Prostatic multi-parametric magnetic resonance imaging (MP-MRI) has recently had a wide development becoming a key tool in the diagnostic and therapeutic decisions in prostate cancer (Pca). The fast development both in technology and in reading (PIRADS V2) requires a continuous updating of knowledge within this area. The aim of this article is to present an updated revision of technical aspects, reading patterns and prostatic MP-MRI in Pca, with a multidisciplinary approach. Currently guidelines establish the use of the MP-MRI when there is a high PSA and a negative prostatic biopsy; tumor staging; evaluation in candidates to active surveillance; focal treatments plans and tumoral recurrence evaluation. Although it is used in other indications in some centers, like its use in patients suspicious of Pca but with no previous biopsy, there is still the need of a cost/benefit assessment for its use to be wider.
La resonancia magnética multiparamétrica (RMmp) prostática ha tenido recientemente un extenso desarrollo, convirtiéndose en una herramienta clave en el diagnóstico y la toma de decisiones terapéuticas en relación al carcinoma prostático (CaP). El rápido desarrollo tecnológico, así como de lectura (PIRADS V2), exigen una permanente actualización del conocimiento en esta área. El objetivo de este artículo es presentar una revisión actualizada sobre los aspectos técnicos, los modelos de lectura y las indicaciones de la RMmp prostática en relación al CaP, en el marco de una visión multidisciplinaria. Actualmente está establecida la utilidad de la RMmp ante un antígeno específico de próstata elevado y una biopsia prostática previa negativa; para estadificación tumoral; en la evaluación de candidatos a vigilancia activa; en la planificación de tratamientos focales y para la evaluación de la recurrencia tumoral. Otras indicaciones, como su uso en pacientes con sospecha de CaP pero sin biopsia previa, aunque se realizan en algunos centros, aún requieren una exhaustiva valoración coste-beneficio para extender su empleo.
In Europe, prostate cancer (PCa) is the most common non-skin cancer in men over 70 years of age, and it has become a major health concern in developed countries given the aging population indexes.1 Until recently, its detection and treatment were based almost exclusively on the prostate-specific antigen (PSA), the findings of digital rectal examination and the anatomopathological results of prostate biopsy. However, these parameters have important limitations.
During the last few years, multi-parametric magnetic resonance (MP-MRI) has become a very useful tool when it comes to diagnosing PCa. There are important and permanent technological advancements includingthe reading model of the MP-MRI. Very recently, the European Society of Urogenital Radiology (ESUR) and the American College of Radiology have updated the key diagnostic aspects associated with the MP-MRI and PCa, included in version 2 of the Prostate Imaging Reporting and Data System (PIRADS v2).2
Some indications for its use have been incorporated to the clinical guidelines or endorsed by extensive systematic reviews, such as the assessment when in the presence of a prior negative prostate biopsy with persistent suspicion of PCa; the assessment of candidates to active surveillance based on initial PSA criteria/first biopsy, and tumor staging (mainly in patients with PCa who present clinical doubts about possible extraprostatic spread and whose presence would condition the therapeutic decision).3,4 Other indications, such as focal treatment plans,5 tumor relapse6 and patients with suspicion of PCa but without a prior biopsy7,8–though not fully established, are being more and more endorsed by the medical literature.
To provide all the necessary information, the radiologist should know all the main anatomopathological and clinical and therapeutical aspects of the PCa.
The goal of this paper is to update the actual role of prostatic MP-MRI, from an interdisciplinary approach, while highlighting the advancements made in the technical aspects, the reading model and the indications of this modality.
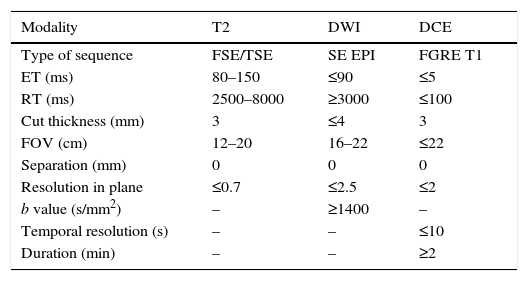
Technical aspects and sequencesThe MP-MRI protocol should be followed closely related to the clinical indication, the characteristics, and the MRI machine. The technical developments allow us to obtain high-resolution images in T2-weighted sequences and diffusion weighted imaging with high factor b, which improves the diagnostic performance. The study protocol should include contrast, diffusion-, T1- and T2-weighted sequences.9 The T1-weighted sequence should be performed with a field of vision (FOV) capable of visualizing the pelvis up to the aortic bifurcation. The study of the prostate and the seminal vesicles in T2-weighted, diffusion and contrast-enhanced sequences (DCE) requires a reduced FOV, as described in the recommendation of the PIRADS v2 guidelines (Table 1). It is useful to add an additional diffusion sequence of the entire pelvis for the analysis of the osseous pelvis and the lymph nodes. The examination requires an MRI machine of at least 1.5T. The use of an endorectal coil combined with the superficial (surface) coil is not essential though it should be done in old machines. There are other technical factors that influence the technical quality of the study, in addition to the endorectal coil, such as bandwidth, the coil channels and radiofrequency power. The results of the study can be optimal in both 1.5T and 3T machines, with or without endorectal coils. The advantage of 3T machines is the possibility of obtaining images with better temporal and spatial resolution; however, depending on the sequence, there can be more susceptibility artifacts. The machines that have been updated with a multichannel surface antenna with 16 or more elements can obtain good image qualities without the need for endorectal coils. 15T machines have fewer air artifacts in the endorectal coil with the diffusion sequence than 3T machines. To minimize them, liquids such as barium sulfate can be used in order to avoid distortions of susceptibility.
Technical characteristics recommended for the multiparametric magnetic resonance imaging study in the PIRADS v2 guidelines.
| Modality | T2 | DWI | DCE |
|---|---|---|---|
| Type of sequence | FSE/TSE | SE EPI | FGRE T1 |
| ET (ms) | 80–150 | ≤90 | ≤5 |
| RT (ms) | 2500–8000 | ≥3000 | ≤100 |
| Cut thickness (mm) | 3 | ≤4 | 3 |
| FOV (cm) | 12–20 | 16–22 | ≤22 |
| Separation (mm) | 0 | 0 | 0 |
| Resolution in plane | ≤0.7 | ≤2.5 | ≤2 |
| b value (s/mm2) | – | ≥1400 | – |
| Temporal resolution (s) | – | – | ≤10 |
| Duration (min) | – | – | ≥2 |
DCE: IV dynamic contrast-enhanced sequence; DWI: diffusion weighted imaging; FGRE: fast gradient echo imaging; FOV: field of view; FSE: fast spin echo imaging; SE EPI: spin-echo-planar imaging; TSE: turbo spin-echo imaging.
Morphological sequences consist of T1- and T2-weighted acquisitions in spin echo (SE) or fast spin echo (FSE). T1-weighted sequences allow us to detect possible pelvic adenopathies, analyze the osseous pelvis to rule out metastasis and assess the possibility of hemorrhagic changes in the prostate gland post-biopsy. T2-weighted sequences should be performed with high resolution (3mm thickness, without separation) in order to be able to assess the normal anatomy of the prostate, preferably on the three planes.9
Functional sequences consist of diffusion and dynamic contrast-enhanced sequences (DCE). Diffusion weighted imaging (DWI) is essential in the analysis of the prostate, together with the independent analysis from the ADC (Apparent Diffusion Coefficient) in the form of a map, in order to be able to detect clinically significant cancer in the peripheral area. DWI images should be available with b factor ≥1400s/mm2, as recommended by the guidelines. Images with high b factor can be obtained directly from acquisition or computed (extrapolated) from those obtained with a lower b factor.2 For the ADC map images, if only two b values can be obtained, it is preferable for the lowest b value to be 50–100s/mm2 and for the highest to be 800–1000s/mm2. That is, if it is not possible to acquire multiple high b values in a single sequence, it is preferable to acquire two diffusion sequences, one with a high b value and another one with two b values so that the ADC can be estimated.
