Natalizumab is very effective at reducing relapses and delaying disease progression in patients with relapsing-remitting multiple sclerosis (RRMS). However, treatment has also been associated with a risk of progressive multifocal leukoencephalopathy (PML). The aim of this article is to provide a consensus view on the assessment and stratification of these risks, and to improve the management of natalizumab-treated patients.
DevelopmentAt an initial meeting of experts on multiple sclerosis (MS) (the authors of this consensus), the relevant topics of the consensus were decided and assigned for development. Topics included how to establish benefit and risk in general, stratification for risk of PML, informing patients of benefit/risk, monitoring treatment, and treatment withdrawal and follow-up. During the drafting phase, all available information published or presented at international meetings was reviewed. After a series of review rounds and meetings, the final draft was produced.
ConclusionsAlthough natalizumab is a very effective drug, its use needs to be considered carefully in view of possible adverse effects and the risk of PML in particular. The neurologist should carefully explain the risks and benefits of treatment in terms the patient can best understand. Before starting treatment, baseline laboratory test and magnetic resonance imaging (MRI) should be available for comparison purposes in the event of suspected PML. The risk of PML should be stratified into high, medium and low risk groups according to antibodies against JC virus status, prior immunosuppressive therapy, and treatment duration. The follow-up, in particular, the frequency of MRI scans, should depend on the risk group to which patient belongs. As our understanding of the risk factors for PML develops, it should be possible to offer patients increasingly individualised therapy. This is a consensus that establishes general recommendations, but neurologists must use their clinical expertise to treat and follow individual patients.
Natalizumab es un tratamiento que ha demostrado ser muy eficaz en los pacientes con esclerosis múltiple recurrente-remitente (EMRR) en cuanto a la reducción del número de brotes y al enlentecimiento de la progresión de la enfermedad. Sin embargo, el fármaco se ha asociado con el riesgo de desarrollar leucoencefalopatía multifocal progresiva (LMP). El objetivo de este artículo es proporcionar una posición consensuada sobre la valoración y estratificación de este riesgo y mejorar el manejo de los pacientes tratados con natalizumab.
DesarrolloEn una reunión inicial de expertos en EM (los autores de este consenso), se perfilaron los temas de interés que fueron asignados a los asistentes para su desarrollo ulterior. Los temas incluían cómo establecer el beneficio y el riesgo en general, la estratificación para el riesgo de LMP, cómo informar a los pacientes de los beneficios y riesgos, cómo realizar el seguimiento del paciente en tratamiento y tras la suspensión del fármaco. Durante la fase de redacción, se revisó toda la información disponible, publicada o presentada en reuniones internacionales. Después de varios ciclos de revisión y de reuniones, se produjo el borrador final.
ConclusionesA pesar de ser un fármaco muy eficaz, la decisión de prescribir natalizumab debe ser tomada con cuidado por los posibles efectos adversos y en particular, el riesgo de LMP. El neurólogo debe explicar al paciente en detalle los riesgos y beneficios del tratamiento, en términos comprensibles para el paciente. Antes de empezar el tratamiento, deben estar disponibles las pruebas de laboratorio y las imágenes de resonancia magnética (RM) que permitan comparaciones en el futuro, en caso de sospecha de LMP. El riesgo de LMP debe estratificarse en alto, medio y bajo de acuerdo con la presencia o ausencia de anticuerpos frente al virus JC, antecedente de tratamiento inmunosupresor y duración del tratamiento. El seguimiento clínico y la frecuencia de la RM dependerá del grupo de riesgo al que pertenece el paciente. A medida que mejore nuestra comprensión de los factores de riesgo, será posible ofrecer a los pacientes una terapia cada vez más personalizada. El presente consenso establece unas recomendaciones generales, pero los neurólogos deben aplicar su experiencia clínica para hacer un seguimiento individualizado de los pacientes.
Natalizumab (Tysabri®) is a recombinant humanised monoclonal antibody that binds to the α4 subunit of α4β1 integrin (VLA-4) on the surface of lymphocytes. This action blocks the binding of the subunit to its receptor (VCAM-1), which is present in the endothelium. This prevents lymphocytes from entering the central nervous system, thereby reducing the pathological process of MS. In initial drug trials,1 natalizumab was shown to be highly effective in patients with relapsing-remitting multiple sclerosis (RRMS). However, 2 cases of progressive multifocal leukoencephalopathy (PML) were reported in the SENTINAL study which compared natalizumab plus interferon beta-1a with interferon beta-1a monotherapy.2
For the reason listed above, natalizumab is indicated as a RRMS disease modifying agent that is very effective in the following patient groups: patients with high disease activity despite interferon beta treatment, and patients with severe and rapidly progressing RRMS.
Patients with treatment failure following a complete course of interferon beta were defined as those who experienced at least 1 relapse in the past year while on treatment and had at least 9 hyperintense lesions in T2-weighted brain MRI sequences, or at least 1 gadolinium-enhancing lesion.
Patients with severe, rapidly progressing RRMS were defined as those who experienced 2 or more disabling relapses in a year and had 1 or more gadolinium-enhancing lesions in the brain MRI, or a significant increase in lesions in T2-weighted sequences compared to an earlier MRI.3,4
Our knowledge of treatment risks and benefits has increased considerably since the first cases of PML were reported, and continues to grow thanks to the efforts of researchers. Today, given the new information which is available, the patient and the neurologist can make decisions that are tailored to the patient's individual case. In this consensus statement, we will present a survey of current knowledge to be used as a guide when making decisions concerning natalizumab treatment.
