Hashimoto encephalopathy (or steroid-responsive encephalopathy associated with autoimmune thyroiditis) is a rare disease characterised by symptoms of encephalopathy in patients with mild thyroid disease, no specific neuroimaging findings, high serum antithyroid antibody titres, no antineuronal antibodies in the serum or CSF, and no other likely aetiology. As the underlying pathophysiology is unclear, particularly with regard to the pathogenic role of antithyroid antibodies (present in up to 13% of healthy individuals), it should be classified as probable autoimmune encephalitis.1
Although autoimmune encephalitis is generally considered a paraneoplastic or post-infectious disease, it has been suggested that several vaccines may exacerbate immune-mediated neurological diseases. In the United States in the period 1990-2010, 708 cases of encephalitis were reported within 2 weeks of vaccination, mainly against hepatitis B, influenza, measles, mumps, rubella, and Haemophilus influenzae type B.2 As a consequence of the recent COVID-19 pandemic (with more than 500 million recorded cases and more than 6 million deaths), vaccine administration under emergency use authorisation has become generalised, with more than 11 billion doses administered.3
We present the case of a 36-year-old man with history of autoimmune hypothyroidism and no history of psychiatric disorder. He was administered the first dose of Spikevax vaccine (Moderna) against SARS-CoV-2 in July 2021, and the second dose 28 days later; in the 24 hours after the second dose, he presented self-limited febrile syndrome and mild postural tremor. Six days later, in the absence of any other precipitating factor, he presented a first focal seizure in the left hemisphere, progressing to bilateral tonic-clonic seizure, and finally to convulsive status epilepticus that led to ICU admission. A CT scan and CT angiography study ruled out vascular or neoplastic aetiology. An EEG showed symmetrical diffuse slowing. In the brain MRI study, diffusion sequences revealed cortical hyperintensity in the left temporal pole, attributed to postictal signal alterations.
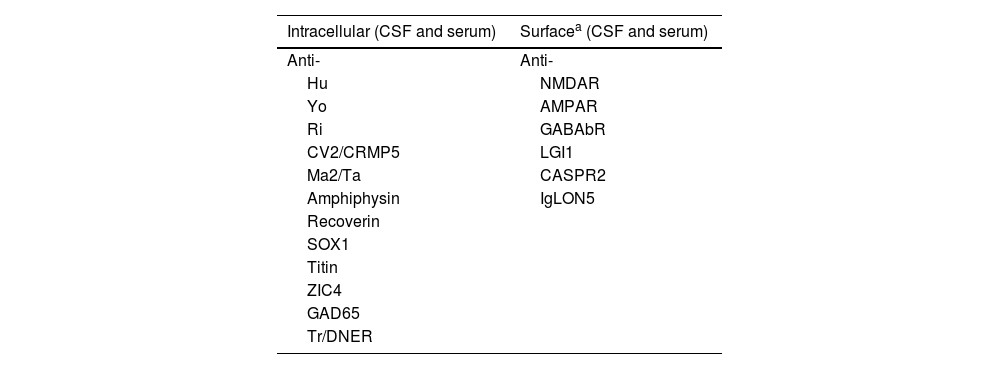
A CSF analysis revealed normal cell count and mildly elevated protein levels (98 mg/dL), no glucose uptake, and negative culture findings; PCR testing for neurotropic viruses yielded negative results. Testing for antineuronal antibodies, initially determined in the serum, showed negative results (Table 1).
Antineuronal antibodies analysed
| Intracellular (CSF and serum) | Surfacea (CSF and serum) |
|---|---|
| Anti- | Anti- |
| Hu | NMDAR |
| Yo | AMPAR |
| Ri | GABAbR |
| CV2/CRMP5 | LGI1 |
| Ma2/Ta | CASPR2 |
| Amphiphysin | IgLON5 |
| Recoverin | |
| SOX1 | |
| Titin | |
| ZIC4 | |
| GAD65 | |
| Tr/DNER |
PCR results for SARS-CoV-2 were negative (at baseline and in all subsequent tests). No pathological findings were observed in the blood analysis, including biochemistry, complete blood count, kidney and liver function, vitamin B12, folic acid, ceruloplasmin, ammonia, and autoimmune study (erythrocyte sedimentation rate, extractable nuclear antigens, ANA, pANCA, cANCA, anti-DNA and anticardiolipin antibodies, and immunoglobulin A).
