Different animal models for Alzheimer disease (AD) have been designed to support the hypothesis that the neurodegeneration (loss of neurons and synapses with reactive gliosis) associated with Aβ and tau deposition in these models is similar to that in the human brain. These alterations produce functional changes beginning with decreased ability to carry out daily and social life activities, memory loss, and neuropsychiatric disorders in general. Neuronal alteration plays an important role in early stages of the disease, especially in the CA1 area of hippocampus in both human and animal models.
MethodsTwo groups (WT and 3xTg-AD) of 11-month-old female mice were used in a behavioural analysis (nest building) and a morphometric analysis of the CA1 region of the dorsal hippocampus.
ResultsThe 3xTg-AD mice showed a 50% reduction in nest quality associated with a significant increase in damaged neurons in the CA1 hippocampal area (26%±6%, P<.05) compared to the WT group.
ConclusionsThe decreased ability to carry out activities of daily living (humans) or nest building (3xTg-AD mice) is related to the neuronal alterations observed in AD. These alterations are controlled by the hippocampus. Post-mortem analyses of the human hippocampus, and the CA1 region in 3xTg-AD mice, show that these areas are associated with alterations in the deposition of Aβ and tau proteins, which start accumulating in the early stages of AD.
Se han diseñado diferentes modelos animales de la enfermedad de Alzheimer (EA) para apoyar la hipótesis de que la neurodegeneración (pérdida de neuronas, sinapsis y gliosis reactiva) asociada al depósito de Aβ y tau en estos animales es similar a la del cerebro humano. Estas alteraciones producen cambios funcionales que se inician con el deterioro en la habilidad para realizar actividades de la vida cotidiana, pérdida de la memoria y, en general, trastorno neuropsiquiátrico. La alteración neuronal desempeña un papel importante en las etapas tempranas de la enfermedad, especialmente en el área CA1 del hipocampo de animales y humanos.
MétodosSe utilizaron ratones WT y 3xTg-AD hembras de 11 meses de edad, para el análisis conductual (construcción del nido) e histológico en la región CA1 del hipocampo dorsal.
ResultadosLos ratones 3xTg-AD mostraron deficiencia del 50% en la calidad de construcción del nido asociado a un aumento del 26±6% (p<0,05) de neuronas dañadas en comparación con el grupo WT.
ConclusionesEl deterioro de la capacidad para llevar a cabo las actividades de la vida diaria (en el hombre) y la construcción del nido (en el ratón 3xTg-AD) están relacionados con las alteraciones en los circuitos nerviosos observados en la EA. Estas alteraciones son controladas por el hipocampo que en el análisis post mortem (en el humano), así como en la región CA1 (en el modelo de ratón 3xTg-AD) se han relacionado con alteraciones en el depósito de las proteínas Aß y tau que comienzan a acumularse al inicio de la EA.
Scientists began researching neurological alterations in different neurodegenerative diseases at the end of 19th century.1 Post mortem topographic studies of these alterations in Alzheimer disease (AD)2 were first used in the mid-1950s. Characteristic changes were diffuse cerebral atrophy, ventricular dilatation, and increased sulcal depth, as well as decreased brain weight and volume associated with neuronal reduction.3–5 Neuronal atrophy mainly affects the entorhinal, temporal, and frontal cortices as well as hippocampal region CA1.6–8 Formation of neurofibrillary tangles (NFT), and of neuritic plaques (NP) due to amyloid beta (Aβ), begins in these areas and probably results in neuronal alteration. With this in mind, we decided to study neuronal death and correlate it to the chronological progression of dementia.9 The studies relating memory and Aβ in transgenic rats expressing the amyloid precursor protein (APP) and its variants have suggested that ageing promotes the formation of soluble Aβ assemblies, which are heterogeneous peptides with carboxyl-terminal residues (Val40 [Aβx-40], Ala42 [Aβx-42], and Thr43 [Aβx-43]). Of these, Aβx-42 and Aβx-43 are deposited in the earliest stage of disease development, and they have a negative impact on memory.10 Recent years have witnessed an increasing need for a better-quality procedure for early diagnosis in humans. This is because, in addition to memory impairment, the neuropsychiatric impairment that occurs includes social changes related to alterations in synaptic activity11 and