We must point out that although this document lists the most recent general recommendations for managing patients treated with natalizumab, neurologists must use their best judgement when prescribing treatment and monitoring patients. This is all the more true due to the rapidity of changes in this field, and the tendency to make use of increasingly personalised treatments.
MethodsSpanish experts in MS, the authors of this article, held a meeting to identify the relevant topics to be addressed in this consensus document. Chosen topics were as follows: how to evaluate risks and benefits in general, PML risk stratification, how to inform patients regarding treatment risks and benefits, and how to monitor patients during treatment or after discontinuing the drug, if applicable. Each of the topics was assigned to the expert in the group who would write that section. Those responsible for each topic reviewed all available literature and any relevant presentations from international congresses.
Randomised trials of natalizumab were carried out in patients whose MS was less severe than in the patient population described in the drug information leaflet. Given the lack of available randomised clinical trials (CTs) conducted with this type of patient, it was not possible to create a hierarchy of evidence such as would normally appear in a consensus statement. Nevertheless, we should clarify that this document covers all relevant articles about natalizumab (phase II and III studies and observational studies), in addition to data, both published and unpublished, that has been presented at international congresses.
During the initial meeting, we designated a coordinator (O.F.) who gathered the texts and prepared a first draft which we then reviewed in the second meeting. After receiving comments, a new version was drafted and presented once again to the group of experts. Once we had reached an agreement concerning the document's content and format, and following several meetings and review sessions, the finished document was sent to the Spanish Society of Neurology's panel on demyelinating diseases for review. Once comments from the review had been taken on board, the final draft of the consensus statement was completed.
The concept of benefit–risk balanceWhen establishing a benefit–risk balance, doctors should be aware of information from CTs testing the drug in question. We should also consider continued exposure to the drug and both short-term and long-term results in the areas of efficacy and safety.
When evaluating benefits, consider that the primary efficacy objectives of CTs are just one of several types of results perceived by patients. We must also evaluate treatment feasibility, and any changes in quality of life caused by use of this treatment.
Benefit was calculated using the statistic ‘number needed to treat’ (NNT) to attain a specific objective, such as number of relapses, progression of disability, or variables determined by MRI. NNT=1/ARR (ARR: absolute risk reduction).
Damage level was determined by closely scrutinising severe adverse events, significant changes in laboratory results, treatment discontinuation, and unknown future risks (for example, the possibility that a patient will suffer infections or neoplasia in the future). These variables are used to calculate the number needed to harm (NNH) in a process similar to that for determining benefit. NNH=1/AHD (AHD: absolute harm reduction).
Once both statistical measurements have been calculated, we can determine the difference between them provided that both benefits and adverse effects have clinically comparable levels of importance. This will be determined by both the doctor and the patient, and perhaps also by health authorities responsible for assigning resources and considering relevant aspects of the case.
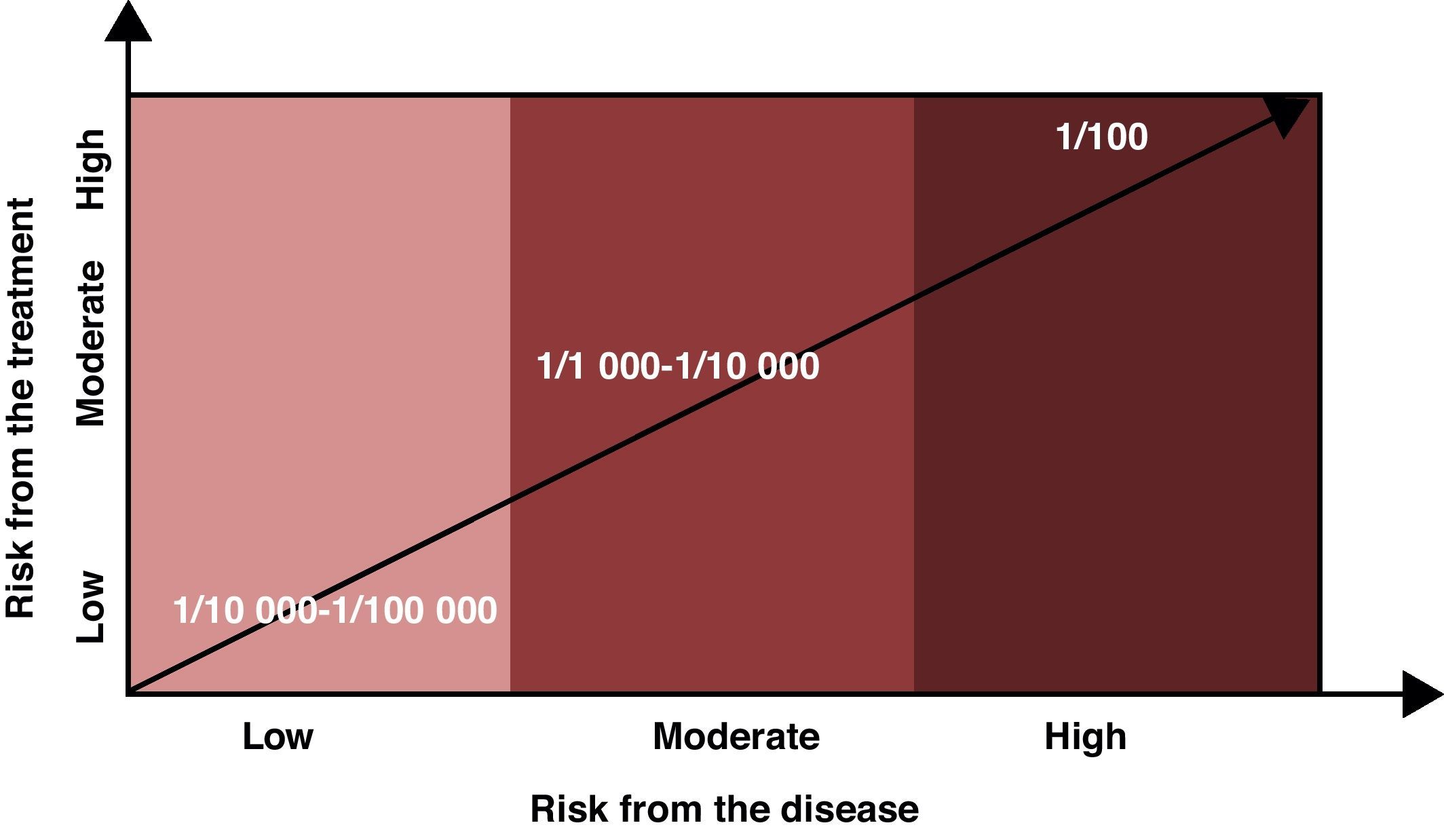
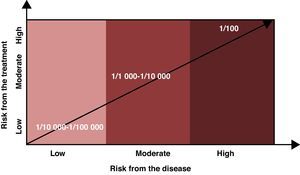
Risk levels considered assumable or acceptable should factor in the risk of the disease being treated in addition to the risks associated with the drug. If the disease does not cause disability or may only produce minor disability, the assumable risk should be low (1/10000 or lower); on the other hand, if the disease can result in major disability, the assumable risk may be higher (1/1000 to 10000), and if the disease entails grave clinical risks (risk of death, for example), the assumable risk for adverse effects may be even higher (1/100 or greater). The patient must be informed of any risks, and the final decision must be made between the two parties (Fig. 1).
