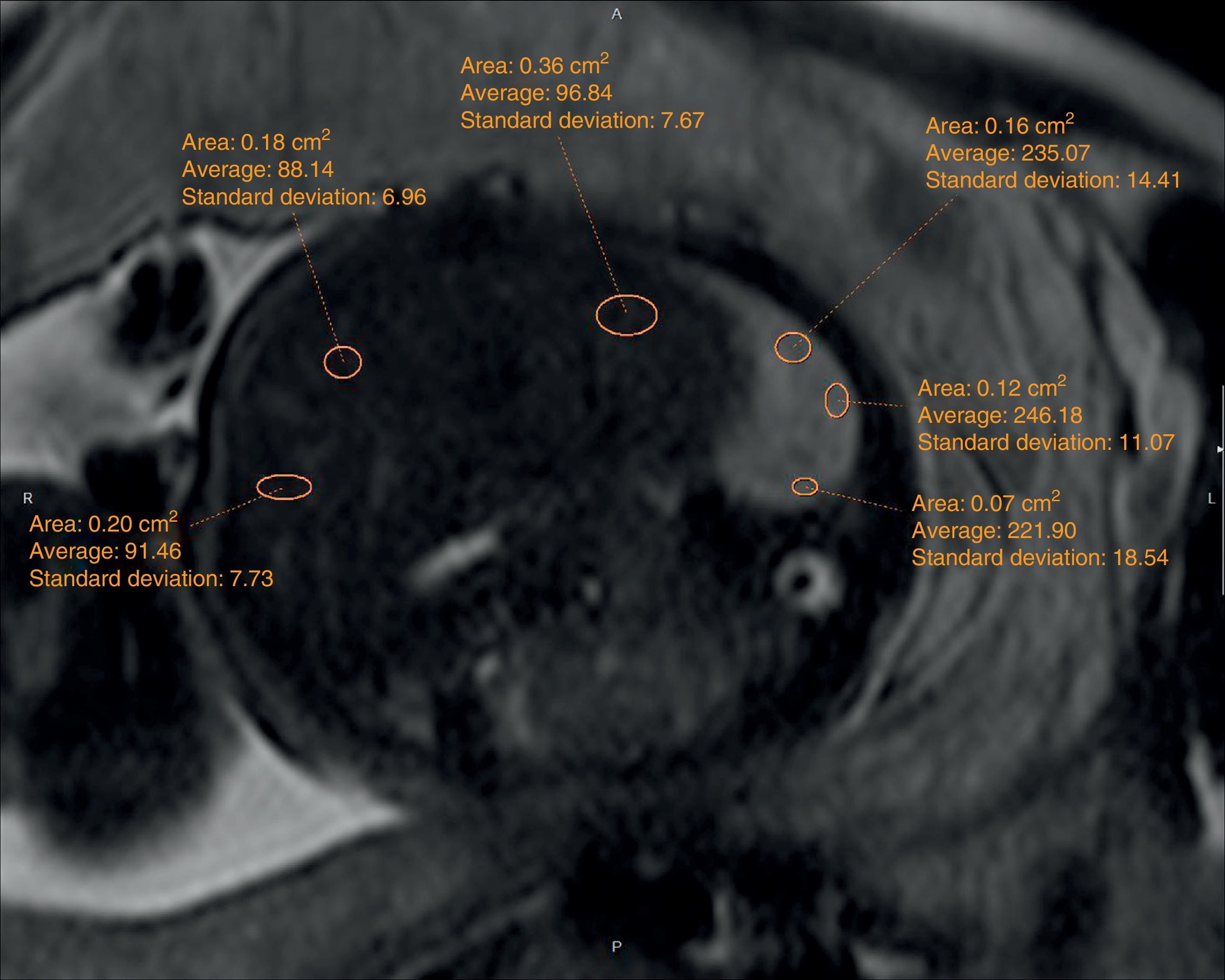
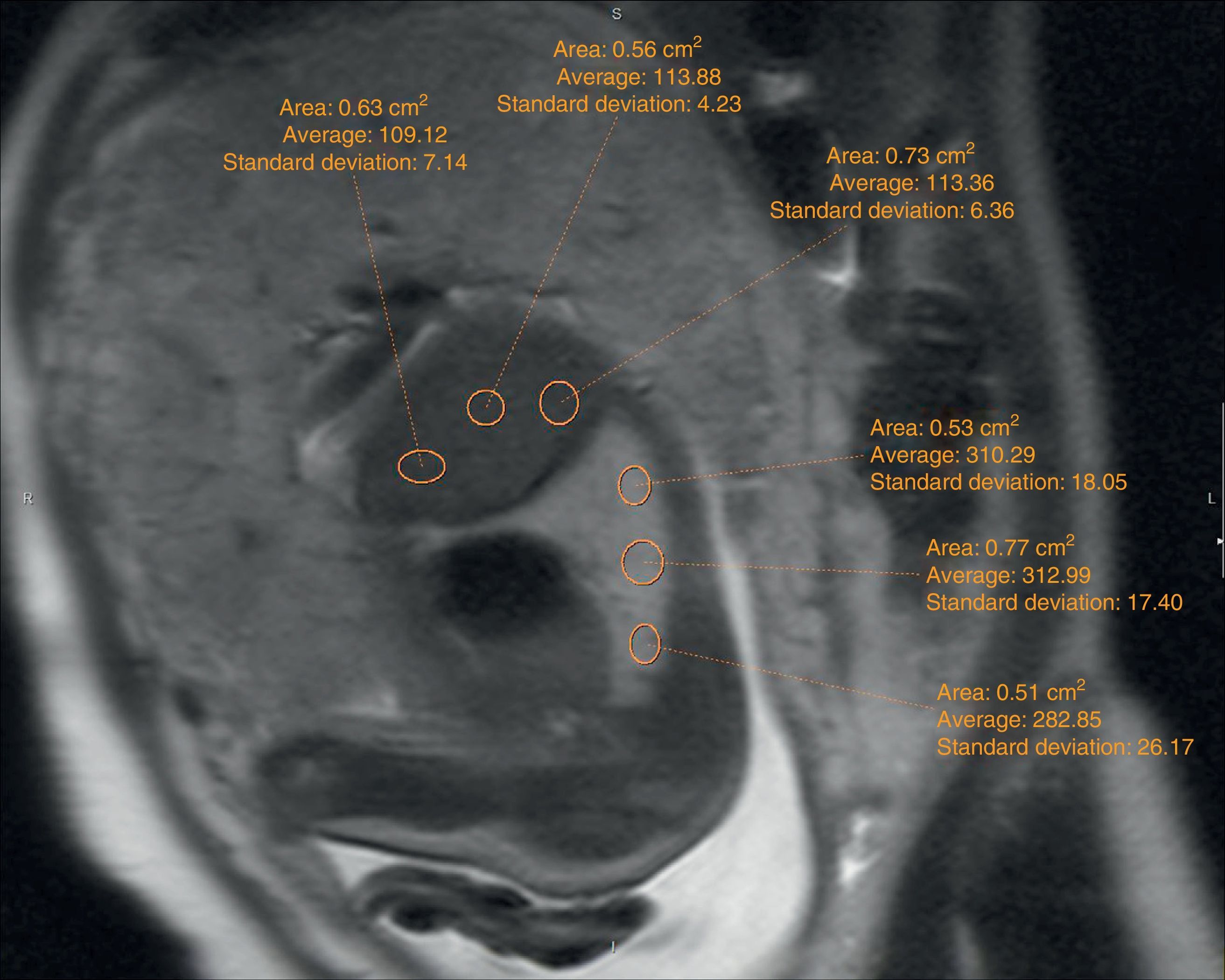
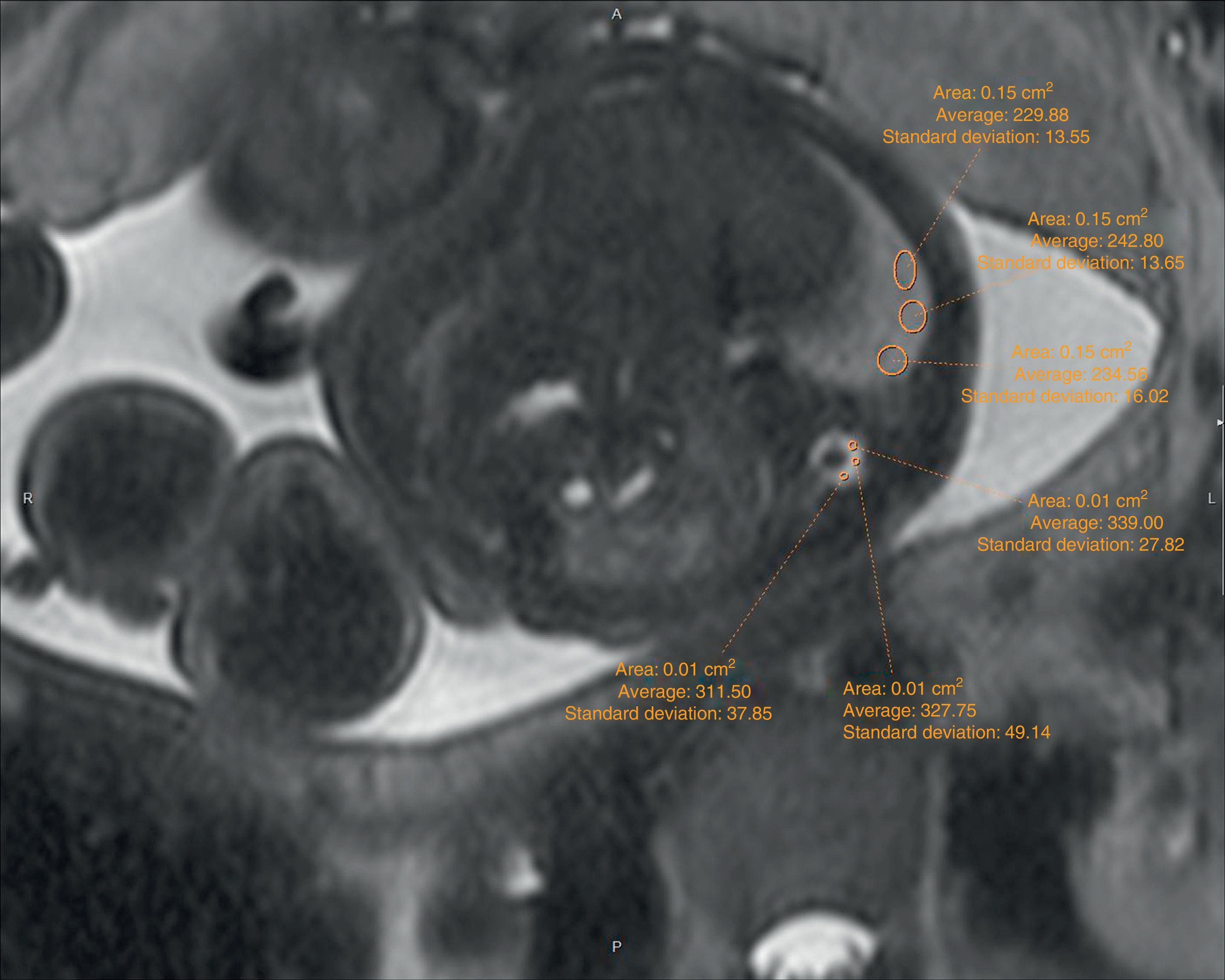
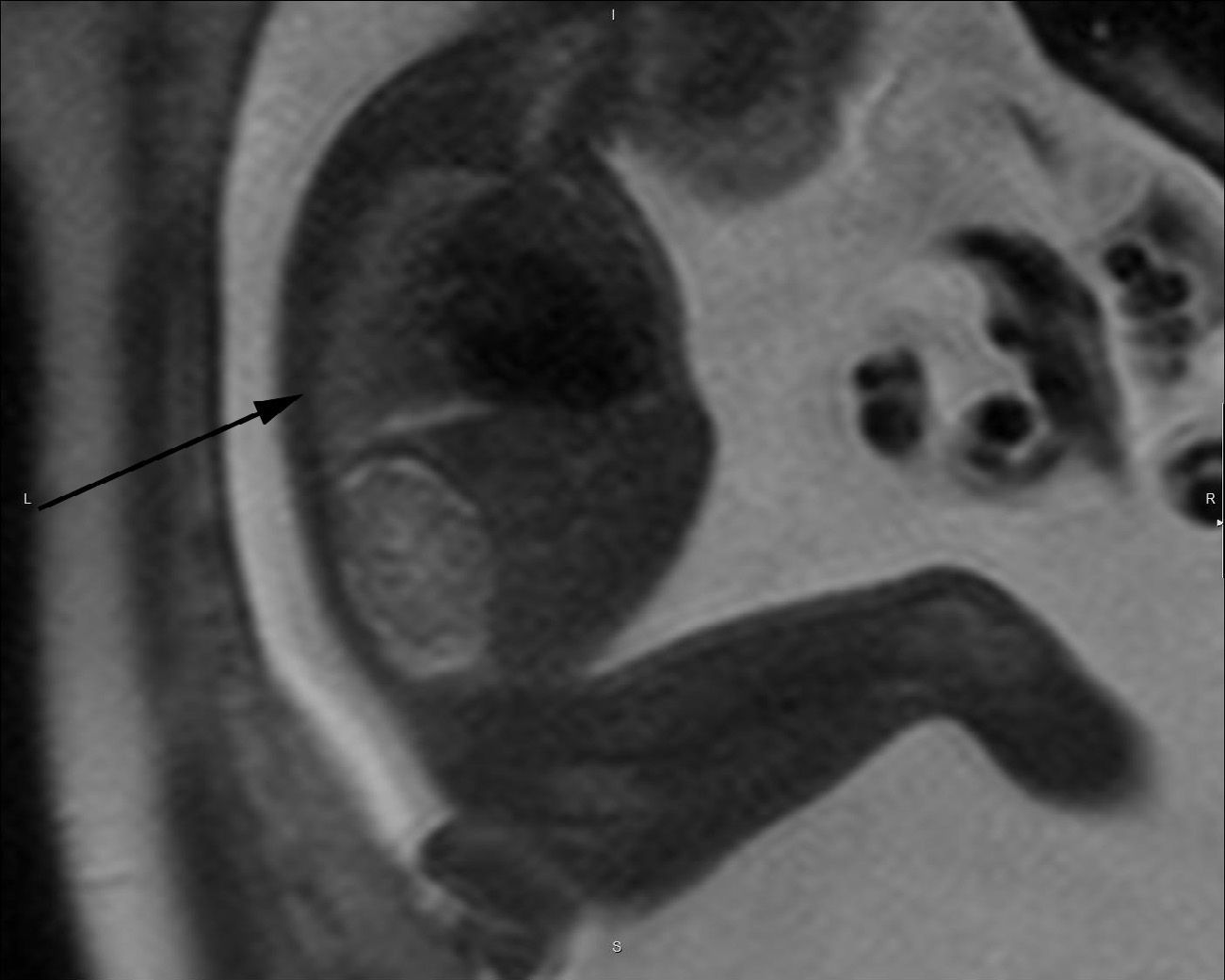
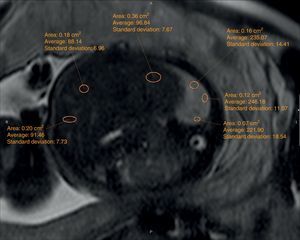
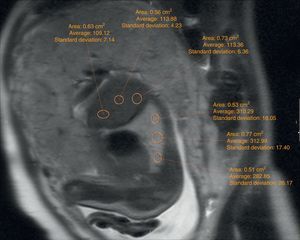
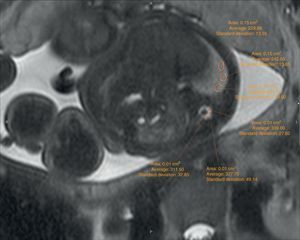
The echographic data were collected retrospectively from the fetal ultrasound report (O/E LHR and hepatic location) and one 5-year experienced radiologist in fetal MRIs who did not know about the postnatal evolution of fetuses evaluated hepatic location and drew the regions of interest (ROI) in lung, liver, and