To evaluate the diagnostic performance of acoustic radiation force impulse imaging (ARFI) in detecting significant hepatic fibrosis in children.
Material and methodsOur hospital's ethics committee approved the study and all patients or their representatives provided informed written consent. We included 96 children (50 boys, 46 girls; mean age, 8 years). We also studied 16 volunteers without liver disease as controls and 80 patients with diseases that can lead to fibrosis and cirrhosis of the liver. The final sample included 31 patients with biopsies and the 16 controls. All patients underwent abdominal ultrasonography including Doppler imaging and elastography with ARFI. The ARFI value, expressed as velocity (m/s) of shear wave propagation through the tissue, was calculated by averaging 16 measurements in both liver lobes. We used one-way analysis of variance to compare means between groups; we set statistical significance at P<0.05. We used Student's t-tests and chi-square tests for categorical data.
ResultsThe ARFI value in children with fibrosis ≥F2 was higher (1.80±0.45m/s) than in controls and higher than in patients with F0–F1 (1.38±0.22m/s). The difference was significant (P<0.001) for detecting ≥F2. Steatosis was not related with the ARFI value (Student's t-test, P>0.84). Necroinflammatory activity was strongly associated with the ARFI value (Student's t-test, P<0.01). Fibrosis and necroinflammatory activity were strongly associated with each other (chi-square test, P<0.0001).
ConclusionThe speed of shear wave propagation is significantly associated with the degree of hepatic fibrosis in children.
Evaluar el rendimiento diagnóstico de ARFI para detectar fibrosis hepática significativa en la edad pediátrica.
Material y métodosEl estudio fue aprobado por el comité de ética hospitalario, con el consentimiento informado de los pacientes o sus representantes. Estudiamos 96 niños (50 varones, 46 hembras; edad media 8 años); 16 voluntarios sin enfermedad hepática conocida y 80 con patologías que pueden evolucionar a fibrosis y cirrosis hepática. La muestra final incluyó 31 pacientes con biopsia y 16 controles sanos. En todos los casos se realizó ecografía abdominal incluyendo Doppler y elastografía con ARFI. El valor ARFI expresado como velocidad (m/s) de propagación de las ondas transversales a través del tejido se calculó promediando 16 medidas en ambos lóbulos hepáticos. Comparamos las medias con el test de ANOVA de un factor. Los tests t de Student y chi cuadrado se usaron para datos categóricos. La significación estadística se estableció para una p<0,05.
ResultadosLa velocidad en niños con fibrosis ≥ F2 fue significativamente más alta (1,80±0,45m/s) que en controles y pacientes con F0-F1 (1,38±0,22m/s) (p<0,001). La esteatosis no se relacionó con la velocidad. La actividad necroinflamatoria se relacionó muy significativamente con la velocidad (p<0,01). Fibrosis y actividad necroinflamatoria se relacionaron muy significativamente (p<0,0001).
ConclusiónLa velocidad de propagación de las ondas ARFI se relacionó significativamente en los niños con el grado de fibrosis hepática.
When chronic diffuse liver disease is assessed, estimating the degree of fibrosis is key for staging the disease, making therapeutic decisions, monitoring the disease and establishing a prognosis. The liver biopsy is a bloody, expensive technique, with associated risks of serious complications,1,2 which in addition to inter- and intra-observer variability during the estimation of the degree of fibrosis, may not reflect the disease's global activity.3–5 On the other hand, serological biomarkers and transitional elastography (FibroScan®, Echosens, Paris, France)6 to measure liver rigidity are bloodless methods developed to assess hepatic fibrosis. Both techniques are simple and well tolerated by the patients. But FibroScan® also has limitations and in approximately 5% of the patients, especially in cases of obesity and ascites, it cannot measure reliably.7 Among the alternatives there are some imaging techniques being developed today such as the elastography, the magnetic resonance hepatic diffusion, computed tomography digital optical analysis of liver images and real time tissue elastography.8,9 Although the preliminary results are encouraging, these techniques are more expensive, they are not available in all healthcare centers and it takes too much time to apply them clinically in hepatic fibrosis.