The DCE sequence should be acquired as T1-weighted with a high temporal resolution (≤10s per phase) after the administration of IV gadolinium for a period of time of no less than 2min, and covering the gland and the seminal vesicles. The recent PIRADS version gives little added value to post-contrast sequences in the detection of clinically significant cancer in conjunction with T2-weighted and diffusion sequences.10 In any case, its inclusion in the protocol for MP-MRIs of the prostate is recommended to help detect small foci of clinically significant cancer, diagnose benign causes that justify the increase of PSA (for example, those of inflammatory origin) or provide additional information when the diffusion sequence has not come out technically optimal. Also, the DCE sequence is essential both in therapeutic monitoring and in the assessment of post-prostatectomies.
If one of the sequences of the MP-MRI (T2, DWI, ADC, DCE) turns out to be technically non-assessable, it should be indicated in the report. This is more common in DWI, especially due to the presence of hip prostheses or due to the greater susceptibility to movement. If this happens, and given that this sequence is crucial in the analysis, such sequence should be repeated and only if it were not possible, or if the second acquisition were also suboptimal, the analysis should be performed with the remaining sequences only.
The spectroscopy sequence is not included in the protocol of the PIRADSv2 guidelines. Its lowest availability, the complexity of the acquisition and post-processing, together with the fact that it has not proven to have improved the diagnostic capacity provided by the combined parameters T2-weighted and DWI/ADC, justifies its exclusion from the systematic protocol of prostate MP-MRIs.
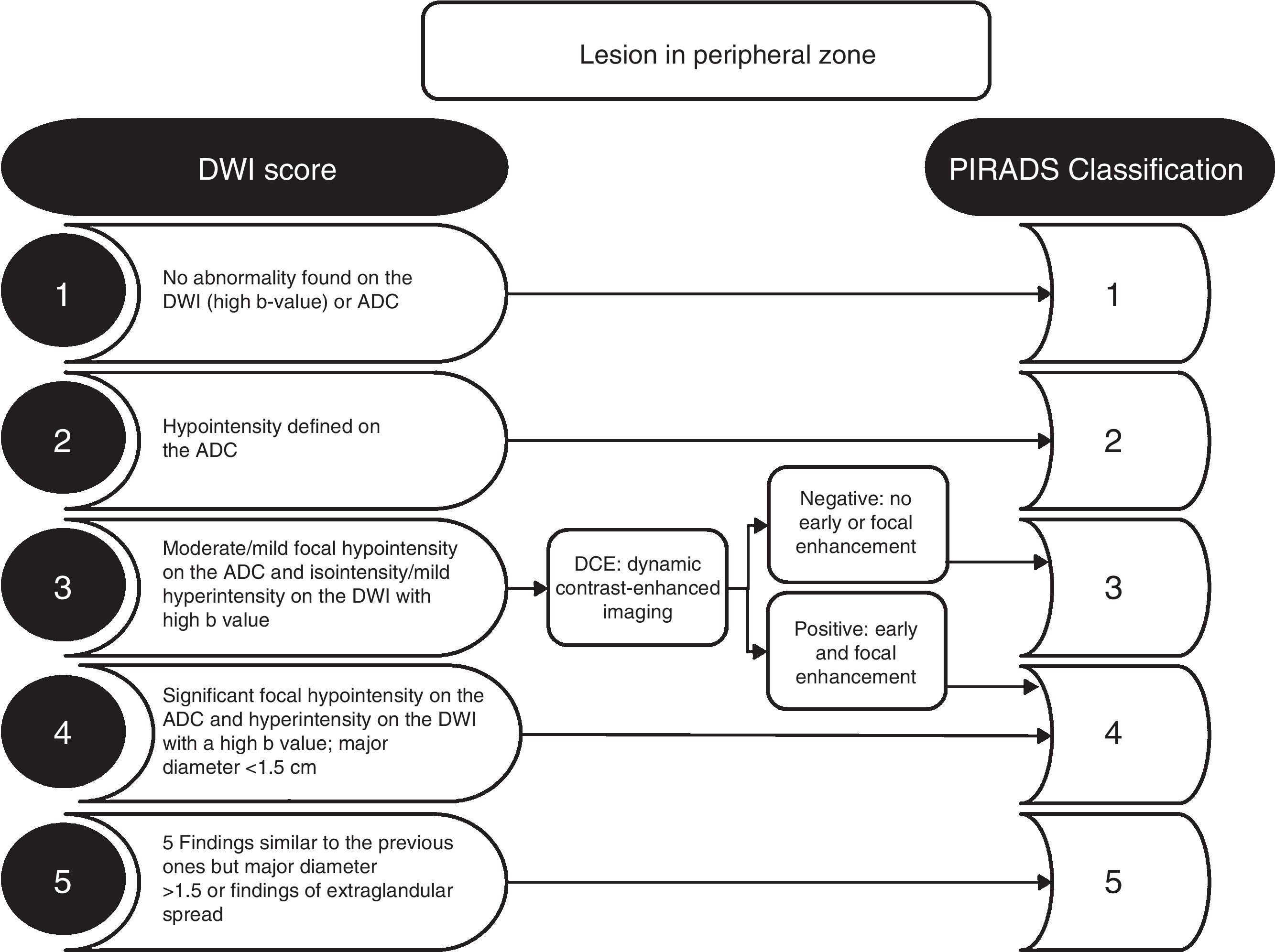
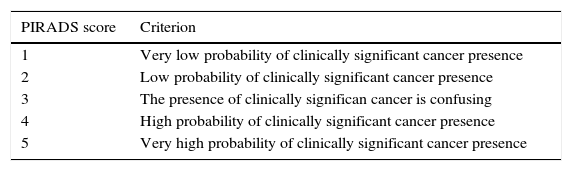
PIRADS reading modelOne of the goals in the reading of an MP-MRI, according to the standardization of the PIRADS v2 guidelines, is to identify and locate alterations consistent to the presence of clinically significant cancer for diagnosis purposes that is. The PIRADS v2 guidelines do not establish any criteria on therapeutic monitoring. Significant cancer is defined as the cancer that histologically is consistent to Gleason scores ≥7 or volumes ≥0.5cc or with extraglandular spread.9 The PIRADS v2 guideline use five categories (Table 2), that indicate the probability of the presence of clinically significant cancer on the MP-MRI on a scale from 1 to 5 (from lower to higher degree of probability). The decision on the PIRADS categorization should be based only on the imaging findings of the MP-MRI, without taken other factors into consideration like the PSA value, the digital rectal examination, the medical history or prior therapies. Although it is recommended to perform a biopsy when in presence of PIRADS 4 or 5, but not in the presence of PIRADS 1 or 2, the PIRADS v2 guidelines do not establish any management recommendations for PIRADS 3.
PIRADS v2. Classification. Probability categories of the presence of significant cancer.