the ability to perform activities of daily living. According to Deacon,12 it is possible to model this disease in laboratory animals if we consider their natural habits, such as burrowing and nesting. Both behaviours are controlled by the hippocampus, and this region is where neuronal alteration begins in AD. These factors enable animal models to contribute to our knowledge of disease development. Several transgenic mouse models have been elaborated to this end.13 Thus, genetic manipulation is a strategy for researching the function of mutated human proteins, such as amyloid precursor (APP), presenilin (PSEN), apolipoprotein E, and tau protein. These proteins, which are overexpressed in the mice, form NP and NFT.14,15
The triple-transgenic mouse model (3xTg-AD) was created in the LaFerla laboratory by Oddo in 2003. His team simultaneously microinjected two genes (APP and tau) into single-cell PS1M146V mouse embryos (transgenic mice that overexpress human or wild-type [WT] APP, and are hybrids of 129/C57BL6 types). The 3xTg-AD type expresses APPSWE and MAPT301L, and it is also a PSEN1M146L knock-in (PSEN1-KI).16–18 These mice exhibit age-dependent Aβ plaques in specific brain regions, plus cognitive impairment related to intraneuronal Aβ. This being the case, the model lets us research the development and treatment of neurodegeneration in AD.13 Moreover, it allows us to draw conclusions about Aβ plaques in the brain and how those plaques precede the disease in which tau protein plays a role (in the animal model). Experiments have also shown that intraneuronal deposition of Aβx-42 is associated with early synaptic impairment in APP/PS1KI mice19 in brain areas related to memory. These include the association cortex, limbic system, and most significantly, the hippocampus, which shows elevated levels of APP mRNA in subjects between 13 and 16 months of age, and high Aβ levels after the age of 6 months. To date, there are no published analyses of innate behaviour and morphology in the region of dorsal hippocampus in this triple-transgenic mouse model (3xTg-AD) for AD. For that reason, our study aims to establish a relationship between nesting behaviour (a natural rodent activity) and anatomical and functional alterations of the hippocampus. This will increase our understanding of specific neuronal damage and death processes that have been observed in this disease.
MethodSubjectsThe protocol designed for this study follows international standards established by National Institutes of Health (NIH) and the National Academy of Science for the handling and use of experimental animals. It was presented to and approved by the Bioethics Committee of the Institute of Neurobiology (INB) pertaining to Universidad Nacional Autónoma, Mexico. Mice used as controls were hybrids of 129 and C57BL6 strains; the 3xTg-AD mice were also hybrids of those strains. Both groups were kept in Animal Services of the INB in polycarbonate cages (12×12×25cm) connected to clean air racks; rooms had a stable temperature (22°C) and relative humidity (50%), with 12:12 light/darkness cycles starting at 8.00am. Water and food were available for ad libitum consumption and the diet was suitable for a laboratory mouse (Purina rodent chow blend 5001).
Only female mice aged 11 months were used for the study; these hybrids of the original reproductive units were weaned at 30 days of age. Mice in the 3xTg-AD group, which were homozygous for AD, possessed the three genes (APPSWE, tauP301L and PS1M146V knock-in), while non-transgenic mice (129-C57BL/6 or WT) did not. They were housed 3 to a cage from weaning to the start of the experiment.
Genotyping the 3xTg-AD mouseDNA was extracted from the most caudal segment of the tail (approximately 0.3cm long) of the 3xTg-AD mouse. Tissue was placed in 1.5mL Eppendorf tubes with 500μL of 0.05M NaOH. The fragments were incubated at 95°C for 15minutes before being added to 50μL of stock solution (1M Tris, 10mM EDTA, pH 8). A polymerase chain reaction (PCR) was performed to check for presence of APP (β-APP), tau protein, and presenilin-1 (PS1PSEN1) genes. Genotyping required 2 reactions. First, two PCR products were amplified with oligonucleotide primers specific to β-APP and tau (500bp and 350bp, respectively) and assessed by 1% agarose gel electrophoresis. In a second reaction, a PCR product of 530bp was amplified and then left to digest for 2hours at 37°C with the BstEII restriction enzyme. The process generated two fragments of 180bp and 350bp.