The Acoustic Radiation Force Impulse (ARFI) technique is a method to virtually quantify hepatic rigidity through integrated software (Virtual Touch Tissue Quantification, Siemens, Erlangen, Germany) in a conventional ultrasound scanner. It emits a series of short (≈100μs), high-energy, low-repetition frequency pulses (pulse repetition frequency) with a modified transducer. The pulses alter the tissue mechanically causing waves that produce local perpendicular (transverse) micro-displacements toward the acoustic pulses. ARFI allows us to measure the wave propagation speed, a biological parameter considered to be analogous to elasticity. Several studies conducted in adults have evaluated ARFI's clinical utility in the estimation and categorization of liver fibrosis,10–14 in the assessment of thyroid nodes, breast lumps, hepatic and renal tumors, in the characterization of atheroma and in the monitoring of the radiofrequency ablation outcomes.15–18 In children, a varied group of liver diseases can produce fibrosis and advance into mid- or long-term cirrhosis.19 Liver fibrosis is not a static lesion20,21 and it can go better or worse depending on how active the disease really is this is why its monitoring is warranted.
Studies on the ARFI utility in the follow-up of chronic liver disease in children are scarce.22–27 The purpose of this article is to assess the diagnostic performance of hepatic elasticity measured through ARFI to detect significant fibrosis in children with diffuse progressive chronic liver disease.
Material and methodsPatientsThe study was approved by the hospital ethics committee and the patients’ or their representatives’ informed consent was obtained.
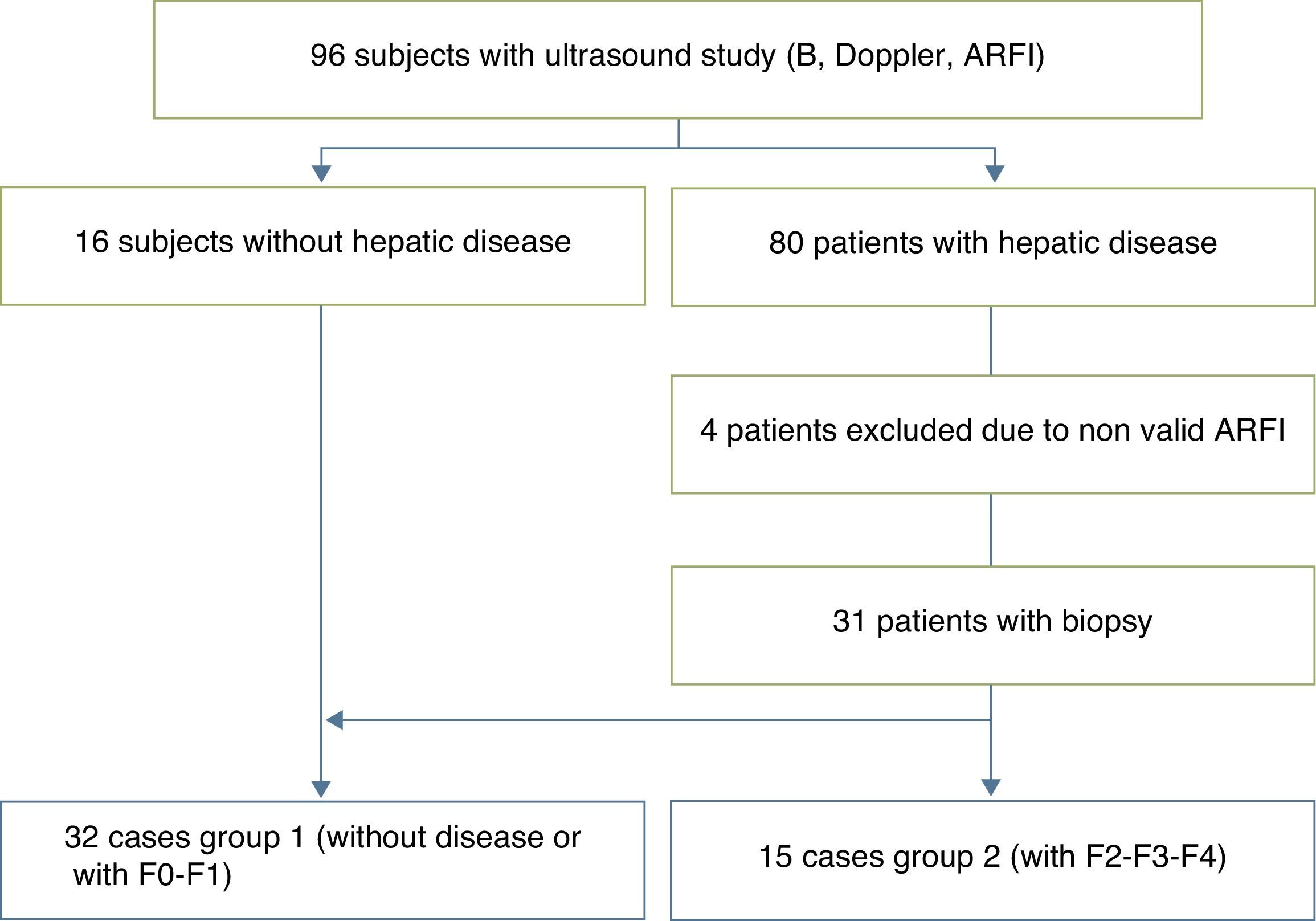
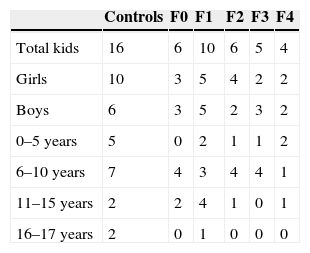