At discharge, he presented naming difficulties, progressive memory impairment, and postural tremor with mild gait instability. In November 2021, he presented a generalised tonic-clonic seizure with progression to convulsive status epilepticus, and was admitted to the ICU. The EEG revealed acute left frontal epileptiform activity. A new brain MRI study showed subtle T2/FLAIR hyperintensities in the bilateral medial temporal and diencephalic region. CSF analysis showed normal values for IgG and ADA antibodies, with no oligoclonal bands. Serology testing for HIV, hepatitis virus B and C, syphilis, Borrelia, and Brucella yielded negative results, with the exception of IgG antibodies against CMV and EBV. CSF results for antineuronal antibodies were negative. We administered a short course of methylprednisolone.
After partial improvement, he presented 2 further generalised tonic-clonic seizures, increased postural tremor, high-frequency myoclonus, increased gait impairment, needing a walker, and clear immediate memory deficit (Montreal Cognitive Assessment [MoCA] score of 21/30). Serum analysis revealed thyroid-stimulating hormone level of 4.4 mIU/L, T4 level of 1.0 nmol/L, T3 level of 2.7 nmol/L, antithyroglobulin (ATG) antibody level of 986 IU/L, and antithyroid peroxidase (TPO) antibody levels of 538 IU/L. The CSF analysis revealed ATG and TPO antibody levels of 4.2 IU/L and 60.9 IU/L, respectively. The ultrasound study revealed normal thyroid gland morphology, with a heterogeneous, diffuse hyperechoic signal compatible with chronic thyroiditis. A chest, abdomen, and pelvis CT scan detected no malignancy, and no increase in any tumour marker was observed. A second course of methylprednisolone was administered, which decreased tremor and improved gait, with the patient walking independently; the patient was prescribed maintenance treatment with prednisone at discharge. At 6 months, the patient was seizure-free and able to walk independently, presenting mild postural tremor and cognitive improvement (MoCA score of 27/30). The patient had returned to work.
We report one of the first cases of Hashimoto encephalopathy as a probable complication of vaccination against SARS-CoV-2, meeting the Graus criteria (with antithyroid antibodies in CSF4) and presenting the typical manifestation of this encephalopathy.5 We also ruled out alternative causes after an extensive aetiological study. Fortunately, corticotherapy achieved excellent results.
Autoimmune encephalitis is infrequent and its pathophysiology is largely unknown. Its association with vaccines is controversial and lacks a clear causal relationship. In the case of vaccines against SARS-CoV-2, several cases have been described of post-vaccination encephalitis in patients receiving the vaccines developed by Pfizer/BioNTech,6–8 Moderna,9–11 and AstraZeneca.12,13 Other severe post-vaccination neurological events have been identified, including Guillain-Barré syndrome, cerebral venous thrombosis, transverse myelitis, and acute disseminated encephalomyelitis; these complications are less frequent with mRNA vaccines than with adenovirus vaccines, such as the Janssen/Johnson & Johnson vaccine14 (with the United States Centers for Disease Control and Prevention warning about the high incidence of associated Guillain-Barré syndrome), and the AstraZeneca vaccine15 (with the European Medicines Agency warning of the incidence of acute disseminated encephalomyelitis and encephalitis). An underlying molecular mimicry with the spike protein has been proposed16: it has been suggested that the loss of the transmembrane anchor of that protein and its secretion may trigger these adverse events.17
However, these events are extremely rare (less than 1 case per million patients), with an incidence up to 617 times lower than those caused by a natural viral infection.14 Therefore, the benefits of vaccination clearly surpass the potential risks.