Behavioural studyNesting behaviour was tested in ten WT mice and ten transgenic mice (3xTg-AD), all females aged 11 months. The test was performed from late afternoon to the following morning (8.00am). Researchers placed a 5cm square of pressed cotton (nestlet) in the cage at 7.00pm, 1hour before the light cycle or resting phase began. Several images were taken 15minutes before the active phase to evaluate nesting behaviour. We used 3 of the 5 nest evaluation categories described by Deacon20: (1) nestlet is largely untouched (>90% intact), (2) nestlet is mostly shredded (<90%) but there may be no identifiable nest site (if nest area is present, it is flat); and (3) a nearly perfect nest (>90% of the nestlet is torn up and the nest is a crater, with walls higher than mouse body height on more than 50% of its circumference (Fig. 1).
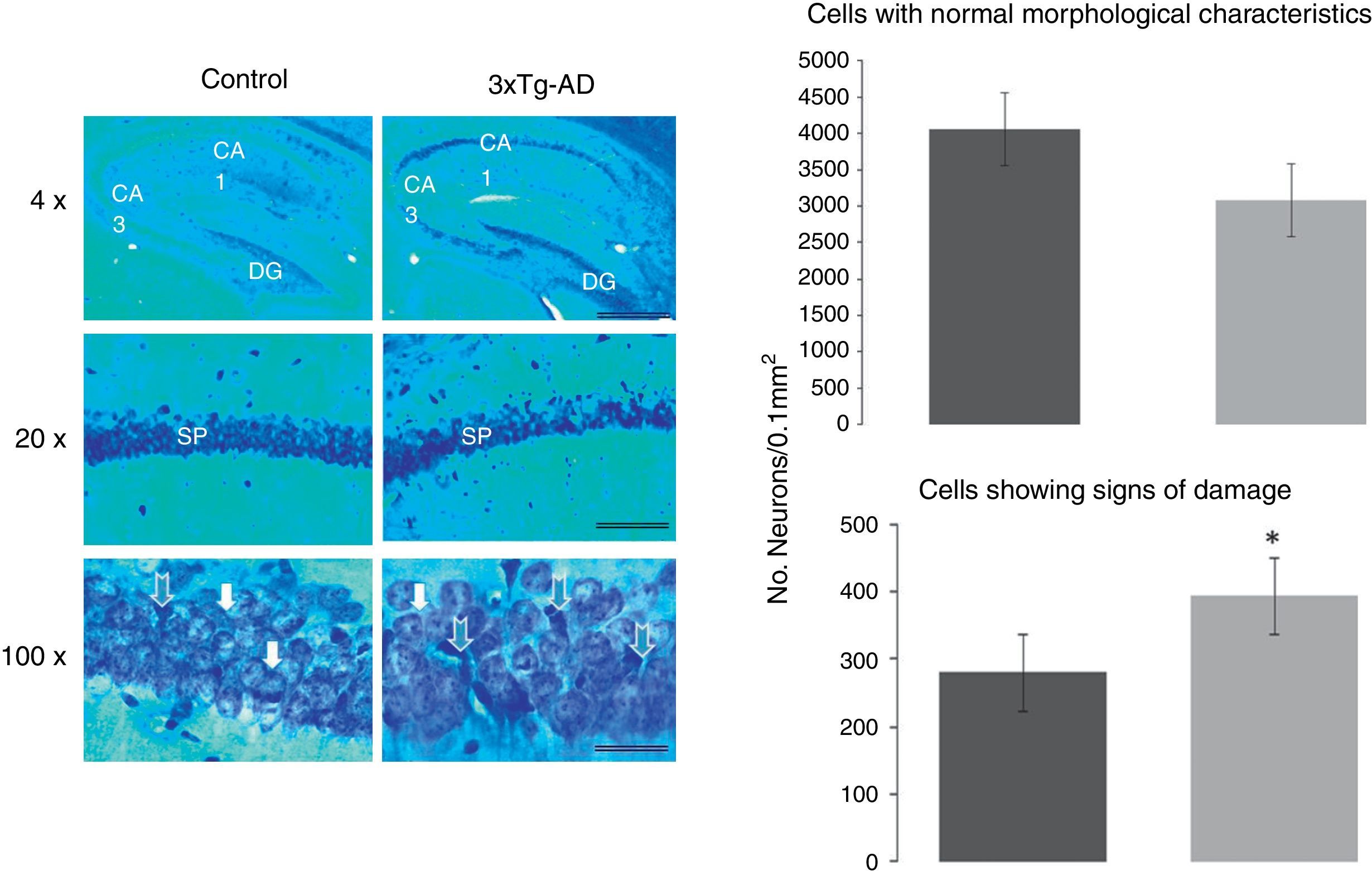
Morphometric analysisSix WT individuals and six 3xTg-AD individuals were randomly selected from the 20 animals employed for the behavioural study (all females). Animals were anaesthetised i.p. with pentobarbital (300μg/kg body weight) and intracardiac perfusion was performed with 4% buffered paraformaldehyde solution (pH 7.4, 0.1M) and 1000U of 0.1% heparin and procaine. Brains were extracted from the skulls and fixed in 4% paraformaldehyde solution for 24hours at room temperature. Brains were cut into sagittal slices 50μm thick by vibratome (microslicer model 3000, Pelco International). Both hemispheres were examined. They were stained with the conventional Klüver-Barrera technique to visualise neuromorphology and myelinated fibres from hippocampal region CA1 (1.92–2.28mm lateral to bregma).21
Morphometric analysis entailed counting neurons in the CA1 hippocampal region, which had an area of 262.5×125μm. Neurons were viewed using a NIKON optical microscope, model Eclipse 50i, equipped with a DS-U2 S camera. Q-Win and ImageJ software programs (NIH, USA) were used to quantify both normal cells and those showing neuronal damage.
Cells in these images were counted and recorded as the number of neurons per established unit of area (μm). Neurons with a normal appearance were counted, identifying those showing signs of damage (hyperchromatic cytoplasm, cellular shrinking, and nuclear fragmentation).