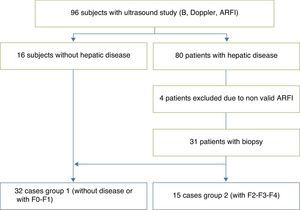
We studied 96 children prospectively (50 boys, 46 girls; median age, 8 years; range, 4 months to 17 years), divided into 16 voluntary controls without known liver disease and 80 patients followed up due to diseases that can produce liver fibrosis and cirrhosis. Four of them were excluded due to invalid ARFI results. Among the remaining, 13 patients had cystic fibrosis; 13 chronic viral hepatitis (3 virus B liver disease; 7 virus C liver disease; 3 cytomegalovirus and Epstein Barr virus liver disease); 10 biliary duct atresia; 6 metabolic syndrome; 5 multifactorial hypertransaminasemia; 4 patients vascular disorders (common atrioventricular canal and portal cavernomatosis); 4 alpha-1 antitrypsin deficit; 4 cryptogenetic cirrhosis; 3 Wilson's disease; 3 congenital liver fibrosis; 3 autoimmune cirrhosis; 3 patients were treated with chemotherapeutic drugs; 2 with tyrosinemia; 2 glycogenosis; 2 with hepatic steatosis, and 3 were isolated cases of hepatitis of unknown etiology, recessive autosomal kidney polycystosis and Fanconi's anemia accompanied with hemosiderosis. Of this sample, 31 patients were selected who had undergone biopsy one year before the beginning of the ARFI study (5 patients with biliary duct atresia; 2 with Alagille syndrome; 2 with glycogenosis ix; 1 with tyrosinemia; 1 with congenital liver fibrosis; 2 with autoimmune hepatitis; 1 with nodular regenerative hyperplasia; 6 with chronic hepatitis C; 3 with chronic hepatitis B; one with Epstein Barr virus hepatitis; 3 with Wilson's disease; 1 with hypobetalipoproteinemia; 1 with common atrioventricular canal; 1 with a type IVa choledochal cyst; 1 with Fanconi's anemia) who together with the 16 controls made up the final series of 47 children selected for the study.