| PIRADS score | Criterion |
|---|---|
| 1 | Very low probability of clinically significant cancer presence |
| 2 | Low probability of clinically significant cancer presence |
| 3 | The presence of clinically significan cancer is confusing |
| 4 | High probability of clinically significant cancer presence |
| 5 | Very high probability of clinically significant cancer presence |
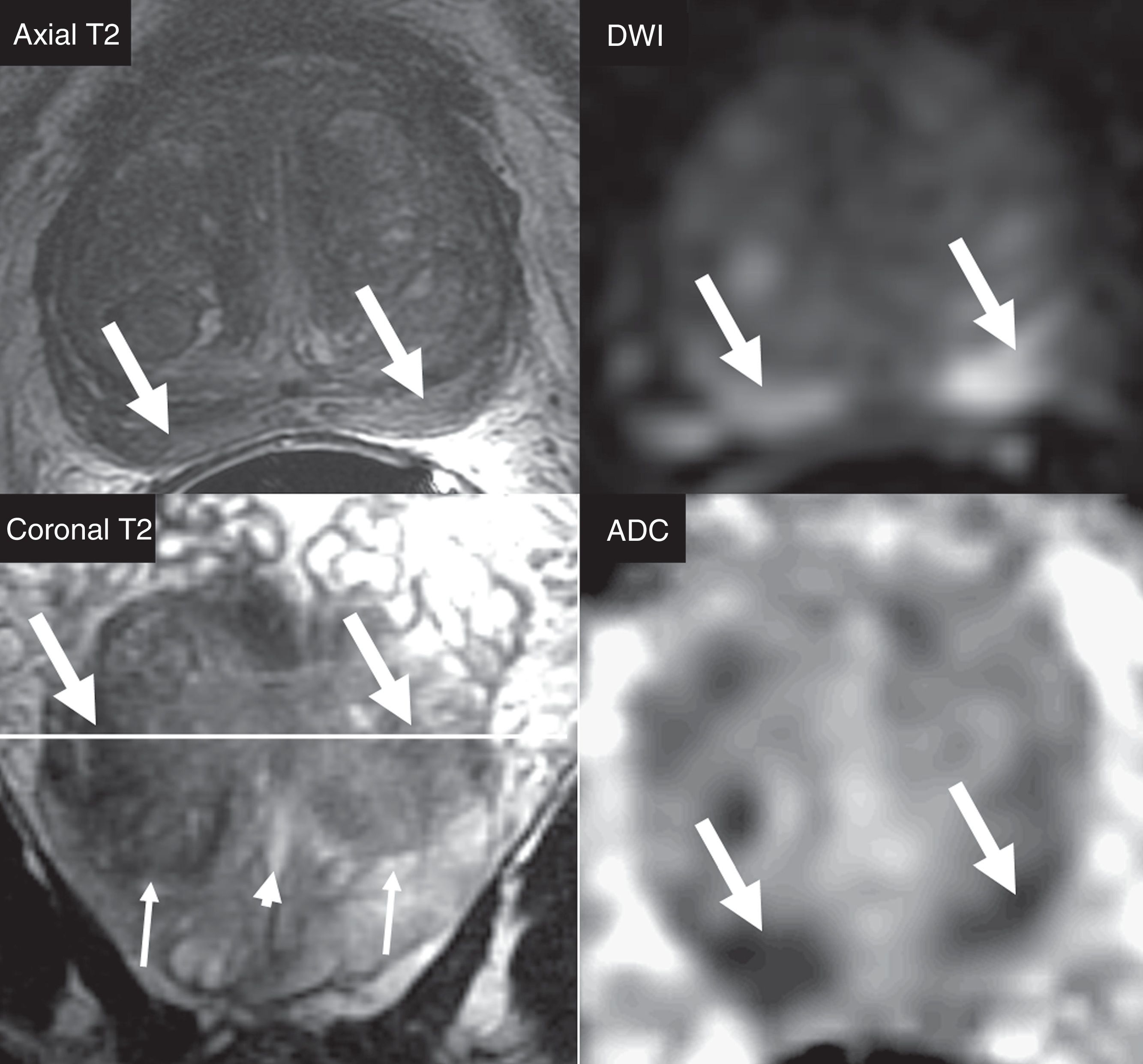
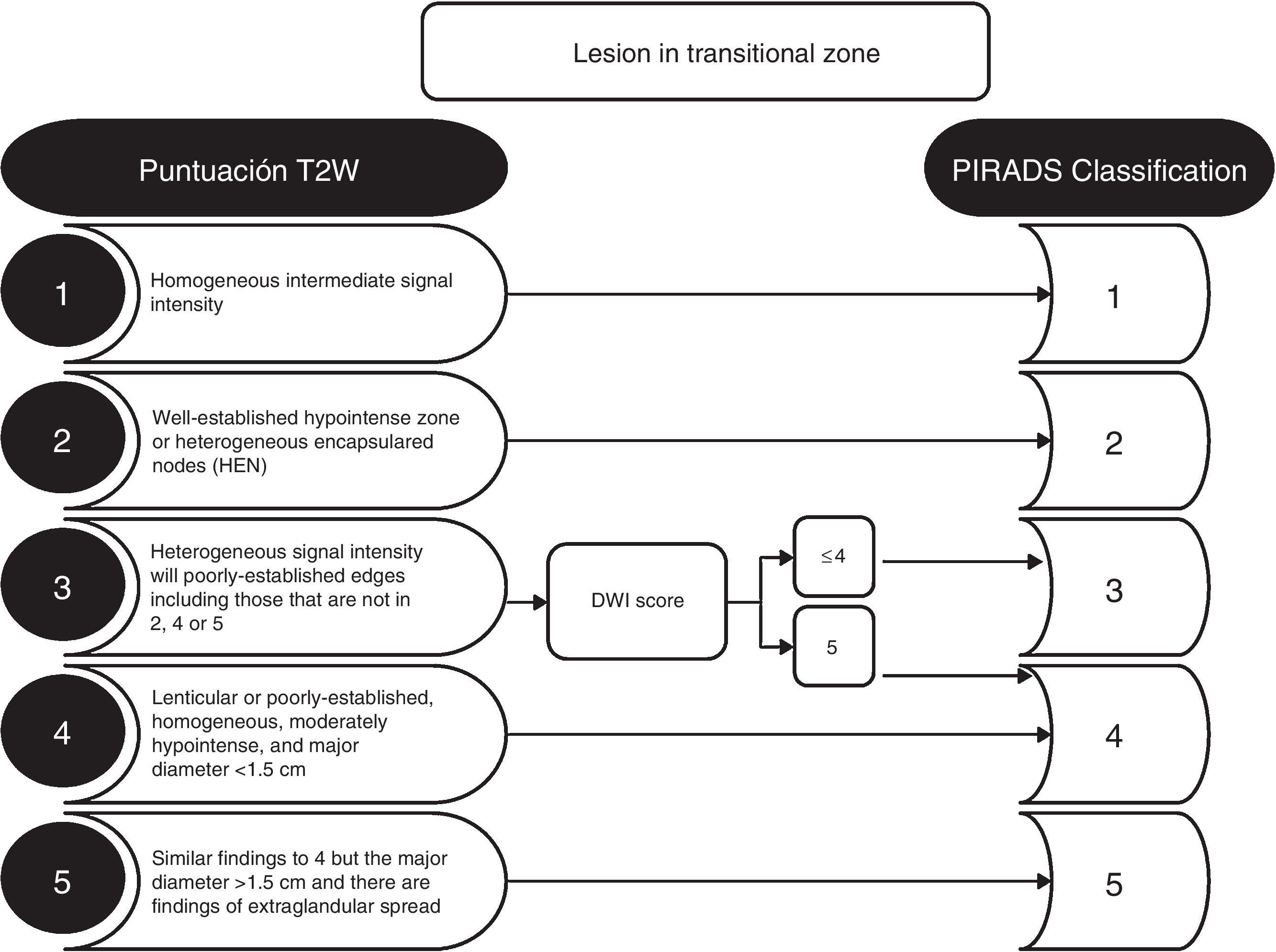
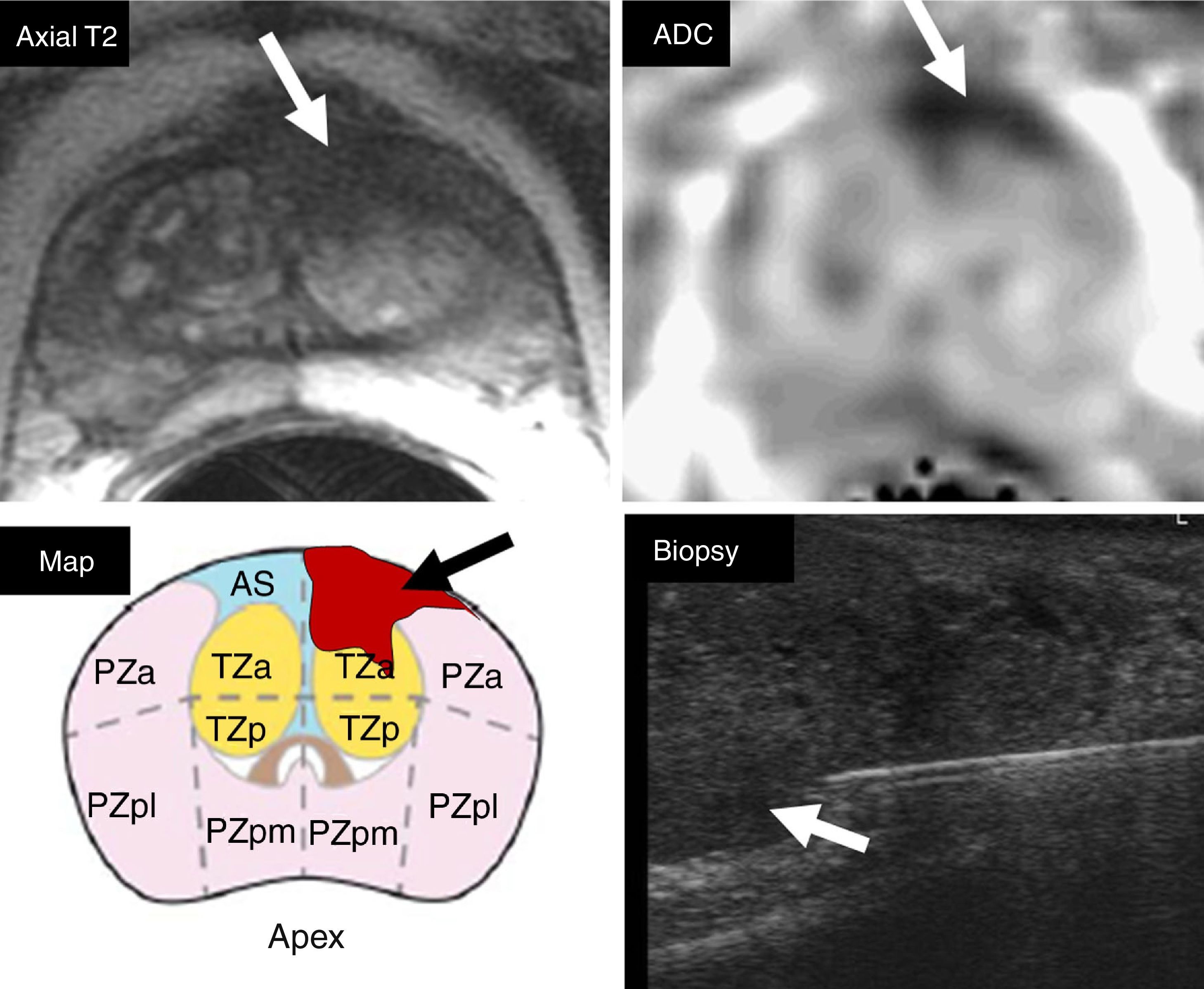
The criterion for significant lesion is to evaluate the location of the zonal anatomy and the findings of the dominant sequence for the given area. It is essential to locate the lesion in the correct area, and to avoid choosing incorrectly the dominant sequence for its interpretation (Fig. 1). The problematic anatomic areas are the transition between the central area and the peripheral area on the base (Fig. 1), and the interface between the anterior peripheral area, the transitional area and the fibrostroma. In the peripheral area, the dominant sequence to determine significance is DWI, and in the transitional area, it is T2. That is, a lesion in the peripheral area with a score of 4 on the DWI (markedly hyperintense focal lesion in DWI with high b value and markedly hypointense in the ADC <1.5cm), regardless of any criterion in the other sequences, will be categorized as PIRADS 4 (Fig. 2). Also, a lesion with a score of 2 in the T2 in the transitional area (hypointense or heterogeneous encapsulated node), regardless of any other criterion in other sequences, will be categorized as PIRADS 2 (Fig. 3).
Selection of dominant sequence with respect to the anatomical area. Diffuse posterior Iso/hyposignal in axial T2-weighted sequence (arrows) with focal bilateral hypersignal in DWI (b=1400s/mm2) and hyposignal in ADC (arrows). The incorrect location of focalizations as peripheral areas would lead to the assessment of lesions in the DWI sequences (dominant sequence in the peripheral area), with PIRADS category 4. The findings are located in the central area, in the proximal posterior base of the gland. The coronal slice in T2 allows us to locate the reference slice of the axial plane (white line), the right area as the central area (glandular base), but not as peripheral area. Anatomically, the central area, in general, does not spread distally to the verumontarum (short arrow), posteriorly to the gland. The coronal slice allows us to assess the transition between the central area (thin arrows) and the posterior distal peripheral area. The correct location of the central area establishes the T2 sequence as the dominant one (and not DWI) - being the homogeneous isointense pattern normal in the T2 of the central area. The restriction of diffusion of the central area is physiological, showing a relatively symmetrical, bilateral appearance, described as mustache sign, due to its appearance on the axial plane in ADC or DWI (arrows).
The DCE sequence is only relevant in the PIRADS v2 guidelines for lesions in the peripheral area with scores of 3 in the DWI (Fig. 2). It is also useful in cases of technical deficiency, such as artifacts in diffusion sequences.
DWI sequences are relevant in the transitional area for lesions categorized as PIRADS 3 in the T2-weighted sequences. A score of 5 in the DWIs increases PIRADS categorization to 4 in the transitional area (Fig. 3).
PIRADS v2 guidelines recommend providing measurement of the lesion in its greatest diameter and in the axial plane, on the ADC map for peripheral area lesions and in the T2-weighted sequences for the transitional area. If the lesion is greater on another plane, both the plane and the size should be indicated.
It is necessary to do a simultaneously reading of the axial T2-weighted, axial DWIs (high b value), axial ADC, and coronal or sagittal T2-weighted sequences (Fig. 4). The ADC maps have a narrow dynamic range; therefore the correct adjustment of the visualization window is crucial.10 Given the subjectivity in the interpretation of DWI and ADC images to establish the signal alteration criterion (hyperintensity in DWI or hypointensity in ADC) as mild, moderate or significant that will be useful to determine the PIRADS score, it is necessary to underscore that the suspicion criterion in DWI and T2-weighted images in the peripheral area is having nodular morphology (Fig. 2); that is, a non-nodular lesion in the peripheral area and with mild restriction of the DWI will be categorized as PIRADS 2 (Fig. 2). Slight non-nodular, poorly-established, linear, lobular or diffuse signal changes are not probably of neoplastic origin in the peripheral area.