ResultsQualitative analysis of nesting behaviourSixty percent or 6 of the 10 control/WT mice (all females aged 11 months) built nests meeting criterion 3. One mouse (10%) made a poor-quality nest. In the 3xTg-AD group, 5 mice (50%) built a poor-quality nest (score of 2); only 2 individuals (20%) made a good-quality nest (score of 3). Three females from each group (30%) did not build nests (Fig. 1). Of the total of 20 animals, 12 were randomly selected for the morphometric study of the CA1 dorsal hippocampal region.
Morphometric analysis of pyramidal neurons from the CA1 region of the hippocampusTo determine the proportions of normal cells and cells with signs of damage in the CA1 hippocampal region, we quantified both types of neurons in the experimental (3xTg-AD) and WT groups. Damaged neurons were more numerous in 3xTg-AD mice. A one-way ANOVA study revealed a statistically significant difference (F1430=56074, P<.0001): the 3xTg-AD group had 26±6% more abnormal cells than the WT group, as shown in Fig. 2.
Representative sagittal CA1 hippocampal slices taken from 11-month-old female mice and stained with the Klüver-Barrera method. The bars in each micrograph represent 1000, 100, and 25μm; images were taken with 4, 20, and 100× lenses, respectively. Blue arrows indicate neurons with signs of damage in the stratum pyramidale (SP) from CA1 in the hippocampus, the dentate gyrus (DG), and CA3. The graphs show a significant increase in the density of abnormal cells in 3xTg-AD mice. White arrows show normal pyramidal neurons in both groups. The graphs indicate the number of neurons with a normal appearance (above) and neurons with signs of damage (below), calculated in an area of 0.01mm2 in both groups of mice (WT and 3xTg-AD). Values represent the mean±SEM. *P<.0001.
The results point to a relationship between the impairment of natural nesting behaviour (regulated by the hippocampus) and neuronal alterations in the CA1 dorsal hippocampal region in female 3xTg-AD mice aged 11 months with a developed form of the disease. This relationship is important because the process of neuronal damage and death originate in this important area that regulates spatial memory. Parallels could be drawn between these results and altered performance of activities of daily living in humans at the onset of the disease.