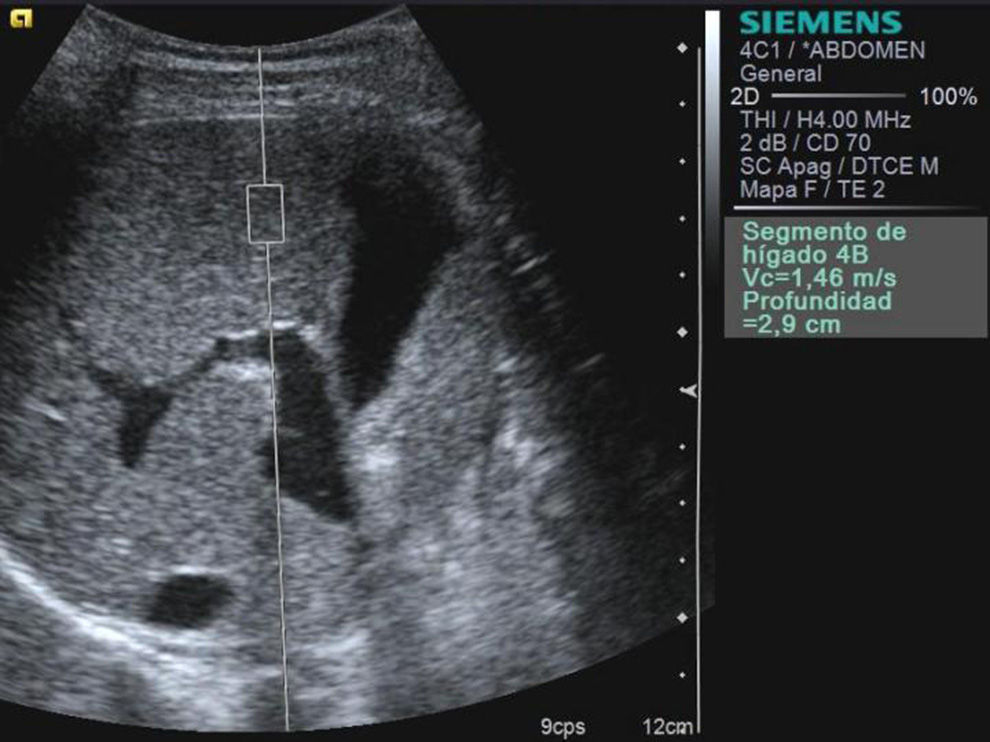
MethodAll the participants were studied in a same session though a B-mode abdominal ultrasound, Doppler and hepatic elastography with the ARFI technique with an ultrasound ACUSON S2000™ US system (Siemens Healthcare, Erlangen, Germany) and a conventional 4MHz, high-energy ultrasonic, short-lived pulses conventional transducer. The assessment was performed independently by 3 radiologists with over 10 years of experience doing abdominal ultrasounds and 2 years of experience using the ARFI modality. To this end, the operator fixed a rectangular region of interest (ROI) of a predetermined size (10mm long×6mm wide; and 2mm3 of volume) more than 1cm under the surface of the liver and at a maximum depth of 8cm from the cutaneous surface (Fig. 1). Areas without any large blood vessels were selected. Patients were examined in the supine position with their right arm in maximum abduction. Sixteen overall measurements were taken in both hepatic lobes, with the patient breathing smoothly and avoiding the areas proximal to the heart, entering through the intercostal spaces when it came to the right hepatic lobe and through the sub-xiphoid space when it came to the left one. The transverse wave propagation speed–expressed in m/s–was regarded as the marker for elasticity in the ROI.28–30 The result of each study was the average of all values obtained during the assessment.
