We present an update of the Spanish Society of Neurology’s recommendations for prevention of both primary and secondary stroke in patients with dyslipidaemia.
DevelopmentWe performed a systematic review to evaluate the main aspects of the management of dyslipidaemias in primary and secondary stroke prevention and establish a series of recommendations.
ConclusionsIn primary prevention, the patient’s vascular risk should be determined in order to define target values for low-density lipoprotein cholesterol. In secondary prevention after an atherothrombotic stroke, a target value <55 mg/dL is recommended; in non-atherothombotic ischaemic strokes, given the unclear relationship with dyslipidaemia, target value should be established according to the vascular risk group of each patient. In both primary and secondary prevention, statins are the drugs of first choice, and ezetimibe and/or PCSK9 inhibitors may be added in patients not achieving the target value.
Actualizar las recomendaciones de la Sociedad Española de Neurología para la prevención del ictus, tanto primaria como secundaria en pacientes con dislipidemia.
DesarrolloSe ha realizado una revisión sistemática en Pubmed evaluando los principales aspectos relacionados con el manejo de las dislipidemias en la prevención primaria y secundaria del ictus, elaborándose una serie de recomendaciones relacionadas con los mismos.
ConclusionesEn prevención primaria se recomienda determinar el riesgo vascular del paciente con el fin de definir los objetivos de LDLc. En prevención secundaria tras un ictus de origen aterotrombótico se recomienda un objetivo de LDLc < 55 mg/dl, mientras que en ictus isquémicos de origen no aterotrombótico dado que su relación con dislipidemias es incierta se establecerán los objetivos en base al grupo de riesgo vascular de cada paciente. Tanto en prevención primaria como secundaria las estatinas son los fármacos de primera elección, pudiendo asociarse ezetimiba y/o inhibidores de PCSK9 en aquellos casos que no alcancen los objetivos terapéuticos.
Several studies have shown an association between high levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C) and increased risk of ischaemic stroke1. This association is evident in atherothrombotic strokes, although it is uncertain with other aetiologies2–5. However, several studies have reported an increased risk of haemorrhagic stroke with low levels of total cholesterol and LDL-C6–8. High density lipoprotein cholesterol (HDL-C) presents an inverse association with the risk of ischaemic stroke9. Elevated triglyceride levels increase the risk of stroke by 10%10.
Management of dyslipidaemias is based on implementing healthy lifestyles11–13 and pharmacological treatment. The decision to start pharmacological treatment depends on each patient’s vascular risk. Statins constitute the treatment of choice. Other effective lipid-lowering drugs include ezetimibe combined with statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. Such other drugs as fibrates and omega-3 fatty acid supplements are useful in the management of triglycerides, although their effectiveness in preventing vascular diseases is not well defined.
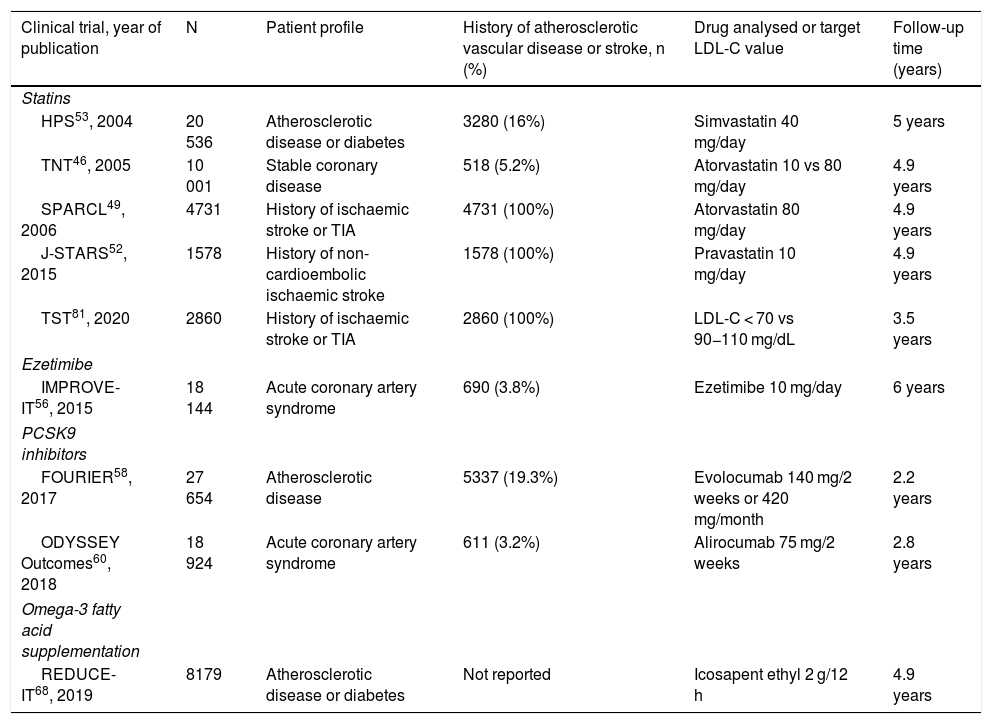
We performed a systematic review of the main aspects related to the pharmacological treatment of dyslipidaemias in primary and secondary stroke prevention, and issue a series of recommendations based on the available evidence, according to the American College of Cardiology/American Heart Association criteria14. Table 1 lists the characteristics of the clinical trials analysed.