cephalo-rachidian liquid (CRL) in the T2-weighted sequences. For the assessment of the contralateral pulmonary signal we followed the most popular method described in literature by Kuwasima and then reproduced by Brewerton and Balassy.10–12 Three measurements of the lung contralateral to the hernia while avoiding vessels and bronchi were taken with an ROI between 0.5 and 2mm2, and three ROIs in the liver also avoiding the hepatic vessels of between 1.5 and 3mm2. The pulmonary and liver measurements were taken in the same cut in one axial or sagittal image choosing both the plane and image with the least possible artifacts. The average of the three pulmonary measurements divided by the average of the three hepatic measurements is called the LLSR (lung to liver signal ratio) (Figs. 1 and 2). For the measurement of the CRL signal the axial plane was used in which no artifacts were seen while measuring the lung as described before and also drawing three ROIs in the CRL while avoiding the bone marrow and proximal bones of between 0.1 and 1mm2 (Fig. 3). The average of the three pulmonary measurements divided by the average of the three CRL measurements is called the L/SF SR (lung to spinal fluid signal ratio).
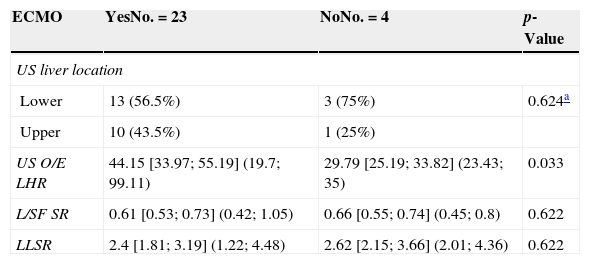
We studied the correlation between the echographic prenatal parameters (liver located above or under the diaphragm and O/E LHR) and the MRI-based echographic prenatal parameters (LLSR and L/SF SR) and the existence–or not of pulmonary hypertension assessed through echocardiography, also the postnatal survival (death before or after the first 45 days from delivery attributable to CDH), the need for oxygen (> or <30 days), and the need–or not for extracorporeal membrane oxygenation (ECMO) (Figs. 4 and 5).