Reading of the multiparametric magnetic resonance imaging study. Visualization of the four sequences (axial, coronal T2, DWI [high b value] and ADC) in the same window for an effective assessment of morphology, signal and zonal localization of a PIRADS 4 type of lesion (arrow) in the left anterior transitional area. The optimal level of the window should be adjusted to visualize the image correctly both in DWI and on the ADC map.
Today, the capacity to reliably detect and characterize clinically significant PCa using MP-MRIs in the transitional area is smaller than in the peripheral area.11 The presence of homogeneous or heterogeneous nodes in the transitional area–round or oval, well-outlined and encapsulated nodes, is a common finding in males over 40 years of age. In the DWI/ADC, these nodes can show restriction and enhancement with DCE; also criteria for benignity in the context of benign hypertrophy of the prostate. These findings do not require allocation in the PIRADS assessment; otherwise, almost all the allocations would be PIRADS 2. In any case, it is advisable to describe in the final conclusion, in addition to the allocation of the PIRADS category, the presence of the prostatic hypertrophy grade. Moderate or significant prostatic hypertrophy grades may explain high PSA values. The presence of bilateral symmetrical signal alterations in any sequences is often due to normal prostate anatomy or benign changes (Fig. 1). In these cases it is useful to quantify the ADC value. It is believed that clinically significant cancer shows ADC values <750–900μm2/s, unlike chronic inflammatory processes, that usually show higher ADC values.10
The location of the significant lesion should appear in the template according to the 39 topographic zonal segments of the gland, defined in the PIRADS v2 guidelines, and included in the report for an optimal communication when the biopsy is performed, to be able to perform a correct cognitive fusion or software-guided procedure (Fig. 5), and then obtain the material from the suspected area defined on the MP-MRI.11
Cognitive topographic localization. The PIRADS category 5 lesion in the anterior transitional segment of the left apex shown in T2 and DWI (arrows) should be located in the sectorial grid of the topographic map together with the report (arrow). This information is essential to perform cognitive fusion during the ultrasound biopsy procedure, and thus be able to guide the needle to the suspicious segment (arrow) detected on the MP-MRI.
Over 90 per cent of PCa have an acinar phenotype, which means that they are made up of small glands of neoplastic secreting cells. The degree of a neoplasm measures the number of genetic alterations accumulated; higher number of alterations equals more aggressiveness. The accumulation of genetic alterations can be measured by the increase and irregularity of the nucleus or by the progressive loss of architecture. The Gleason grading system is based on the loss of architecture.12 Thus, a series of patterns are defined with differences in the size of glandular lumens and an accumulation of nuclei that can be correlated with hydric content, alterations in extracellular space and vascularization. These histological and architectural differences among the different patterns would be the foundation of the different appearance of lesions on the MP-MRIs; hence the importance for the radiologist to have at least superficial knowledge of such patterns. Pattern#3 is characterized by individualized glands of different sizes with intermediate stroma that can be infiltrated in between non-neoplastic glands. Pattern#4 can have several morphologies, ranging from groups of glands with absent or deformed lumen and prominent, fibrous stroma (malformed gland pattern) to gland aggregates that are not entirely separated from one another, or sieve-like distribution with stromal bands circumscribing large neoplastic aggregates. Pattern#5 is also variable, since it can be made up of accumulations of neoplastic cells without hardly any stroma and collagen areas with arrays of neoplastic cells. This accentuation in the distortion of architecture as the tumor becomes more aggressive probably explains the differences seen in diffusion sequences (DWI/ADC) among well-differentiated tumors, with low Gleason scores, that show none or little restriction to diffusion, and the most aggressive tumors, with higher Gleason scores, that show an accentuated restriction to diffusion.