In a risk/benefit analysis, low NNT indicates a good treatment response (few patients are required in order to obtain a response to treatment). High NNH indicates a good safety profile (a high number of patients must be treated before an adverse event occurs). The measurement can be expressed as a quotient or as a difference. The first approach delivers a benefit/risk ratio or risk-benefit probability.
Risk difference or absolute risk reduction is the simple difference between adverse event rates (40%−30%=10%; NNT=10). The difference in relative risk in this case is 25%. Keep in mind that if the relative effect of the treatment is the same, absolute risk reduction may be different despite the fact that the relative risk reduction is similar in both cases.
When it comes to evaluating benefits against harm or risk, quotients may be misleading if we do not know the baseline risk. It is therefore preferable to use statistics that account for differences in risk, and this is why we must always consider absolute benefit or absolute risk.5–8
In MS, the efficacy measures for NNT calculations include relapse prevention, keeping a patient progression-free, and absence of MRI findings suggesting activity/progression. NNH factors include the presence of severe adverse events and treatment discontinuation due to such events.
MS is a chronic disease with a risk of progression. In cases of this disease, the goal is for a treatment to benefit a specific patient by reducing the number of relapses and slowing or stopping disease progression, while itself causing the least possible harm.
Although patients have different wishes regarding the sharing of information, a growing number of patients prefer to be fully informed and make shared decisions with their doctors.
We can help patients understand the process by using NNT and analysing gains in disease-free periods. However, explaining the concept of benefit–risk balance is an art, and when providing explanations, we must allow for ample participation by the patient and evaluate his/her preferences regarding information, plus the patient's expectations, concerns, and capacity for assuming risks. Lastly, we must determine how the final decision will affect the patient's quality of life, which will enable the patient and the doctor to make a joint decision regarding treatment and follow-up.9 We must always be aware of which treatment alternatives are available to a specific patient.
In any case, the benefit–risk balance depends on both the drug and the disease being treated. The NNT/NNH figures are important for informing patients about benefits and adverse effects that have been demonstrated by CTs. We must define the right process for informing each patient, considering the fact that although patients generally prefer to be informed, they tend to trust their doctor's final decision, which should ideally be supported by both parties.
Information on the benefits associated with the treatmentIn the phase III AFFIRM study,1 treatment with natalizumab in MS patients reduced the risk of steady disability progression by 42%–54% over 2 years, the relapse rate decreased by 68% in 1 year, the number of new or worsening lesions in T2-weighted MRI sequences decreased by 83%, and the number of gadolinium-enhancing lesions fell by 92%. The number of patients who remained relapse-free after 2 years increased by 57%. NNTs for these variables are as follows:
- •
NNT for relapses (per year): 2.0
- •
NNT for being relapse-free (2 years): 4
- •
NNT for being progression-free (2 years): 8
Later observational studies of patients who responded poorly to conventional interferon and/or glatiramer acetate showed natalizumab to be highly effective.10,11
Information on the risks associated with the treatmentIn the AFFIRM study, patients in the natalizumab group experienced more adverse effects than those in the placebo group; these were mostly fatigue and allergic reactions. Hypersensitivity reactions of different types appeared in 4% of the patients, and were serious in 1%.1 In the SENTINEL study, adverse reactions caused by combination therapy with IM interferon beta 1a were anxiety, pharyngitis, sinus congestion and peripheral oedema. There were 2 cases of PML in the natalizumab group, and 1 was fatal.2 In addition, another CT reported a case of PML in a patient with Crohn's disease previously treated with immunosuppressants (IS).12
PML is a subacute form of encephalitis caused by the JC virus. It is listed as an opportunistic infection since it occurs almost exclusively in patients whose immune systems are severely compromised, whether due to HIV, immunosuppressant agents, different types of cancer, or certain autoimmune diseases.