Anatomopathological analysisLiver samples were obtained from 31 patients through TruCut puncture biopsy, with an 18G BioPince Full Core Biopsy Instrument automatic needle. At least one pass through subcostal access was performed to obtain a 23–33mm long cylinder. Only the biopsy samples with more than 4 intact portal tracts were used. The necro-inflammatory activity, hepatic fibrosis and steatosis were assessed semiquantitatively based on the METAVIR system. Fibrosis was staged on a 0–4 scale: F0, without fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with some septa; F3, numerous septa, without cirrhosis; and F4, cirrhosis. The patient sample was classified into 2 groups based on this scale: Group I, controls and patients F0–F1, and Group II, patients F2–F4 (Fig. 2). The necro-inflammatory activity was classified into: A0, without necro-inflammatory activity; A1, scarce; A2, moderated; and A3, significant activity. The steatosis was classified according to a visual assessment as follows: E0, no steatosis; E1, steatosis in less than 33% of the hepatocytes; E2, steatosis between the 33 and 66% of the hepatocytes; and E3, steatosis in more than 66% of hepatocytes.
Statistical analysisThe shear wave speed values were expressed as median±standard deviation and normal distribution was confirmed through the Kolmogorov Smirnov test (p<0.0001). The differences among the medians were analyzed through the one-factor ANOVA test. A p<0.05 was considered statistically significant. Once the ARFI measurement differences between groups I and II were confirmed, and after their graphic representation in a Cartesian plane, the centroids were defined (one for each group) that in this case since this is a monofactorial study coincide with the measurement of the group's median. The cut point was determined though the identification of the intermediate point of the line joining both centroids (Euclidean distance). Once it was