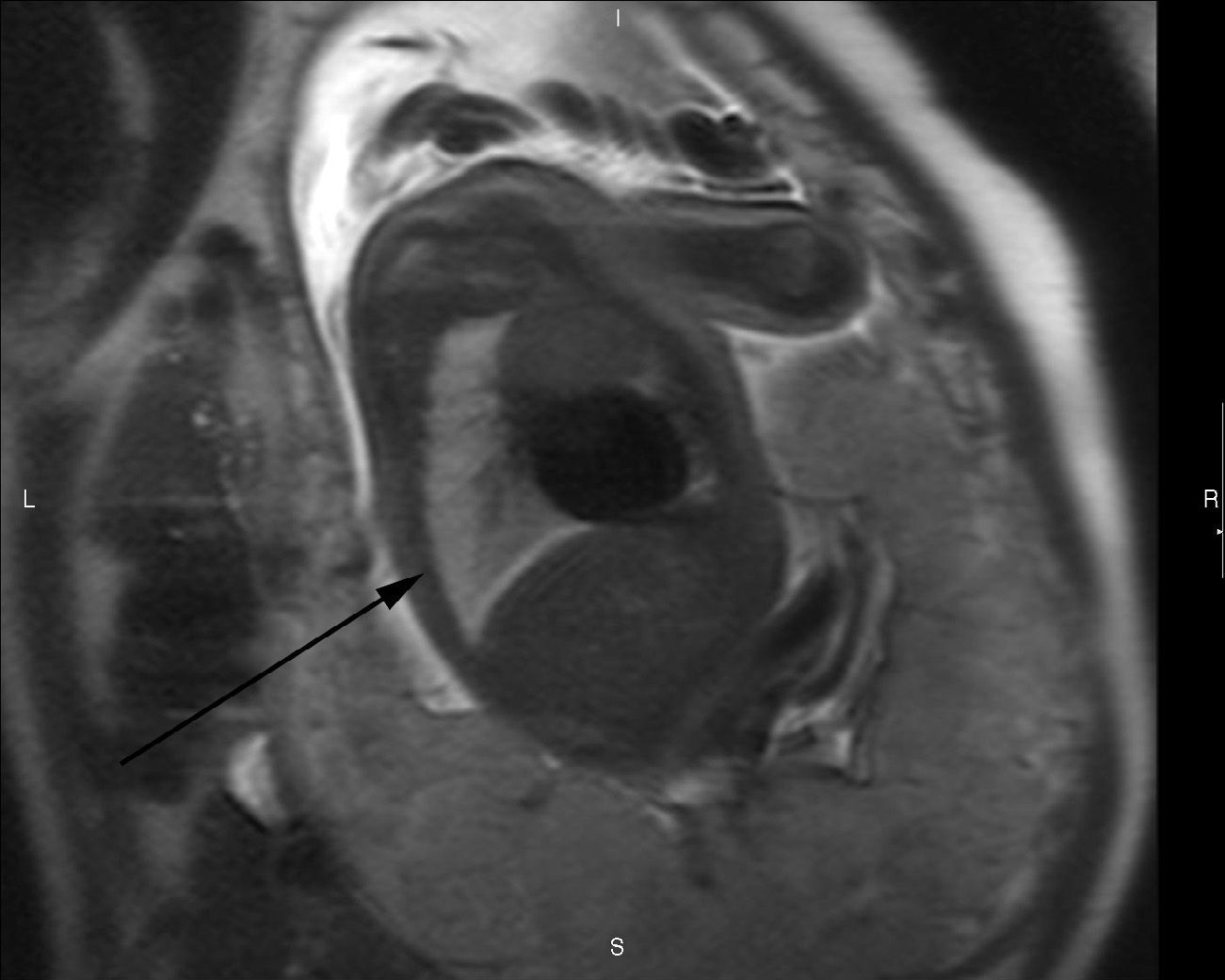
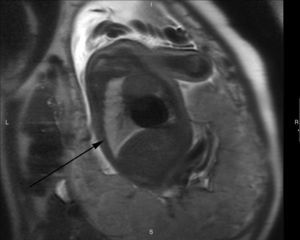
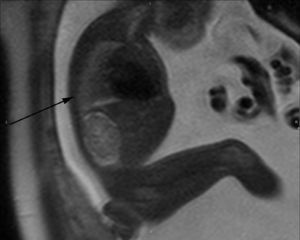
T2-weighted sagittal image corresponding to the contralateral lung of one left congenital diaphragmatic hernia (arrow) of a 35-week old fetus with a lung to liver signal ratio (LLSR) of 3.7 and a lung to spinal fluid signal ratio (L/SF SR) of 0.87 with an excellent postnatal evolution.
T2-weighted sagittal image corresponding to the contralateral lung of one left congenital diaphragmatic hernia (arrow) of a 27-week old fetus with a lung to liver signal ratio (LLSR) of 1.52 and a lung to spinal fluid signal ratio (L/SF SR) of 0.52. The mother refused intrauterine therapy and the fetus died due to respiratory failure at birth. The autopsy confirmed bilateral pulmonary hypoplasia. Note the difference of signal between Figs. 4 and 5.
The quantitative variables were described as maximum, minimum, median and interquartile ranges and compared with the non-parametric Mann–Whitney U test. The quantitative variables were described as absolute and relative frequencies and analyzed through the Fischer's exact test.
To obtain a cutting point of variables LLSR and L/SF SR the positive coefficient of probability (CP+) for mortality was assessed and defined as the ratio sensitivity/(1−especificity). Compared with an ROC analysis the value of CP+ in this study allows us to quantify the goodness of the cutting point proposed because the ratio of CP+ also expresses the relation of true positives vs false negatives for a determined cutting point.