After the drug began to be marketed and used in monotherapy, this complication continued to occur in a small number of patients. However, their number has increased alongside the number of patients treated with the drug. Different analyses have shown that the overall risk of developing PML is estimated to be about 1 per 1000 treated cases. They have also shown that risk increases with years of treatment; the effect is more noticeable after 2 years, and it is greater still in patients with a prior history of treatment with IS. NNH for these variables are as follows:
- •
NNH for discontinuing treatment due to adverse effects: 50
- •
NNH for a case of PML to occur (de novo treatment, treatment duration >2 years and no prior IS): 1/3000.
Treatment duration is one of the main risk factors for PML. Data from patients who developed PML reveal that it rarely appears prior to 1 year of treatment, and that the mean time before onset in most cases is about 2 years in patients with treatment durations of up to 4 years. We do not know if risk continues to increase after this time period or if it remains stable, given that the number of patients being treated is still low.
Prior immunosuppression treatment is another of the fundamental factors involved in risk of PML; it was present in nearly half of the cases that developed this complication. The recorded types of IS vary greatly, and there are no specific patterns related to drug type or posology.
Data derived from the TYGRIS study suggest that regarding the 2 factors listed above, patients with no prior history of IS treatment and less than 2 years on natalizumab are at low risk, while those with a prior history of IS use and a treatment duration of more than 2 years are at high risk.13
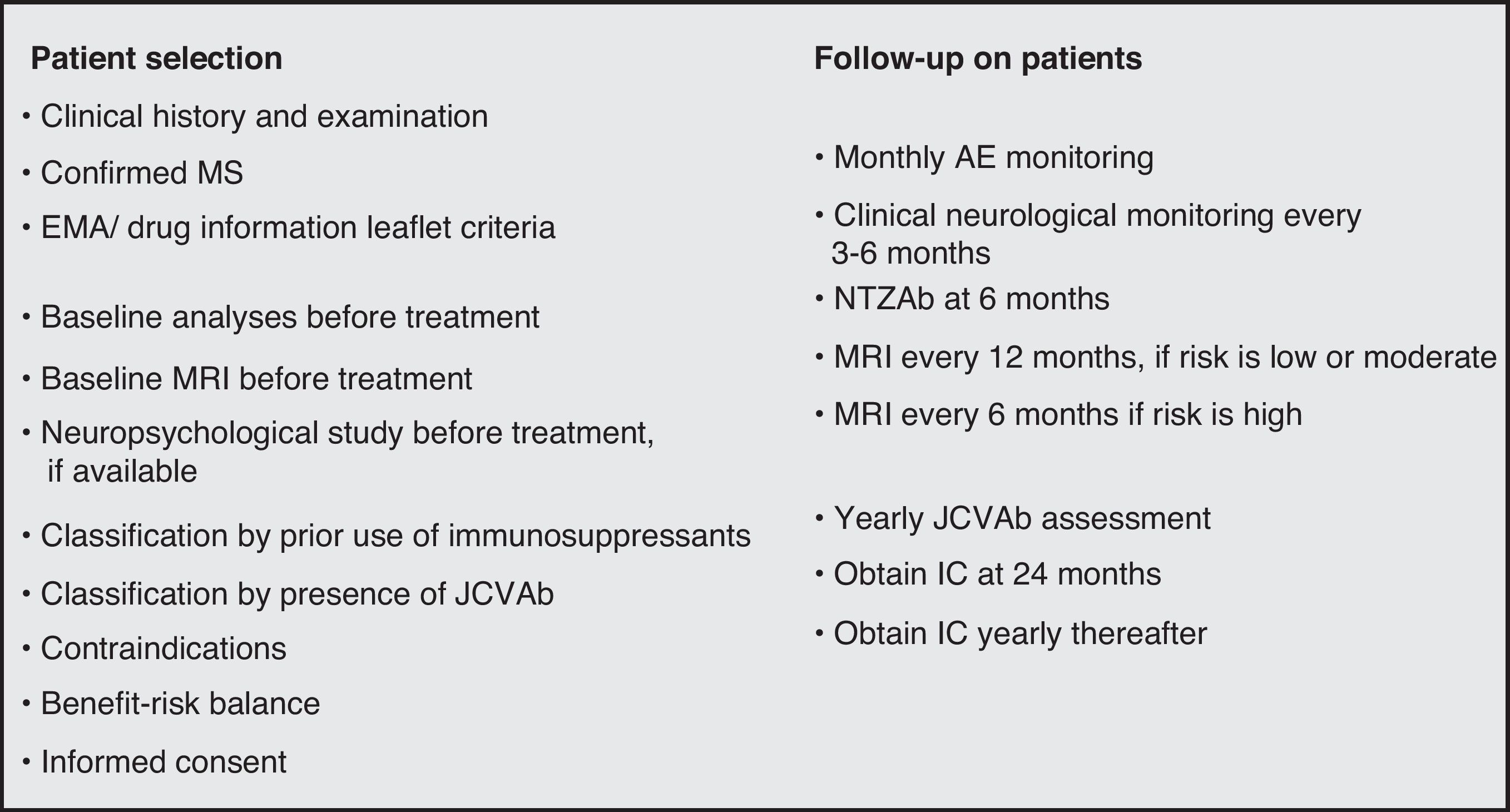
Stratifying risk and beginning treatmentAt present, there is no single tool capable of predicting the individual risk of PML in an MS patient beginning treatment with natalizumab. Proper patient selection and follow-up will make it possible to achieve a favourable benefit–risk balance in the patients in whom the treatment is indicated.14–16
When selecting patients, doctors should consider different sections of each candidate's medical history, including confirmed diagnosis of MS, disease activity, presence of comorbidities, prior history of IS treatment, and the baseline values from laboratory analyses. Natalizumab is contraindicated in patients who are HIV positive or have a history of immunodeficiency or lymphoproliferative