It is interesting that this basic and natural activity in mice would provide specific information about the tissue damage that begins in the hippocampus and its relay zone the subiculum, where Aβ plaques and tau tangles can be observed in animals aged 11 months. Only 3 out of Deacon's 5 criteria were used to evaluate nest-building, and we found that just 3 animals (30%) of the total (WT and 3xTg-AD combined) did not build a nest correctly. The real difference was the quality of the construction, which was good in 60% in the WT group, indicating no tissue damage in the hippocampus. This is a good test of an animal's well-being. In contrast, only 20% of the transgenic animals built nests correctly, and 50% built poor nests, indicating neuronal disconnection and loss associated with the appearance of these proteins. This may even indicate plaque formation between CA1 and the subiculum, which is one of regions incurring the most damage as the disease progresses.22
The study did not reveal significant changes in the number of normal cells. This coincides with data reported for other entities, such as Huntington disease.23 On the other hand, in the CA1 area of 3xTg-AD mice, we found diverse types of neuronal degeneration in addition to a higher density of abnormal cells. Signs of degeneration included clear symptoms of pyknosis, condensation, and intracellular Aβ and tau deposits at this age (results forthcoming). These changes have been also observed post mortem in the CA1 hippocampal region of humans in advanced stages of AD.6,7,24,25 The specific characteristics of AD are associated with the presence of NP and NFT, which are predominantly found in this brain region.23 Furthermore, our results are consistent with those from the PSAPP transgenic mouse model, which revealed dysfunction of CA1 hippocampal neurons.26 A 14% decrease in neurons was reported in the APP23 model, associated with the presence of Aβ NP.26,27
This neuronal dysfunction with cell death performs an important role in early to intermediate stages of the disease.27 Intraneuronal Aβ deposits originate the cascade of pathological events that elicit neurodegeneration in AD, resulting in classic clinical symptoms such as memory loss and personality changes.28,29 It has been reported that these Aβ deposits precede the formation of the NFT accompanied by loss of hippocampal and cortical neurons. This in turn yields the pathological alterations described in the brains of AD patients, resulting in decreased brain weight and volume.5 This neuronal loss stems from synaptic changes,11 and it correlates to progressive cognitive dysfunction. This dysfunction arises because the reduced number of neurons determines the functional capacity of brain structures like the hippocampus.29
A 2006 study by Spires et al.4 demonstrated neuronal decrease in this area of the hippocampus in a mouse model of tauopathy. This makes it possible to correlate specific characteristics of AD to the neuronal damage that begins on the intracellular level and continues progressively.24 Other studies have reported a neuronal loss of 25% to 70% in various hippocampal areas due to AD,30,31 especially in the CA1 area, where the brains of patients with AD show a significant neuronal decrease compared to normal brains.21,30–33
In this 3xTg-AD mouse model we have found that diseased females present difficulty completing the natural task of nest-building as a behavioural manifestation. This sign was associated with neuronal alteration in the hippocampus, a structure that regulates spatial memory, and WT animals without this alteration had difficulty building nests. Moreover, there is a correlation between intracellular formation of Aβ and abnormal tau in the dorsal hippocampus, which can be seen in this model starting at 7 months of age. Doctors require further experiments that focus on preventing deposition of these proteins in the hippocampus, whether by using antioxidants or oestrogens that protect females from neuronal loss in this part of the brain, or through other approaches.
FundingThis work was partially funded by CONACYT (grant no. 295523; project no. CB-2012/178841) and UNAM-DGAPA (IN201613).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the Proteogenomic Unit at INB and laboratory technicians A. Aguilar Vázquez and M. Servín García for their help caring for the animals.
Please cite this article as: Orta-Salazar E, Feria-Velasco A, Medina-Aguirre GI, Díaz-Cintra S. Análisis morfológico de la región del hipocampo asociado a una tarea conductual innata en el modelo de ratón transgénico (3xTg-AD) para la enfermedad de Alzheimer. Neurología. 2013;28:497–502.