The statistical analysis was performed through the SPSS 20 software (IBM) using a type I error rate of 5% bilateral. p<0.05 was considered significant.
ResultsIn the 28 cases we had the O/E LHR and the ultrasound-based hepatic location and the measurements of the contralateral, hepatic pulmonary signal and that of the CRL. In summary, the results can be seen in Table 2. In 27 out of 28 cases we had information on the need–or not for ECMO. In one case in which delivery happened outside the hospital we did not have the corresponding information so the total number of cases in which we compared the radiologic findings with the need for ECMO was 27. In 20 of the 28 cases we knew exactly the number of days during which the newly born baby needed oxygen. In the 28 cases we could assess postnatal survival and the diagnosis of pulmonary hypertension.
Results.
| ECMO | YesNo.=23 | NoNo.=4 | p-Value |
|---|---|---|---|
| US liver location | |||
| Lower | 13 (56.5%) | 3 (75%) | 0.624a |
| Upper | 10 (43.5%) | 1 (25%) | |
| US O/E LHR | 44.15 [33.97; 55.19] (19.7; 99.11) | 29.79 [25.19; 33.82] (23.43; 35) | 0.033 |
| L/SF SR | 0.61 [0.53; 0.73] (0.42; 1.05) | 0.66 [0.55; 0.74] (0.45; 0.8) | 0.622 |
| LLSR | 2.4 [1.81; 3.19] (1.22; 4.48) | 2.62 [2.15; 3.66] (2.01; 4.36) | 0.622 |
| Need for O2>30d | ≤30No.=16 | >30No.=4 | |
|---|---|---|---|
| US liver location | |||
| Lower | 9 (56.2%) | 2 (50%) | 1a |
| Upper | 7 (43.8%) | 2 (50%) | |
| US O/E LHR | 44.7 [34.35; 55.75] (23.43; 99.11) | 41.66 [30.38; 49.5] (26.95; 49.5) | 0.376 |
| L/SF SR | 0.6 [0.54; 0.69] (0.43; 0.86) | 0.69 [0.53; 0.75] (0.42; 0.77) | 0.617 |
| LLSR | 2.32 [1.68; 3.24] (1.22; 4.48) | 2.98 [2.64; 3.77] (2.51; 4.36) | 0.148 |
| Pulmonary hypertension | YesNo.=8 | NoNo.=20 | |
|---|---|---|---|
| US liver location | |||
| Lower | 4 (50%) | 13 (65%) | 0.671a |
| Upper | 4 (50%) | 7 (35%) | |
| US O/ELHR | 44.7 [38.08; 55.75] (33.82; 56.62) | 40.42 [29.79; 49.5] (19.7; 99.11) | 0.299 |
| L/SF SR | 0.61 [0.54; 0.74] (0.46; 0.86) | 0.61 [0.51; 0.7] (0.32; 1.05) | 0.709 |
| LLSR | 3.13 [2.14; 3.78] (1.81; 4.48) | 2.37 [1.68; 2.79] (1.22; 4.36) | 0.11 |
| Survival at 45 days | Dead (45d)No.=6 | Alive (45d)No.=22 | |
|---|---|---|---|
| US liver location | |||
| Lower | 3 (50%) | 14 (63.6%) | 0.653a |
| Upper | 3 (50%) | 8 (36.4%) | |
| US O/E LHR | 28.77 [21.94; 35] (19.7; 48.48) | 44.7 [34.73; 55.19] (23.43; 99.11) | 0.01 |
| L/SF SR | 0.56 [0.45; 0.64] (0.32; 0.8) | 0.62 [0.55; 0.73] (0.42; 1.05) | 0.309 |
| LLSR | 1.91 [1.52; 2.14] (1.22; 2.96) | 2.56 [2.28; 3.41] (1.34; 4.48) | 0.039 |
ECMO: extracorporeal membrane oxygenation; L/SF SR: lung to spinal fluid signal ratio; LLSR: lung to liver signal ratio; O/E LHR: observed-to-expected lung area to head circumference ratio; US: ultrasound.
Outcomes are expressed as a median [percentiles 25 and 75] and as (min, max) for quantitative variables and as the absolute frequency and percentage for qualitative variables.
Among all of the assessed data only the relation between O/E LHR and the need for postnatal ECMO (p=0.033) and between O/E LHR and LLSR and postnatal survival (p=0.01 and p=0.039, respectively) were statistically significant. There was no significant relation between the hepatic location or the L/SF SR with any of the postnatal parameters. Neither between the LLSR and the need for oxygen, ECMO or pulmonary hypertension. The LLSR cutting point between survivors and non-survivors was 2.2 with a CP+=2.7; and 0.52 for L/SF SR, with a CP+=3.7.