disease. Although the drug information leaflet does not list pregnancy, trying to conceive, or breastfeeding as contraindications, some consensus statements discourage use of the drug by patients in these situations.17 An MRI should be taken before beginning treatment and scans should be repeated periodically. MRI periodicity will depend on the patient's risk group,14–16 but it normally ranges from 6 to 12 months. Natalizumab must always be used in monotherapy.18
We must inform the patient of any risks entailed by starting treatment. Measuring antinatalizumab antibodies after 6 months of treatment is recommendable, since when these antibodies do appear, they do so within this period in nearly all cases. If the patient is positive for antibodies, the test should be repeated in 4 weeks; if the antibodies persist, treatment should be discontinued, because presence of antibodies is linked to decreased effectiveness of the drug. This enables us to avoid unnecessary exposure to the drug and reduce risk.19
Recently, implementation of a 2-step ELISA procedure with high sensitivity and specificity for detecting anti-JC virus antibodies has brought up the possibility of being able to stratify that risk. There is a Europe-wide programme that analyses samples from MS patients in a central laboratory (Unilabs, Denmark). Individual health centres must register with that programme if they wish to participate. This test, which has a high negative predictive value, confirms prior exposure to the virus or primary infection, which is present in 53.6% of patients with MS; a negative result rules out exposure. In addition, antibodies were detected in previously obtained samples in the 25 cases of confirmed PML which were studied.20–22
If studies currently underway, specifically STRATIFY-1 (clinicaltrials.gov identifier: NCT01070823) and STRATIFY-2 (clinicaltrials.gov identifier: NCT01070836) confirm the hypothesis that a negative test for anti-JC antibodies rules out prior contact with the JC virus, this detection process added to the 2 factors mentioned before – history of immunosuppression and treatment duration – could serve as tools for stratifying PML risk clearly. An additional recommendation would be to complete a serology study annually, since there is a 2% annual seroconversion rate among patients testing negative for antibodies.23
If all these data are confirmed definitively, this will constitute a fundamental step towards providing personalised natalizumab treatment in MS.