Most prostate cancers have different patterns that are shown in the Gleason grading system that consist of the total sum of two patterns. According to the criteria established in 2005, in such total sum, the most frequent pattern present in the sample should be the primary pattern and the least represented pattern should the secondary pattern. Thus, in a Gleason pattern 3+4, pattern#3 is the most represented in the sample. When in presence of a tertiary pattern (normally there are just two patterns, but sometimes, there can be representation of a third pattern), if this tertiary pattern has a higher degree than the secondary pattern thanks to its relevance, then it occupies the second position in the total sum.
In the biopsy, patterns 1, 2 and 3 are unified into one common pattern – number 3. In patients with one Gleason pattern only, the pattern is repeated in the total sum (3+3, 4+4 or 5+5).
As an evolutionary form of Gleason grading system, and in order to be able to adapt the biology of the total sums of the different patterns based on the actual criteria, a grouping of such total sums in five prognostic groups has been proposed: 3+3 (group 1); 3+4 (group 2); 4+3 (group 3); 4+4, 3+5, 5+3 (group 4); and total sums 9 and 10 (group 5).13
With these groupings, good prognostic staging has been observed both in biopsies, prostatectomy pieces and irradiated patients,12–15 being the general consensus the use of Gleason denomination patterns together with the prognostic grade groups.
Implications of prostatic multiparametric magnetic resonance imagingDetection of prostate cancerIt does not seem adequate to carry out one collective screening for the early detection of PCa and it is recommended to carry out individualized strategies based on the risk of every patient. For that purpose, a life expectancy ≥10–15 years should be estimated and the risk of possible overdiagnosis should be informed.16
Until recently, the only diagnostic technique used for the detection of PCa when in suspicion of PCA due to high PSA or suspicious digital rectal examination was thesystematic prostate biopsy. However, this technique has important limitations: the apex, the medial and anterior areas are usually poorly assessed; it provides an important percentage of clinically non-significant cancer, that sometimes leads to overtreatment; and there is 30 per cent discrepancy with respect to the Gleason grading system obtained subsequently depending on the prostatectomy piece, which often leads to mistaken therapeutic decision making. In this setting, the MP-MRI gains the utmost relevance today.
One systematic review of the literature that selects 50 studies for its analysis compares the profitability of the MP-MRI-guided biopsy vs the systematic biopsy.17 The authors conclude that both techniques detect PCa in a similar way (43 per cent), this is why performing biopsies in patients without suspicious lesions on the MP-MRI (approximately one third of the cases) could have been avoided. Thus, efficiency (detected cancers/biopsied patients) would have been clearly favorable to the MP-MRI-guided biopsy (70 per cent) with respect to systematic biopsy (40 per cent). Also, the MP-MRI-guided biopsy skipped the detection of 10 per cent of clinically non-significant cancers.
De Visschere et al.18 retrospectively assessed 830 patients with high PSA and MP-MRI in an effort to evaluate the characteristics of the PCa showed during the follow-up (2 years) of the MP-MRIs that had tested negative. Such follow-up was conducted though PSA control, digital rectal examination, prostate biopsy or a new MP-MRI. The MP-MRI tested negative in 391 patients (47.1 per cent). The PCa was detected in their follow-up in 124 patients (31.7 per cent), most of them (67.7 per cent) scoring and being confined to the organ (96.0 per cent). The negative predictive value for high score PCa was 95.4 per cent. The authors conclude that, with high PSAs and negative MP-MRIs surveillance should be considered instead of immediate biopsies.
Yet despite the fact that there would be evidence to use the MP-MRI as a guide for the first biopsy, most centers still use the MP-MRI systematically only as a guide in cases of persistent suspicion of PCa and prior negative biopsies. In these patients, the MP-MRI is particularly useful because it allows us to target the biopsy to prostate regions that are poorly assessed through systematic biopsy, and given its high negative predictive value it avoids having to re-biopsy unnecessary cases. Roethke et al.19 evaluated 100 patients with at least one prior negative biopsy and persistently high PSAs, performing one MP-MRI as guide. PCa was detected in 52.0 per cent of the cases, 80.8 per cent of which were clinically significant prostate cancers. Numerous studies support the utility of the MP-MRI in this population of patients.20,21
Nevertheless, the limitations of the MP-MRI in the detection of tumors with scarce volumes should be taken into consideration. Vargas et al.22 retrospectively evaluated 150 patients with a history of PCa who had undergone one MP-MRI (PIRADS v2 reading model) and subsequent radical prostatectomies. These authors identify approximately 95 per cent of the tumors ≥0.5ml, regardless of their Gleason grading system. However, they only identify 26 per cent and 20 per cent (peripheral and transitional areas, respectively) of tumors <0.5ml and with Gleason scores ≥4+3 (that is, small, but clinically significant tumors because of their predominance of Gleason pattern#4). The findings from these authors show clear limitations in the technique for the detection of clinically significant small volume-prostate cancers.
A cost-benefit analysis is required to determine the exact role of MP-MRI in the detection of prostate cancers before the first biopsy.
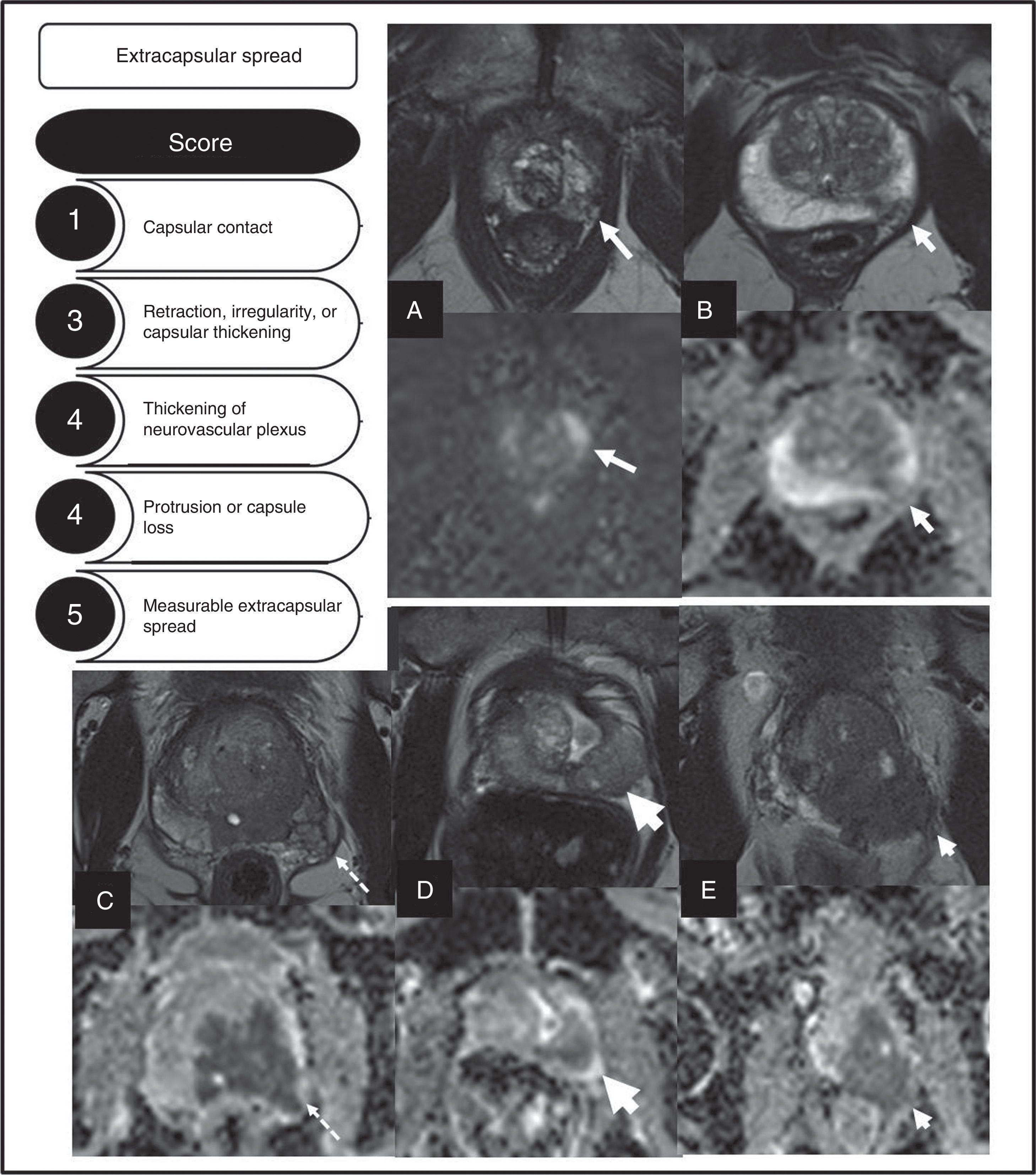
StagingCorrect local staging is essential to decide the most adequate type of treatment. ESUR has proposed a score ranging from 1 to 5 (using T2-weighted sequences) to assess the risk of extraprostatic spread23 (Fig. 6). Boesen et al.24 assessed the utility of this model in 87 patients, using the prostatectomy piece as diagnostic model. The authors state that scores ≥4, according to this scale, has a 81 per cent and 78 per cent sensitivity and specificity, respectively for the diagnosis of extracapsular spreads. On the other hand, scores ≥3 have a 94 per cent sensitivity, 68 per cent specificity and a 95 per cent negative predictive value, which would allow us to exclude, with very high probability, any extracapsular spreads. The results improved significantly when the readers added their personal opinion, including assessments of diffusion sequences. Other authors25 also show the utility of MP-MRIs in the prediction of extracapsular spreads.