DiscussionIn our study we proved that even though the most consistent parameter to predict postnatal prognosis like several studies ratify1,6 is the echographic O/E LHR there is also a statistically significant relation between the highest values of LLSR and the postnatal survival beyond 45 days. Assessing the degree of pulmonary hypoplasia in fetuses with CDH is very important for prenatal advice and to predict postnatal evolution. One-third of the newly born babies with CDH will die from pulmonary hyperplasia and/or pulmonary hypertension.13
Being able to diagnose correctly prenatal pulmonary hypoplasia with image modalities is still a challenge. Today the ultrasound-assessed O/E LHR is considered to be the most consistently correlated parameter with the postnatal prognosis in fetuses with CDH.1 MRIs allow us to study the chemical and structural characteristics of several tissues. Studies published on animal and human experimentation have analyzed the relation between the signal intensity and the pulmonary structure.13–16 There is a significant positive correlation between the T2-weighted signal of healthy lungs and gestational age and there are no significant differences between the signal of the two lungs in healthy fetuses. Different formulae have been proposed to be able to calculate the pulmonary signal intensity in healthy fetuses based on gestational age.13,14,16
Pulmonary hypoplasia is defined anatomopathologically as the shrinking of airways and the number of alveoli with respect to a normal lung.17 Even though the pathogenesis is not clear yet the pulmonary liquid can have an important role in pulmonary development. Lungs with a significant hyperintensity in the T2-weighted sequences are normal while those highly hypointense are hypoplasic while a cutting value for LLSR=2 can help us distinguish hypoplasic from normal lungs.10 This statement is only valid from the gestational week #26–not earlier because the production of liquid in the fetus lungs starts in the second quarter when the pulmonary interstitium refines gradually.8,11 All these have to do with the anatomic studies that consider that the pulmonary development and maturation in the canalicular and alveolar phases are similar in normal and hypoplasic lungs.18 On the other hand, the pulmonary signal of lungs compressed due to CDH is very similar to that of the contralateral lung suggesting that the hernia contralateral signal can be a good marker of global pulmonary signal.19
MRI-based signal intensity of any tissues depends on the sequence used and the distance to the coil–this is why absolute values are not appropriate to assess this. This is why structures at a similar depth are used as a reference. These structures can be the liver, the CRL and the amniotic fluid. To be able to estimate the ratio between the intensity of pulmonary signal and that of a reference structure this second structure should not experience changes with gestational age. The liver signal changes based on pregnancy probably due to the progressive increase of the concentrations of iron and the progressive reduction in the production of hemoglobin20 and can be an excellent parameter of reference. Yet these differences were studied in the 20 and 26th weeks of pregnancy not later. The CRL however does not experience any changes during pregnancy.14
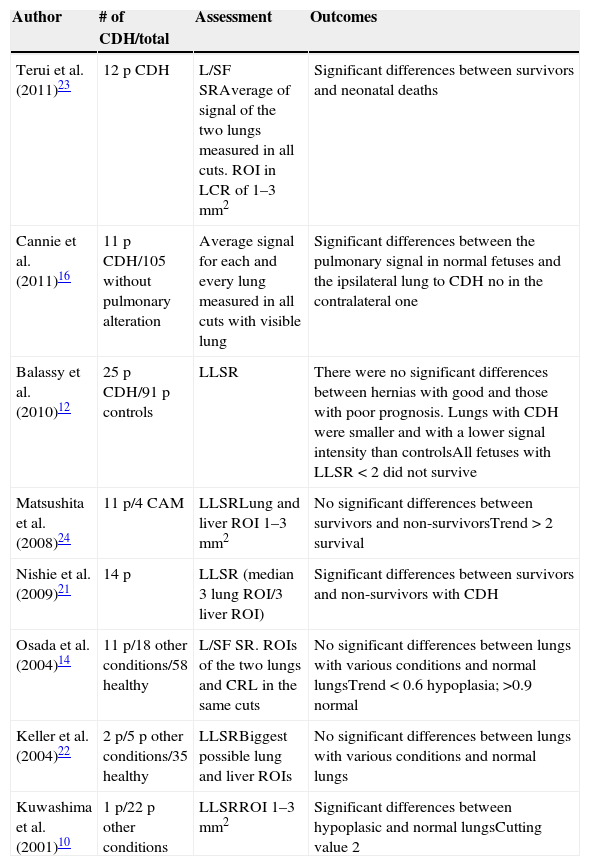
There are very few studies showing the efficacy of measuring the T2-weighted pulmonary signal intensity in fetuses with CDH and its relation to pulmonary hypoplasia and the fetal postnatal prognosis and all of them are based in very few cases and use different methods (Table 3). Even though it is well known that the hepatic signal varies with gestational age before the 25th week of pregnancy most studies support the LLSR,9,11,12,21,22 while other studies have valued the L/SF SR14–23 with contradictory results. Even though no significant differences between the L/SF SR of newly born babies with good or poor prognosis have been found in our study, there is a trend that patients with poor prognosis have a lower ratio with an average signal in these cases similar to that of previous studies of around 0.65. Similarly the differences among patients with good or poor prognosis and the LLSR cutting level are also similar to that of previous studies (around 2).8,24
Summary of bibliographic results.