obtained, the sensitivity and specificity values were analytically determined applying the mathematical formulae that assess true positives, true negatives, false positives and false negatives on the data already acquired. The Student's t-test was used to compare the steatosis and necro-inflammatory activity with the ARFI data, and the chi-squared test (χ2) was used to determine the association of the ARFI data with the degree of fibrosis and the necro-inflammatory activity in the liver. The software used for the statistical analysis was SPSS 12.0 (SPSS Inc., Chicago, USA).
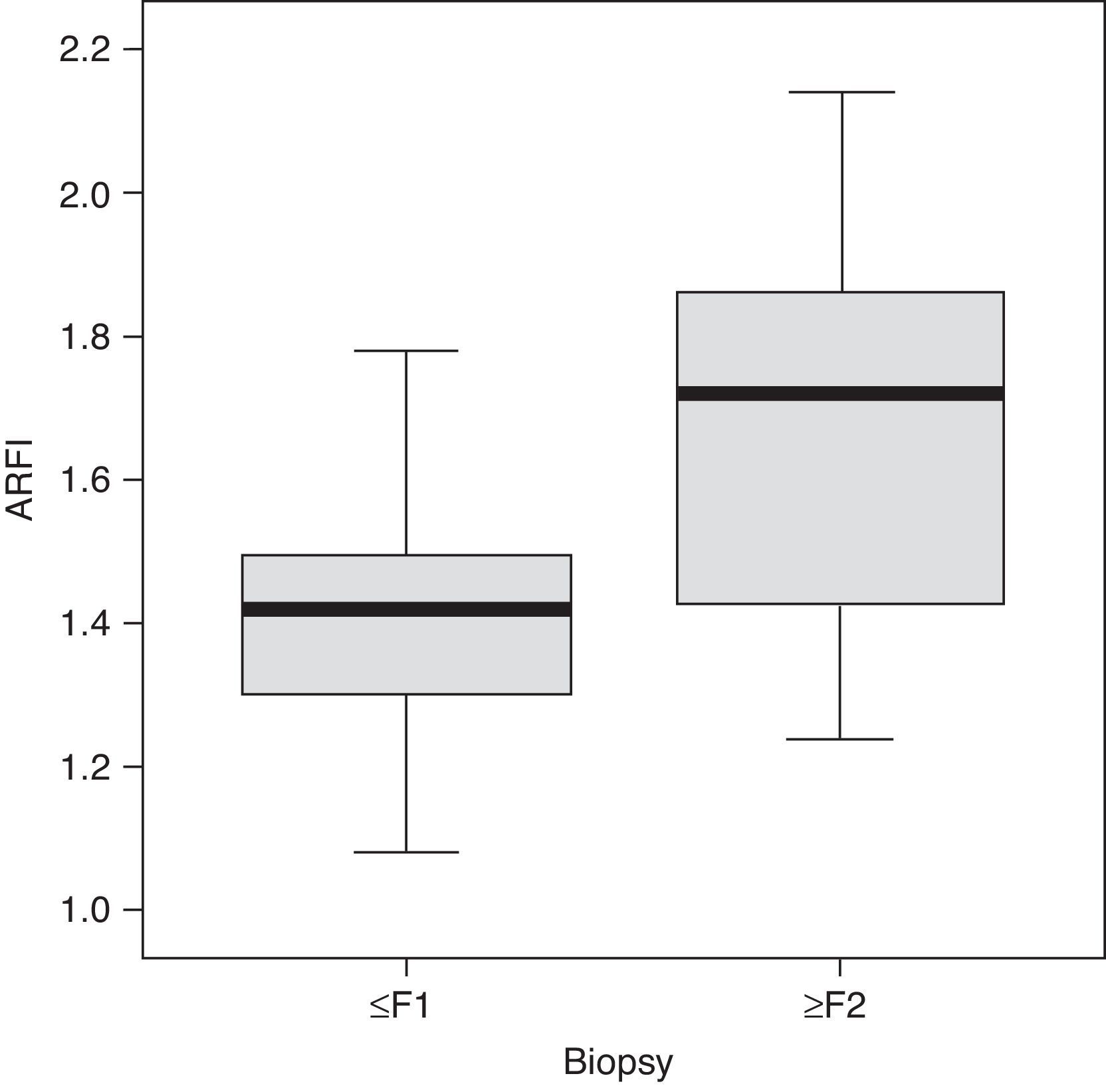
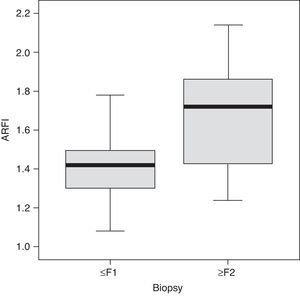
ResultsThe biopsy was performed in 31 patients with chronic diffuse liver disease. In 16 the result was no fibrosis or non-significant fibrosis (F0–F1), which, along with the 16 controls, made up Group I. In 15 patients, the result was significant fibrosis (≥F2) and they made up the Group II case study (Table 1). We saw a trend toward an increase of speed in stage F2 and an evident rise in the most advanced stages (F3–F4). The values overlapped among individuals without liver disease and stages F0–F1 (Table 2), which made it difficult to distinguish them. The variance analysis (ANOVA) showed significant differences (F[1,43]=17,81, p<0.000) between groups I and II. The wave median speed in groups I and II was 1.38±0.22m/s and 1.80±0.45m/s, respectively. The centroids of both groups separated even further with a cut value of 1.52m/s, for which sensitivity, specificity, predictive positive and negative values, and the precision to diagnose hepatic fibrosis ≥F2 were 73.68, 83.33, 73.68, 83.33 and 79.59%, respectively (Fig. 3). Steatosis was not associated with the wave propagation speed but necro-inflammatory activity was very significantly associated (p<0.01). In turn, fibrosis and necro-inflammatory activity were also significantly associated to lobe another (p<0.0001).