Before beginning treatment, neurologists must inform patients of the benefits and risks of natalizumab treatment and explain the benefit–risk balance for each individual patient as clearly as possible. The patient's medical history should contain a record of the information that was provided, especially that regarding PML. Likewise, doctors should inform patients of the risks of discontinuing the drug and record that information.
Doctors should provide patients with treatment warning cards; it is also considered useful, while not mandatory, to have them sign an informed consent form prior to treatment.
Throughout the entire treatment process, we continue informing the patient about the drug's benefits and risks of complications, especially the appearance of PML, according to that patient's situation.
We recommend completing both an analytical and MRI study, plus a neuropsychological study where possible, prior to starting treatment. This allows us to compare values if PML is suspected at some point in the future.
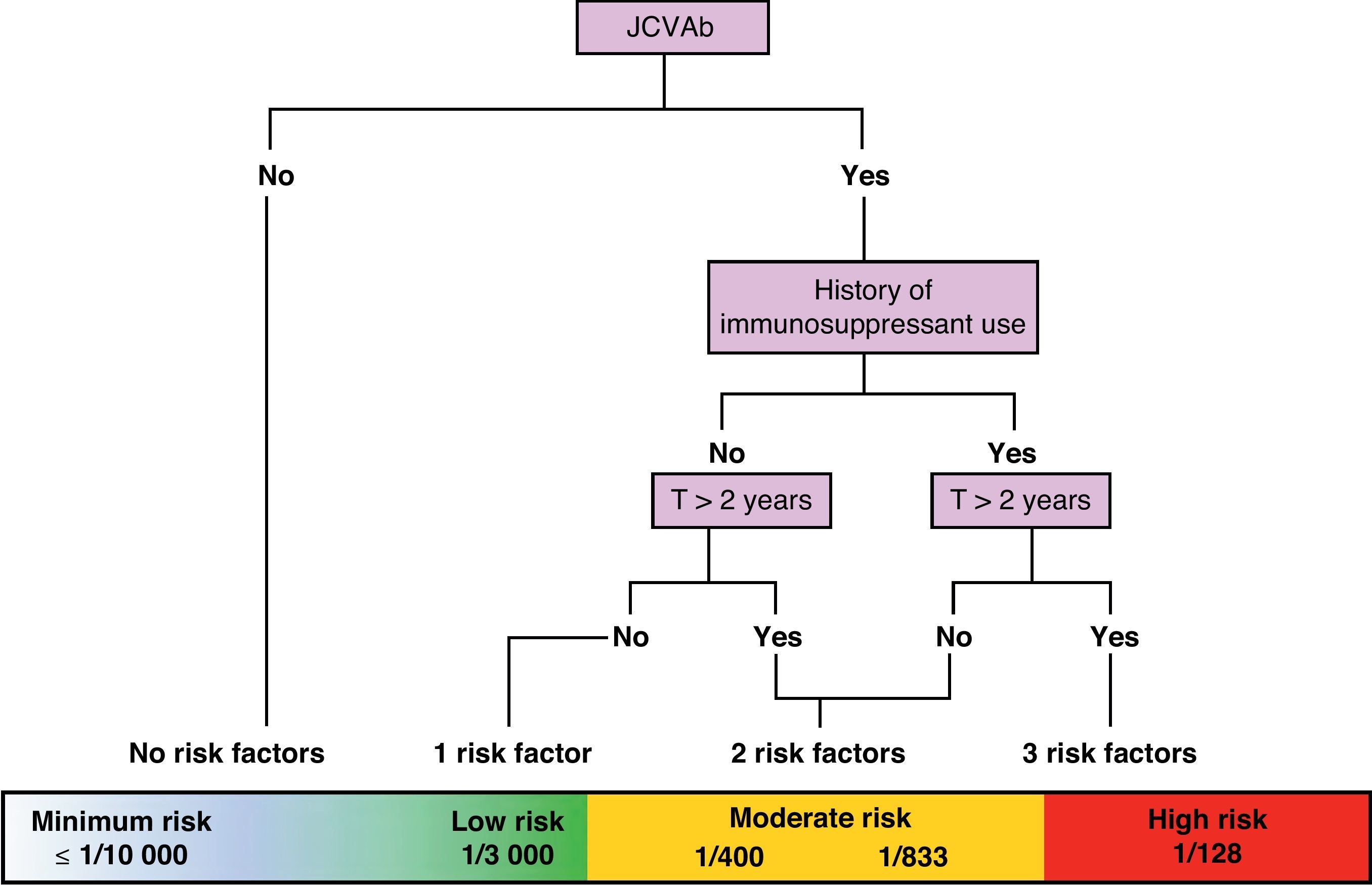
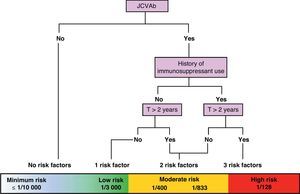
On a practical level, we are currently attempting to stratify risk of PML based on 3 variables: presence/absence of anti-JC virus antibodies, presence/absence of previous IS treatment, and treatment duration in months (greater than or less than 24 months). Using these variables, we can create a risk/benefit algorithm that lets us stratify risk in 3 groups: minimum to low risk, ≤1/10000–1/3000; moderate risk, 1/833–1/400; and high risk, 1/128. This enables us to provide patients with more precise information about the benefit–risk balance. These data are still being polished, but this is the best numerical approximation available at this time (Fig. 2).24
Approximate stratification of risk of PML according to the number of currently identified risk factors (JCVAb: anti-JC virus antibodies; Trt: treatment).24
Monitoring the patients undergoing treatment is the way to ensure an early diagnosis of potential complications and a rapid response that will minimise consequences. This is achieved through clinical vigilance and the use of MRI and laboratory findings.
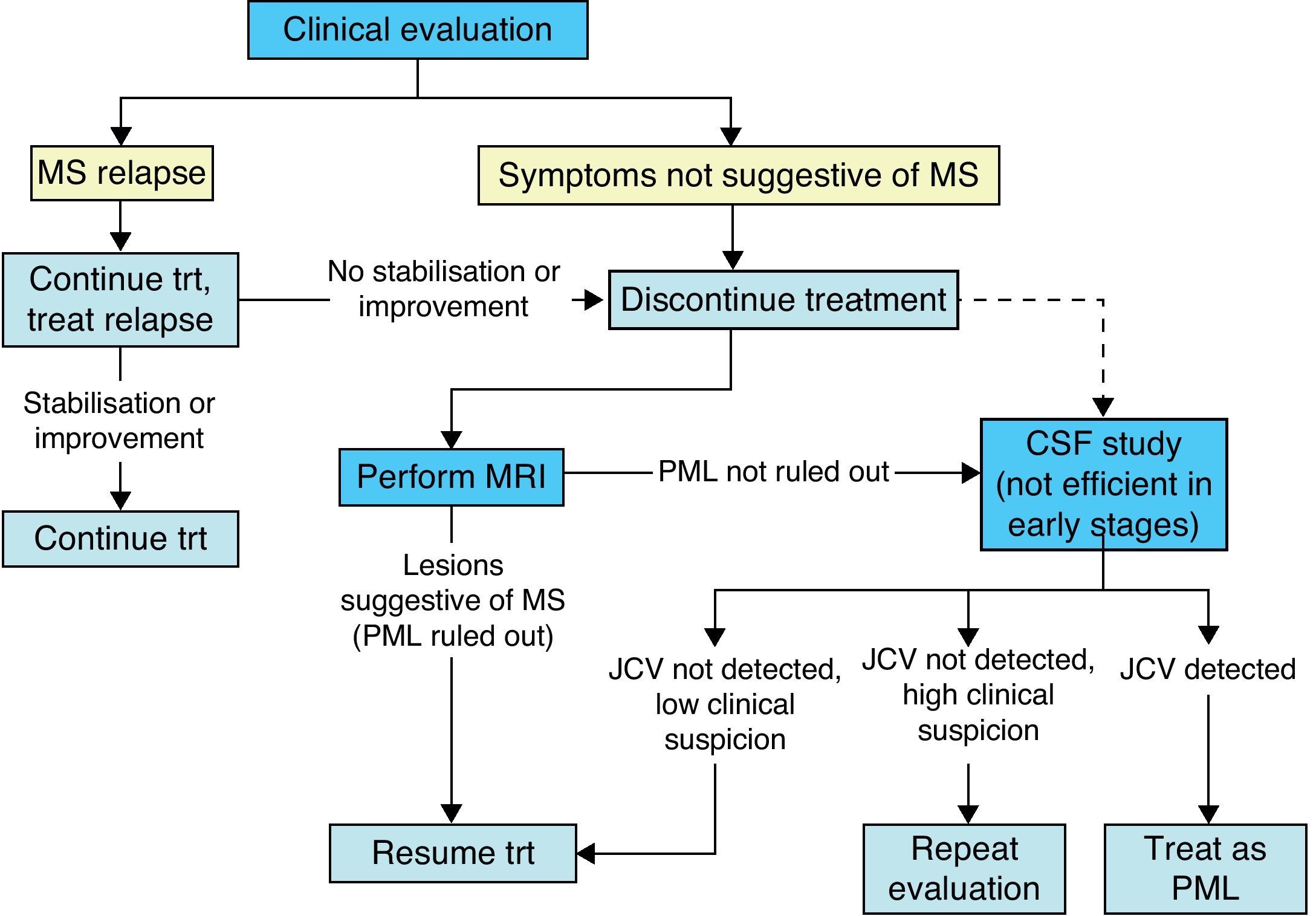
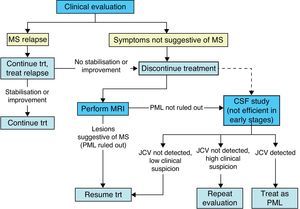
The neurologist's expertise and clinical abilities are the best tools for monitoring patients and detecting PML development as early as possible. The specialist must consider the onset, development and type of symptoms presented by a patient in order to distinguish PML from an MS relapse.