Description of the different types of local spread of prostate cancers defined by the European Society of Urogenital Radiology and their score. Examples of the different categories defined are shown. (A) Capsular contact, T2 and ADC sequences (arrows). (B) Retraction, irregularity of capsular thickening, T2 and ADC images (arrows). (C) Thickening of neurovascular plexus, T2 and ADC images (arrows). (D) Protrusion of the capsule, T2 and ADC images (arrows). (E) Measureable extracapsular spread, T2 and ADC sequences (arrows).
At present, MP-MRIs are accepted by ESUR as a useful modality for the evaluation of local spread,23 while the European Association of Urology proposes its use in intermediate- and high-risk patients.
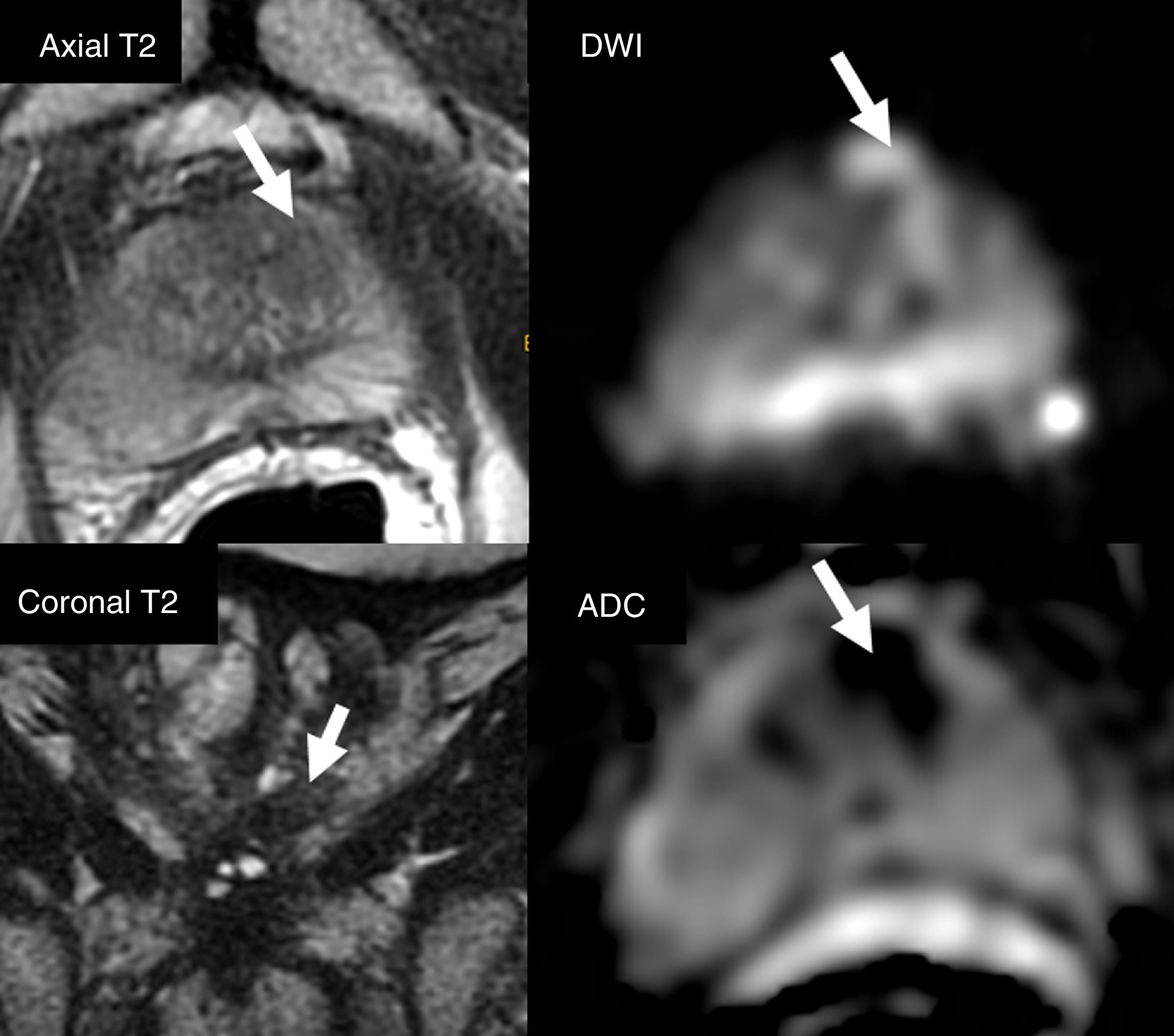
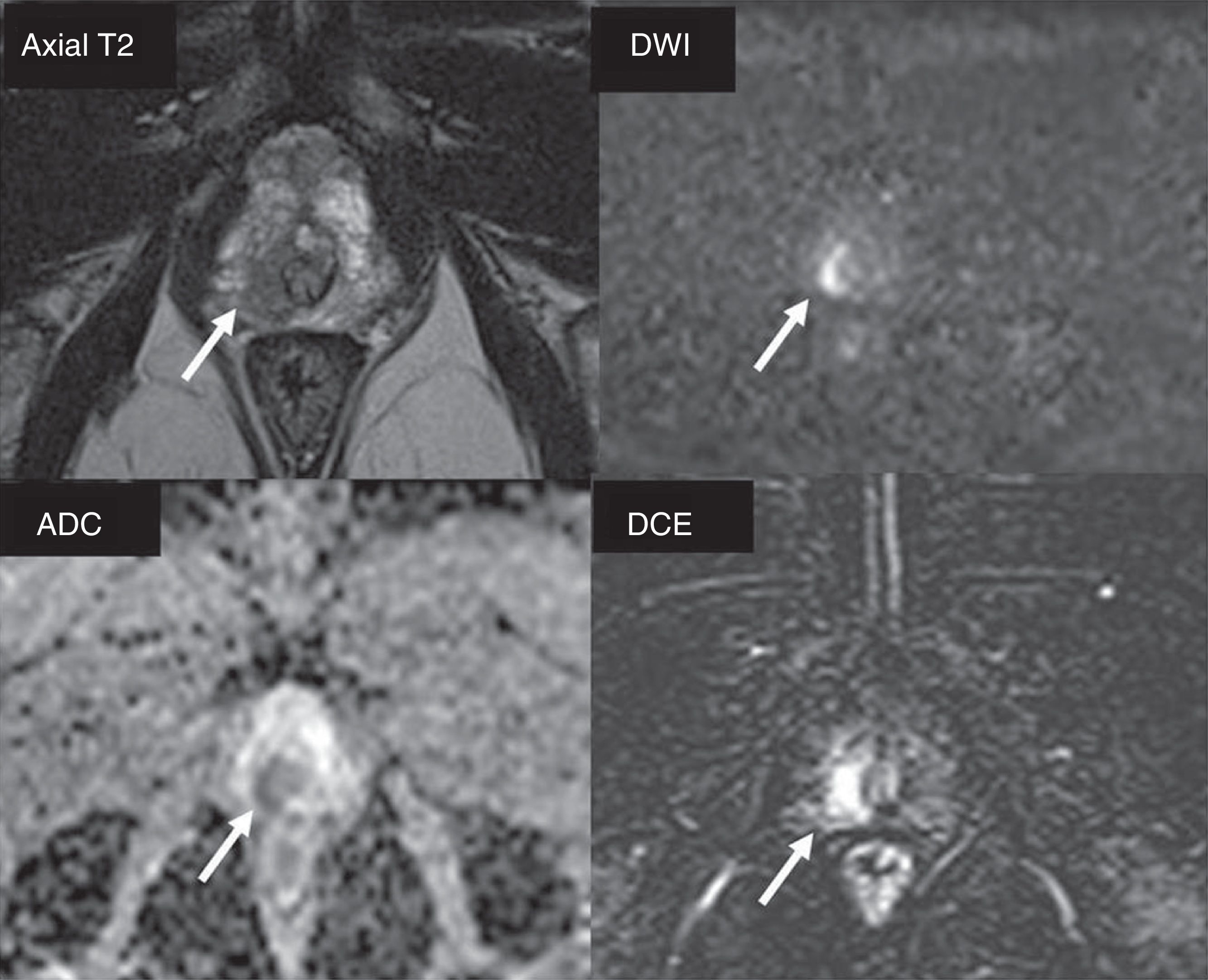
Active monitoringIn low-risk tumors it is possible to carry out active surveillance (also known as active follow-up), and it is not necessary to administer any initial treatments, with very good oncological results. The concept is based on the idea of not producing unnecessary morbidity in patients with clinically non-significant tumors. Active surveillance requires close follow-up of these patients with periodic PSA analysis, digital rectal examination and biopsies and an active treatment when the tumor progresses, either clinically or according to the histological grade. At the beginning, the inclusion criteria for active surveillance were very strict: Gleason scores ≤6 in less than 5 per cent of the biopsy tissue, PSAs <10 and anodyne digital rectal examinations (cT1c). Its good results from from the oncological and quality of life standpoints have made possible that the actual tendency is to widen the criteria and include patients with intermediate-risk localized tumors.26 In an effort to avoid including patients with clinically significant tumors in active surveillance protocols, the European urology guidelines recommend prior MP-MRIs to detect foci of clinically significant prostate cancers that might have been misdiagnosed by the systematic prostate biopsy16 (Fig. 7). The MP-MRI can predict the degree of tumor aggressiveness, especially by diffusion sequences. This is how better differentiated (clinically non-significant) tumors usually have little or no translation into these sequences, unlike what happens as the degree of aggressiveness progresses (clinically significant).27,28
Patient who underwent systematic biopsy due prostate-specific antigen increase. Prostate cancer (PCa) with Gleason 3+3 is diagnosed affecting a small percentage of only one cylinder. Patient is eligible for active surveillance; multiparametric magnetic resonance imaging study is performed. A right apical area is identified in the peripheral area (arrows) with PIRADS category 4 (score 4 in DWI sequence [b=1400s/mm2]) suggesting Gleason ≥7. When the guided biopsy is performed, PCa with Gleason 4+4 is diagnosed affecting three cylinders in 30 per cent of sample tissue.