| Author | # of CDH/total | Assessment | Outcomes |
|---|---|---|---|
| Terui et al. (2011)23 | 12 p CDH | L/SF SRAverage of signal of the two lungs measured in all cuts. ROI in LCR of 1–3mm2 | Significant differences between survivors and neonatal deaths |
| Cannie et al. (2011)16 | 11 p CDH/105 without pulmonary alteration | Average signal for each and every lung measured in all cuts with visible lung | Significant differences between the pulmonary signal in normal fetuses and the ipsilateral lung to CDH no in the contralateral one |
| Balassy et al. (2010)12 | 25 p CDH/91 p controls | LLSR | There were no significant differences between hernias with good and those with poor prognosis. Lungs with CDH were smaller and with a lower signal intensity than controlsAll fetuses with LLSR<2 did not survive |
| Matsushita et al. (2008)24 | 11 p/4 CAM | LLSRLung and liver ROI 1–3mm2 | No significant differences between survivors and non-survivorsTrend>2 survival |
| Nishie et al. (2009)21 | 14 p | LLSR (median 3 lung ROI/3 liver ROI) | Significant differences between survivors and non-survivors with CDH |
| Osada et al. (2004)14 | 11 p/18 other conditions/58 healthy | L/SF SR. ROIs of the two lungs and CRL in the same cuts | No significant differences between lungs with various conditions and normal lungsTrend<0.6 hypoplasia; >0.9 normal |
| Keller et al. (2004)22 | 2 p/5 p other conditions/35 healthy | LLSRBiggest possible lung and liver ROIs | No significant differences between lungs with various conditions and normal lungs |
| Kuwashima et al. (2001)10 | 1 p/22 p other conditions | LLSRROI 1–3mm2 | Significant differences between hypoplasic and normal lungsCutting value 2 |
CDH: congenital diaphragmatic hernia; L/SF SR: lung to spinal fluid signal ratio; CRL: cephalo-rachidian liquid; LLSR: lung to liver signal ratio; CAM: cystic adenomatoid malformation; p: patient; ROI: region of interest.
Our study has some limitations. It is a retrospective study with a small sample yet it needs to be reminded that it is an uncommon process and that our series is the largest one ever published. Another limitation of this study is that the measurement of signal intensity was taken by one radiologist only and we did not calculate the variability induced by the observer. Also in daily routine it is hard to draw one relatively big ROI in the CRL without including the bone marrow or the adjacent vertebra. The possible error of measurement can be the reason for the lack of conclusive results in our study with L/SF SR. Also the signal intensity among images and sequences has been extremely variable. However measurement variability is solved by estimating the ratio. A third limitation is that our sample is biased because mid-and-high risk patients who met the criteria for fetal endoscopic tracheal occlusion underwent surgery and we only have the natural evolution of those fetuses that were not candidates to undergo fetal endoscopic tracheal occlusion or mothers who refused this therapy and moved on with their pregnancy.
In summary, our study shows that the LLSR is a parameter correlated with postnatal survival in fetuses with CDH that potentially can be used as a prognostic parameter in the fetal study of CDH.
Ethical responsibilitiesProtection of people and animalsAuthors confirm that no experiments have been performed on human beings or animals.
Data confidentialityAuthors confirm that the protocols of their institution have been followed on the publication of data from patients.
Right to privacy and informed consentAuthors confirm that in this report there are no personal data from patients.
Authors’ contribution- 1.
Manager of the integrity of the study: CS.
- 2.
Original idea of the study: CS, OG, CN.
- 3.
Study design: CS.
- 4.
Data mining: CS, OG, RG, RS, LB, CN.
- 5.
Data analysis and interpretation: CS, OG, RG, RS, LB, CN.
- 6.
Statistical analysis: RS.
- 7.
Reference search: CS, OG, RG, RS, LB, CN.
- 8.
Writing: CS, OG, RG, RS, LB, CN.
- 9.
Critical review: CS, OG, RG, RS, LB, CN.
- 10.
Approval of final version: CS, OG, RG, RS, LB, CN.
Dr. Carmen Sebastià, MD received a research grant from SERAM Industria. The remaining authors reported no conflicts of interests.
Please cite this article as: Sebastià C, Gomez O, Salvador R, Buñesch L, Garcia R, Nicolau C. Cuantificación de la señal T2 pulmonar por resonancia magnética como factor pronóstico en las hernias diafragmáticas congénitas fetales. Radiología. 2015;57:239–247.
This study has been possible thanks to a research grant from SERAM Industria 2009–2011.