The patient and the family members must also be informed so that they will be able to help detect new symptoms and signs.14–16
If clinical data are not sufficient, an MRI study is used to visualise suspected PML lesions which can then be distinguished from typical MS lesions based on such characteristics as location, borders, and the type of change. This technique is very sensitive, but not very specific, particularly when dealing with the initial PML lesions.25,26
Although cerebrospinal fluid (CSF) analysis to check for JC virus DNA is very specific for diagnosing PML, it often gives negative results during early stages of the disease.26,27 As a result, it is not a good tool for screening patients, and it is only used in patients with suspicious clinical or MRI findings.
Scientific evidence of the efficacy of measuring viraemia and viruria is insufficient. One study describes an increase in JC viral count in the plasma and urine of patients undergoing treatment, which could prove useful for monitoring patients at a greater risk of developing PML,28,29 but this has not been observed in other larger cohorts, probably due to differences in methodology.30–32
The immune response to the JC virus has also been the subject of scrutiny. Some researchers have found no changes in immune response, while others point to a decrease in T-cell activity during treatment with the drug, and still others claim that T-cell activity increases.32
Lastly, the RESTORE study is being carried out in order to research immunological function and disease activity in periods during which treatment has been discontinued. It analyses objectives following suspension of natalizumab treatment during 6 months, and contains 3 arms: no treatment, treatment with corticosteroids at high monthly doses, or treatment with interferon beta or glatiramer acetate (clinicaltrials.gov identifier: NCT01071083).
Practical advice for monitoring treatment14,33–36Clinical monitoringDoctors and nurses should be well-acquainted with the patient and have special training in the use of natalizumab. They should be able to recognise the potential complications of treatment with that drug.
Nurses or doctors should be present during every infusion to watch for any reactions to the drug (fatigue, dizziness, headache, asthenia, nausea, etc.) and be able to distinguish them from allergic reactions or hypersensitivity (urticaria with or without systemic repercussions).
During each infusion (every 4 weeks), specialist doctors or nurses should be near the patient so as to monitor clinical activity and quickly detect potential medication-related complications, especially PML.
During treatment with natalizumab, the patient's clinical course should be monitored with periodic examinations by a neurologist every 3 to 6 months, especially if new symptoms appear; ruling out PML is the main concern (Fig. 3).
MRI monitoringA brain MRI must be taken in the 3 months prior to starting natalizumab treatment. After that, some authors propose taking brain MRI scans in order to detect potential cases of PML in their early stages, establishing MRI periodicity according to the patient's risk group; patients at high risk for PML would have scans every 6 months, and those at moderate to low risk, every 12 months. This proposal may reflect a lack of experience with MS patients treated with natalizumab. (There is no evidence demonstrating that MRI is an effective tool for detecting PML in a subclinical stage, and we cannot therefore recommend use of this technique for early diagnosis of the complication in that stage.)
An emergency brain MRI must be performed if any neurological symptoms or signs appear that would raise doubts as to whether the patient was undergoing a relapse or PML.
MRI should be performed in radiology units by staff members who are knowledgeable about MS and the complications of MS treatment. We recommend that subsequent studies of the patient be taken using similar machines (the same units, if possible) and following standard protocols, including T2-weighted FLAIR images and T1-weighted images with and without contrast.
Laboratory monitoringAn analytical study including a haemogram and a basic biochemical assessment (including liver function) should be completed at treatment onset and at least every 3 months thereafter. While liver alterations are uncommon, we recommend discontinuing the treatment temporarily until liver enzymes normalise, and resuming treatment at a later date. If the patient is suspected to be immunocompromised at treatment onset, measure the neutrophil, CD4, and CD8 counts and the CD4/CD8 ratio. In areas with a high incidence of HIV infection, HIV testing is recommended prior to starting treatment. If there is a risk of tuberculosis, we recommend a chest radiography and a tuberculin test.