A systematic review4 on the MP-MRIs and active surveillance confirms that two thirds of patients eligible to surveillance showed suspicious lesions on MP-MRI. The presence of suspicious lesions on the MP-MRI was most commonly associated with an increase of Gleason score (43 per cent) based on the prostatectomy piece with respect to those cases where there were no visible lesions with this modality (27 per cent). The MP-MRI led to a reclassification of the patient in 1:2 to 1:3 of the cases. This is why we can say that the MP-MRI is a useful modality for the assessment of patients eligible for surveillance based on the initial systematic biopsy. This indication has been endorsed by the guidelines published by the European Association of Urology.16
Although the MP-MRI is accepted for the initial evaluation of patients who are potential candidates to active surveillance,23 there still is no solid evidence to determine its role during follow-up.
Focal therapy planningFocal treatment is indicated in cases of tumors located in a single prostate lobe, usually scoring Gleason ≤7 and without any evidence of extraprostatic spread.29 The strategy is to treat the affected lobe only, but not the remaining gland, in an attempt to minimize the side effects and toxicity of the treatment, and preserve neurovascular bundles, the urethra and the sphincter musculature. The main goal is the eradication of measureable and biologically-aggressive diseases in just one single session. The two most widely used techniques considered as therapeutic options by the European Association of Urology - once brachytherapy and external radiotherapy16 have been excluded, are high intensity focalized ultrasounds (HIFU) and cryotherapy. They both show equivalent oncologic effectiveness, but significantly different treatment-related side effects. The decision on which therapy to use in each case will depend on age, comorbidity and the patient's decision.
Regarding focal therapies, the MP-MRI is valuable to locate and define lesion spread, as well as to rule out bilateralism. Although the PCa is usually multifocal (67–87 per cent) during the assessing of the the prostatectomy piece,30 there is usually an index lesion, defined as the largest, most aggressive lesion and consequently, the one with the highest prognostic value. This way, even though there is a wide spectrum of results in relation with the capacity of the MP-MRI to detect PCa,31–33 it has been confirmed that the diagnostic performance of this modality increases in larger, more poorly differentiated lesions, just as it happens in the index lesion. A recent interdisciplinary consensus panel5 concludes that if conducted with the right technology and is interpreted by experienced radiologists, the MP-MRI is the technique of choice for the planning of such therapies. Saturation biopsies, understood as biopsies with a larger than usual number of shots (approximate range of cylinder between 16 and 26), would be reserved for patients with inconclusive MP-MRIs only. In these cases, the goal would be to determine the location of the tumor, which is not clearly visible through this modality.
Suspicion of relapse after prostate cancer therapyRelapse of local disease can be managed with different therapeutic techniques; therefore, correct assessments and accurate diagnoses to design second treatments are required.