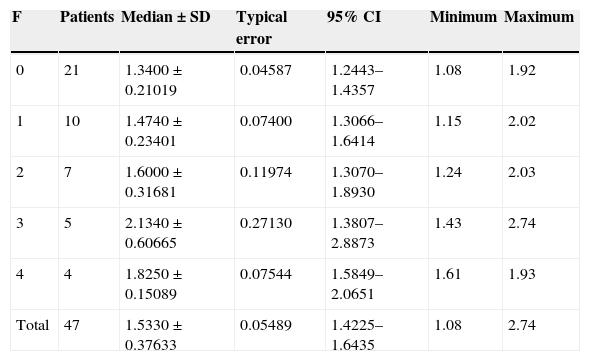
Wave speed related to the anatomopathological status of hepatic fibrosis (METAVIR).
| F | Patients | Median±SD | Typical error | 95% CI | Minimum | Maximum |
|---|---|---|---|---|---|---|
| 0 | 21 | 1.3400±0.21019 | 0.04587 | 1.2443–1.4357 | 1.08 | 1.92 |
| 1 | 10 | 1.4740±0.23401 | 0.07400 | 1.3066–1.6414 | 1.15 | 2.02 |
| 2 | 7 | 1.6000±0.31681 | 0.11974 | 1.3070–1.8930 | 1.24 | 2.03 |
| 3 | 5 | 2.1340±0.60665 | 0.27130 | 1.3807–2.8873 | 1.43 | 2.74 |
| 4 | 4 | 1.8250±0.15089 | 0.07544 | 1.5849–2.0651 | 1.61 | 1.93 |
| Total | 47 | 1.5330±0.37633 | 0.05489 | 1.4225–1.6435 | 1.08 | 2.74 |
SD: standard deviation; F: fibrosis; CR: confidence interval.
According to our data, in order to obtain >95% sensitivity the median wave propagation speed should be <1.24m/s. To obtain >95% specificity this value should be >1.62m/s. According to these data, two safety zones could be defined in which, without the need for biopsy, hepatic fibrosis can be quantified through ARFI with high sensitivity or specificity: up to 1.23m/s, sensitivity is very high; from 1.24m/s to 1.62m/s is an uncertainty region; from 1.62m/s onwards, specificity is very high.
DiscussionOur results indicate that the median speed in children with significant hepatic fibrosis (≥F2) measured through ARFI is significantly higher (1.80±0.45m/s) than in children without fibrosis or non-significant fibrosis (1.38±0.22m/s).
Staging hepatic fibrosis is essential for the early diagnosis, follow-up and appropriate treatment of patients with chronic liver disease. The liver biopsy, a reference pattern,1–5 cannot be considered the only tool for monitoring these diseases in pediatric age because it is bloody, it needs sedation and can entail serious complications. Among the noninvasive techniques developed to assess hepatic fibrosis we have serological markers (Fibrotest, APRI, among others), used in adults to predict hepatic fibrosis but they are not accurate in children,31 and FibroScan, which shows some technical drawbacks (fixed depth of measurement, it is not possible to see the area where the measurement is taken, limited use in case of obesity and ascites). ARFI is a new tool with tested advantages in adults.32–36 ARFI's diagnostic accuracy is similar to that of FibroScan, but it has added advantages: it can be used in patients with narrow intercostal spaces, with ascites and morbid obesity.37 Furthermore, the hepatic steatosis does not seem to have a statistically significant influence in its results.21
Most studies on the usefulness of ARFI to study hepatic fibrosis have been carried out in the adult population. Very few make reference to the normal range of wave propagation speed in healthy children and in groups of children affected with chronic diffuse liver disease.22,31 There is not such a thing as a standardized protocol of frequencies, the number and the depth to measure the speed. Some papers refer greater speeds in the left hepatic lobe and they recommend to take measurements only in the right one.32,38,39 In our study with ARFI, the average speed in Group I was 1.38±0.22m/s. This result was obtained considering the measurements obtained in both lobes. In children, the left hepatic lobe is proportionally larger than the right one and it does not have the limitations described in adults. That is why we also measure segments II, III, IVA and IVB, to assess the overall situation of the liver. This may have introduced an error in the average in our results thus explaining that our values in Group I are higher than those of other studies23,24,26 but we understand that measuring the right hepatic lobe alone would have introduced a sampling error. Nevertheless, taking into account the results published and the recommendations made by other authors, more studies will be required to come to a consensus as to how to use this technique.