Measuring antinatalizumab antibodiesApproximately 9% of patients on natalizumab treatment have persistent neutralising antibodies in their serum which appear during the first 6 months. Most of these neutralising antibodies are associated with allergic reactions or hypersensitivity, especially at the time of the second infusion. Testing for antinatalizumab antibodies is probably recommendable for all patients, but it should be mandatory in the following cases:
- •
Patients with allergic reactions or hypersensitivity to the drug.
- •
Clinical relapse or MRI findings indicative of radiological activity.
If a patient's sample reveals neutralising antibodies, the analysis should be repeated again in 4 weeks. If the patient should test positive again (persistent antibodies), discontinuing treatment is recommended. Presence of antibodies is linked to loss of clinical efficacy of natalizumab and increased incidence of hypersensitivity reactions.1,13
Information for the patientAt 24 months of treatment, all patients should be informed once more about the natalizumab risks and benefits known at that time. The patient should reiterate his or her consent to continue treatment, and this should be reflected in writing in the section of the medical history that includes information provided to the patient. This information should be updated every year and every time new information about the risks of treatment becomes available (Fig. 4).
Treatment discontinuation and follow-upDiscontinuation of natalizumab has been associated with the reappearance of disease activity; the clinical effects of the drug last about 3 months, while the biological effect lasts between 6 and 12 months.37 After discontinuing natalizumab, we therefore recommend immediately starting treatment with an immunomodulator (interferon beta or glatiramer acetate) or corticosteroids.38 As its drug information leaflet states, natalizumab remains in the blood during approximately 12 weeks after the last dose is administered. Therefore, beginning other treatments during that period will result in concomitant exposure to both drugs, and extreme caution must be exercised.
If the patient began natalizumab treatment because an immunomodulator proved to be ineffective, the logical step would be to treat the patient with another drug to which he or she has had no prior exposure. We do not know what role the forthcoming oral drugs might play in this area.
Appearance of PML is a severe complication with high mortality (20%) and morbidity rates. The best treatment is prevention, but this strategy is complex as there are no simple, reliable diagnostic procedures that would allow us to anticipate PML development. As a result, we recommend extreme vigilance and following currently available recommendations.4,12–16 Suspected diagnosis is based on clinical and MRI findings, and the disease is confirmed when the JC virus is detected in CSF.
Treatment for cases of PML associated with natalizumab aims to rapidly eliminate the drug from the bloodstream, fight the virus, and prevent the neurological damage caused by immune reconstitution inflammatory syndrome (IRIS). Since PML is attributed to lymphocytes being prevented from entering the inner CNS as a result of natalizumab's action mechanism, the main priority is removing that mechanism. Approaches include plasmapheresis and immunoabsorption in different dosing regimens.39
Numerous different antiviral or immunomodulating agents and 5-HT2A receptor antagonists, including psychoactive drugs (mirtazapine), have been tested as treatments but their results have been unclear. The efficacy of the antimalarial drug mefloquine is also unclear, but it has been used in a number of cases of PML.
IRIS, which appears several weeks after plasmapheresis or immunoabsorption treatment, may cause extremely severe neurological damage or death. It is characterised by the onset of neurological deterioration with signs of inflammation appearing in neuroimaging studies. Although there is no consensus regarding IRIS prevention and treatment, abundant clinical evidence, most of which comes from experience with patients with HIV, suggests that high doses of intravenous corticosteroids may be useful in both prevention and treatment.
Lastly, there is no evidence identifying the best ‘detox’ duration after discontinuing natalizumab and prior to starting other immunosuppressant agents. We could speculate that 3 to 6 months might be sufficient, but this will have to be confirmed. Likewise, we are unaware of the risks involved in starting another immunosuppressant directly, and in particular, how that action may affect risk of PML. These matters will have to be strictly monitored in the future, especially once the new oral drugs have become available.
ConclusionsIn conclusion, we are currently witnessing very important advances in the treatment of MS with natalizumab as we move towards personalised medicine. We have access to sufficient data regarding treatment benefits and possess a better understanding of its risks now that a very large number of patients have been treated worldwide.
This situation allows us to establish a fairly realistic quantitative approximation of the benefit–risk balance, stratify risks, and inform patients of their risk level at any time in the treatment process, whether at the beginning or during follow-up, based on 3 variables: anti-JC virus antibodies, history of immunosuppression, and treatment duration.
Recommendations for treatment monitoring are beginning to become available.
Our knowledge of the consequences of discontinuing treatment and the steps to take in this case is growing. This enables us to prevent relapses, and can even prevent the appearance of IRIS, which can be devastating.
These advances mean that we can proceed with more confidence in treating MS with natalizumab, which has considerable benefits, since we can now also reduce the risks associated with its use.
Conflicts of interestOscar Fernández has received fees as a consultant to committees and as a moderator or speaker at medical congresses and symposia. He has also participated in clinical trials and other research projects promoted by Biogen-Idec, Bayer-Schering, Merck-Serono, Teva, and Novartis.
Xavier Montalbán has received fees and travel expenses for attending meetings as a speaker. He has acted on the steering committees of clinical trials, and has also recently collaborated with clinical trial committees as a consultant with Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, and Almirall.
We would like to acknowledge the valuable comments made by A. Miralles, C. de Andrés, A. Rovira, L. Landete, F.J. Hernández, J. Meca, B. Casanova, C. Arnal, and V. Pérez de Colosía, and G. Morley's contribution to the drafting of this text.
Please cite this article as: Fernández O, et al. Consenso español sobre la utilización de natalizumab (Tysabri®) – 2011. Neurología. 2012;27:432–41.