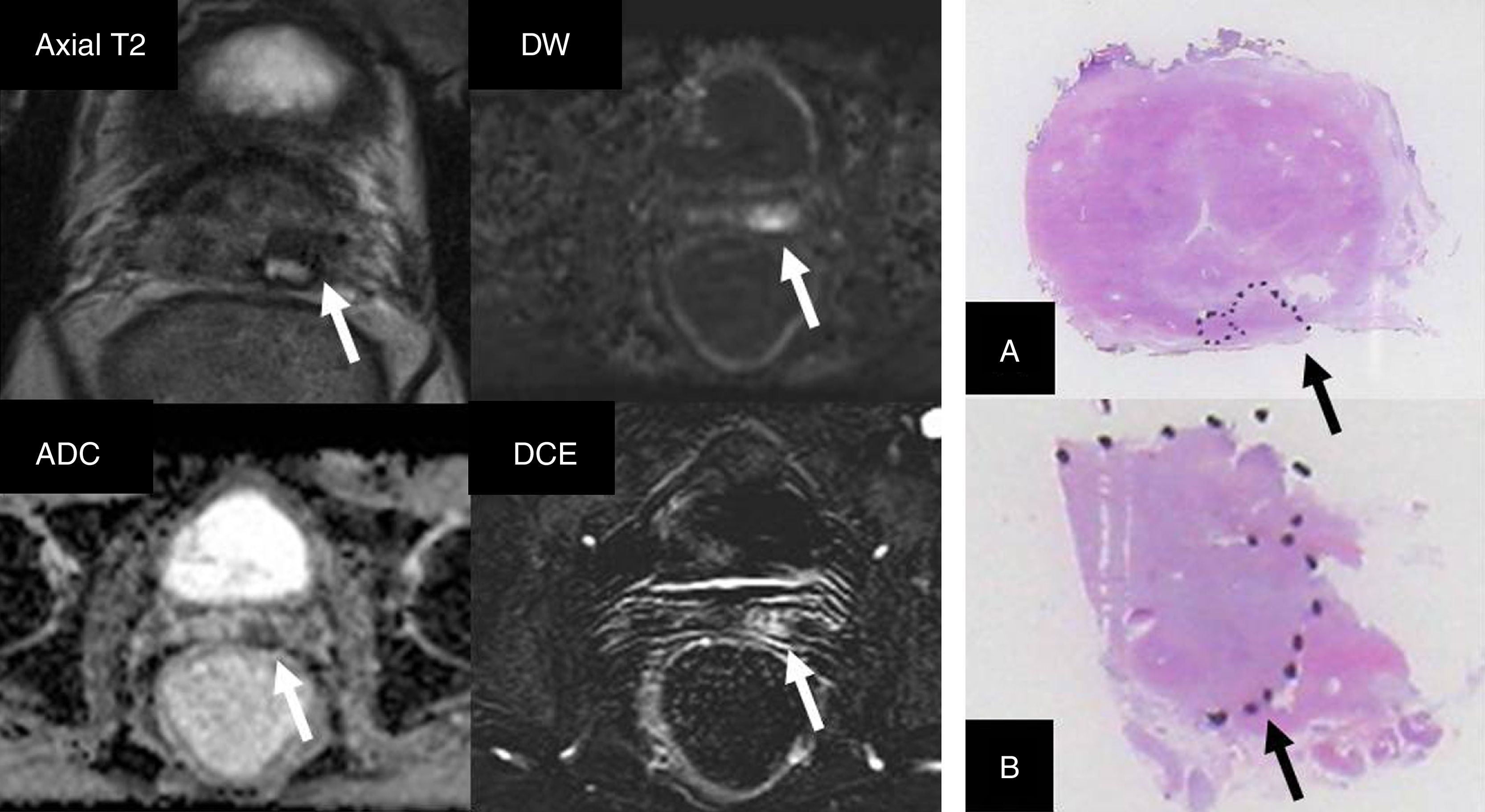
The MP-MRI is a useful tool to assess tumor persistence or recurrence. Regardless of the initial treatment of PCa, the assessment of diffusion and dynamic sequences is relevant, since the area of tumor relapse is usually not very evident in the T2-weighted sequences, while in the diffusion sequences a clear restriction is evident34 and in the dynamic sequences the presence of uptake is evident too.27,35–37 (Fig. 8).
Patient with a history of brachytherapy due to prostate cancer (PCa) with Gleason 3+3. Clinical manifestation of a 8ng/ml PSA (nadir value: 1.94ng/ml). T2-weighted sequence with hypointensity in a small area of the left seminal vesicle (arrow), with water restriction in the DWI and ADC sequences (b=1400s/mm2), and clear contrast uptake (DCE with subtraction) suggesting tumor remains or relapse (arrows). The patient was treated with rescue prostatectomy. Presence of PCa is outlined on the surgical piece (arrows) in the region of the ampulla of the deferent duct (A) and the left seminal vesicle (B).
Kitajima et al.,38 in prostatectomized patients (n=115), compare the performance of pelvic MP-MRIs versus PET/CTs with 11C-choline for the detection of local recurrences (AUC of 0.90 and 0.76, respectively; p<0.05), local adenopathies (AUC of 0.81 and 0.95, respectively; p<0.05) and osseous metastases (AUC of 0.92 and 0.89, respectively; p=NS). The authors conclude that the MP-MRI was better to detect local relapses, while the PET/CT with 11C-choline was better for the assessment of adenopathies, and that both modalities are equally optimal for the detection of pelvic osseous metastases, and provide additional information.
The main purpose of MP-MRIs is to provide information (tumor location and spread) for the planning of possible bailout therapies, like for instance, focal cryotherapy in post-prostatectomy relapses or radical prostatectomies in post-radiotherapy relapses. It is relevant to have information about the initial treatment administered, as well as clinical information and the anatomopathological history, since this allows a better interpretation of the images and gives guidance about the most likely location of relapse.
At present, the MP-MRI is an accepted modality to assess any possible relapses in patients undergoing different therapeutic modalities (Fig. 8).
ConclusionAt present, MP-MRI can be conducted optimally with widely distributed technology as long as the technical parameters are optimized rigorously. The PIRADS v2 reading model is crucial to achieve high performances in the detection of clinically significant carcinomas. It is well-established that the MP-MRI plays a key role in the diagnosis and treatment of patients with PCa, becoming an essential tool in this field. Many of the indications are already established in the clinical guidelines and they are commonplace in everyday care; others are being more consistently endorsed by medical literature. Scientific evidence and cost-benefit analysis will determine the exact place this modality will have in the near future.
Ethical responsibilitiesProtection of people and animalsThe authors declare that while conducting this investigation no experiments have been carried out with human beings or animals.
Data confidentialityThe authors confirm that they have followed their centers protocols on the publication and disclosure of data from patients.
Right to privacy and informed consentThe authors declare that no data from patients have been disclosed in this paper.
Authors- 1.
Manager of the integrity of the study: VC, JCV and TM.
- 2.
Study idea: VC.
- 3.
Study idea: VC.
- 4.
Reference search: VC, JCV, JMG, FA and TM.
- 5.
Writing: VC, JCV, JMG, FA and TM.
- 6.
Critical review of the manuscript with intellectually relevant remarks: VC, JCV and TM.
- 7.
Approval of final version: VC, JCV, JMG, FA and TM.
The authors declare no conflicts of interests associated with this article whatsoever.
FinancingAll authors confirm that there has not been any economic support to finance this paper.
We wish to thank Mr. Ricard Pellejero for helping during the reference research; Ms. Seila Ballerini, for her efficient support helping us adequate the formal aspects of this manuscript. Finally we also wish to thank Mr. Carles Fernández, for his help and contribution providing graphic material.
Please cite this article as: Catalá V, Vilanova JC, Gaya JM, Algaba F, Martí T. Resonancia magnética multiparamétrica y cáncer de próstata: ¿qué hay de nuevo? Radiología. 2017;59:196–208.








![Reading of the multiparametric magnetic resonance imaging study. Visualization of the four sequences (axial, coronal T2, DWI [high b value] and ADC) in the same window for an effective assessment of morphology, signal and zonal localization of a PIRADS 4 type of lesion (arrow) in the left anterior transitional area. The optimal level of the window should be adjusted to visualize the image correctly both in DWI and on the ADC map. Reading of the multiparametric magnetic resonance imaging study. Visualization of the four sequences (axial, coronal T2, DWI [high b value] and ADC) in the same window for an effective assessment of morphology, signal and zonal localization of a PIRADS 4 type of lesion (arrow) in the left anterior transitional area. The optimal level of the window should be adjusted to visualize the image correctly both in DWI and on the ADC map.](https://static.elsevier.es/multimedia/21735107/0000005900000003/v1_201706080033/S2173510717300289/v1_201706080033/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


![Patient who underwent systematic biopsy due prostate-specific antigen increase. Prostate cancer (PCa) with Gleason 3+3 is diagnosed affecting a small percentage of only one cylinder. Patient is eligible for active surveillance; multiparametric magnetic resonance imaging study is performed. A right apical area is identified in the peripheral area (arrows) with PIRADS category 4 (score 4 in DWI sequence [b=1400s/mm2]) suggesting Gleason ≥7. When the guided biopsy is performed, PCa with Gleason 4+4 is diagnosed affecting three cylinders in 30 per cent of sample tissue. Patient who underwent systematic biopsy due prostate-specific antigen increase. Prostate cancer (PCa) with Gleason 3+3 is diagnosed affecting a small percentage of only one cylinder. Patient is eligible for active surveillance; multiparametric magnetic resonance imaging study is performed. A right apical area is identified in the peripheral area (arrows) with PIRADS category 4 (score 4 in DWI sequence [b=1400s/mm2]) suggesting Gleason ≥7. When the guided biopsy is performed, PCa with Gleason 4+4 is diagnosed affecting three cylinders in 30 per cent of sample tissue.](https://static.elsevier.es/multimedia/21735107/0000005900000003/v1_201706080033/S2173510717300289/v1_201706080033/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