When analyzing the categories of fibrosis, we observed a trend pointing at a rise in speed with the increase of fibrosis (≥2), but they do not allow us to differentiate normal children from early fibrosis which is consistent with other works published. Nevertheless, there is discordance between median speed in the group of individuals with chronic diffuse liver disease of our series (1.80±0.45m/s) and the results by Noruegas et al. (1.42±0.07m/s), and Hanquinet et al. (1.99±0.99m/s). This can be due to the different criteria used to establish the study groups.22,24
The main contribution of our study has been determining ARFI's diagnostic performance to detect significant hepatic fibrosis without the need for performing biopsies. However, we have to admit some limitations. In the first place, it is a prospective study, open, but not controlled; inclusion was random and ARFI was performed on patients with or without known liver disease that can advance into fibrosis. On the other hand, we did not study the inter-observer variability or were able to have the anatomic-pathological results of the livers of all children included. The biopsy was only performed on children referred from the department of gastroenterology due to worsening of clinical and biological parameters of the liver function, which presupposes a sample selection bias. Furthermore, necro-inflammatory activity was very significantly associated with the ARFI data (p<0.01), and fibrosis and necro-inflammatory activity were also very significantly associated too (p<0.001). This is why it could not be determined whether the wave propagation speed was altered by the fibrosis or by the necro-inflammatory activity–both were present at the same time and in the same way (the more fibrosis, the more necro-inflammatory activity) in our series. Finally we should also take into account that the advancement from fibrosis to hepatic cirrhosis, atrophy and liver mass loss–which brings closer together portal ducts and hepatic veins–and congestion might have something to do in the loss of hepatic elasticity in the advancement from fibrosis to cirrhosis of the liver, without mentioning the confounding effects of cardiac failure and the cholestasis that can be associated.40 Yet despite of everything, our results and those already published in pediatric series are consistent with the ones found in studies in other age groups15,30,32,33,35 and they show that ARFI is a simple method without any side effects and with good diagnostic performance to determine fibrosis by measuring the transverse wave propagation speed.
In sum, ARFI allows us to discriminate between children with ≥F2 fibrosis and normal children or with slight degrees of fibrosis. Since hepatic fibrosis is a continuous, progressive process that requires repeated controls and that the biopsy (because it is a bloody, risky technique) is not appropriate to monitor the disease, the ARFI elastography is a bloodless, simple and useful modality that is well tolerated by children and that can be repeated many times in the course of the disease.
Authors- 1.
Manager of the integrity of the study: SDPA.
- 2.
Original idea of the study: SDPA, DMV, CSN, LMB.
- 3.
Study design: SDPA, DMV, CSN.
- 4.
Data analysis and interpretation: SDPA, GGM, LMB.
- 5.
Reference search: SDPA.
- 6.
Writing: SDPA, DMV, GGM, CSN, LMB.
- 7.
Critical review of the manuscript with intellectually relevant remarks: SDPA, DMV, GGM, CSN, LMB.
- 8.
Approval of final version: LMB.
- 9.
All authors have read and approved the final version of this article.
Authors declare that the proceedings followed abide by the ethical regulations of the corresponding human experimentation committee and the World Health Organization and the Helsinki Declaration.
Data confidentialityAuthors confirm that in this article there are no personal data from patients.
Right to privacy and informed consentAuthors confirm that in this article there are no personal data from patients.
Conflict of interestsAuthors declare no conflict of interests.
We wish to thank Dr. Fernando Aparici Robles, M.D., and Dr. María Jesús Esteban Ricós, M.D., from the Department of Radiology of the HUP La Fe de Valencia, Spain, who, respectively, helped us with the creation of the database and the performance of hepatic biopsies whose results have been used in the writing of this paper.
Please cite this article as: Picó Aliaga SD, Muro Velilla D, García-Martí G, Sangüesa Nebot C, Martí-Bonmatí L. La elastografía mediante técnica Acoustic radiation force impulse es eficaz en la detección de fibrosis hepática en el niño. Radiología. 2015;57:314–320.