Main characteristics of the included clinical trials evaluating the benefits of lipid-lowering drugs.
| Clinical trial, year of publication | N | Patient profile | History of atherosclerotic vascular disease or stroke, n (%) | Drug analysed or target LDL-C value | Follow-up time (years) |
|---|---|---|---|---|---|
| Statins | |||||
| HPS53, 2004 | 20 536 | Atherosclerotic disease or diabetes | 3280 (16%) | Simvastatin 40 mg/day | 5 years |
| TNT46, 2005 | 10 001 | Stable coronary disease | 518 (5.2%) | Atorvastatin 10 vs 80 mg/day | 4.9 years |
| SPARCL49, 2006 | 4731 | History of ischaemic stroke or TIA | 4731 (100%) | Atorvastatin 80 mg/day | 4.9 years |
| J-STARS52, 2015 | 1578 | History of non-cardioembolic ischaemic stroke | 1578 (100%) | Pravastatin 10 mg/day | 4.9 years |
| TST81, 2020 | 2860 | History of ischaemic stroke or TIA | 2860 (100%) | LDL-C < 70 vs 90−110 mg/dL | 3.5 years |
| Ezetimibe | |||||
| IMPROVE-IT56, 2015 | 18 144 | Acute coronary artery syndrome | 690 (3.8%) | Ezetimibe 10 mg/day | 6 years |
| PCSK9 inhibitors | |||||
| FOURIER58, 2017 | 27 654 | Atherosclerotic disease | 5337 (19.3%) | Evolocumab 140 mg/2 weeks or 420 mg/month | 2.2 years |
| ODYSSEY Outcomes60, 2018 | 18 924 | Acute coronary artery syndrome | 611 (3.2%) | Alirocumab 75 mg/2 weeks | 2.8 years |
| Omega-3 fatty acid supplementation | |||||
| REDUCE-IT68, 2019 | 8179 | Atherosclerotic disease or diabetes | Not reported | Icosapent ethyl 2 g/12 h | 4.9 years |
Recommendations regarding non-pharmacological treatment of dyslipidaemia (lifestyle interventions) are addressed in a specific review by the ad-hoc committee of the Spanish Society of Neurology’s Stroke Study Group15. Furthermore, it is essential to monitor different factors and conditions predisposing to hypertriglyceridaemia (diabetes, alcohol consumption, obesity, nephropathies, hypothyroidism, some autoimmune diseases, drugs).
Assessment of vascular riskIt is important to classify vascular risk when indicating lipid-lowering treatment and setting treatment objectives. Different guidelines on managing dyslipidaemia assess vascular risk according to the presence or absence of several vascular risk factors, atherosclerotic diseases including ischaemic stroke, and the use of vascular risk scoring systems11,12,16.
Vascular risk scoring systems are useful in primary prevention, and the use of scales validated in the target population is recommended. In European populations (including the Spanish population), the validated vascular risk scoring system is the SCORE scale (www.heartscore.org)17. This scale includes the 10-year risk of death from cardiovascular disease and is recommended for use in in asymptomatic patients older than 40 years. It need not be administered in cases of clinically or imaging-documented atherosclerotic disease, diabetes, chronic kidney disease (CKD), presence of a high number of vascular risk factors, familial hypercholesterolaemia or LDL-C ≥ 190 mg/dL, as these cases are automatically included in the high or very high vascular risk groups.
The criteria of the European Society of Cardiology/European Atherosclerosis Society establish 4 vascular risk groups12:
- -
Low risk: calculated SCORE < 1%
- -
Moderate risk: at least one of the following criteria:
- o
Diabetes mellitus (DM) in young patients (DM type 1 < 35 years; DM type 2 < 50 years) with duration < 10 years and no associated risk factors
- o
Calculated SCORE ≥ 1% and < 5%
- o
- -
High risk: at least one of the following criteria:
- o
High number of vascular risk factors, especially if total cholesterol > 310 mg/dL, LDL-C > 190 mg/dL, or blood pressure >180/110 mm Hg
- o
Familial hypercholesterolaemia with no other major vascular risk factors.
- o
DM without target organ damage, with DM duration ≥10 years or another additional risk factor
- o
Moderate CKD (glomerular filtration rate of 30−59 mL/min/1.73 m2).
- o
Calculated SCORE ≥ 5% and <10%
- o
- -
Very high risk: at least one of the following criteria:
- o
Documented atherosclerotic disease: acute coronary syndrome, coronary revascularisation, ischaemic stroke, transient ischaemic stroke (TIA), peripheral arterial disease (including carotid artery stenosis and aortic aneurysm), and significant plaque on coronary angiography or CT scan (multivessel coronary disease with 2 major epicardial arteries having > 50% stenosis) or carotid ultrasound
- o
DM with target organ damage (microalbuminuria, retinopathy, or neuropathy) or at least 3 major risk factors, or early onset of DM type 1 of long duration (>20 years)
- o
Severe CKD (glomerular filtration rate < 30 mL/min/1.73 m2)
- o
Calculated SCORE ≥ 10%
- o
Familial hypercholesterolaemia with associated atherosclerotic disease or another major risk factor
- o
These criteria do not mention the presence of intracranial artery stenosis. Several studies have shown that intracranial artery stenosis of atherosclerotic origin, both in primary and secondary prevention, significantly increases the risk of stroke and other cardiovascular events18–24. Therefore, patients with intracranial artery stenosis of atherosclerotic origin are classified as having very high vascular risk.
Furthermore, vascular risk modifiers have been described in primary prevention that may be useful for reclassifying patients into a higher risk group. Coronary artery calcium (CAC) score in non-contrast CT studies is associated with the risk of cardiovascular events. A score of ≥ 100 Agatston units corresponds to a high risk of cardiovascular events, with a score of 0 suggesting low risk25–27. Another vascular risk modifier is the presence of carotid or femoral artery plaques, detectable in ultrasound studies28–30.
Recommendations- -
In primary prevention, we recommend calculating vascular risk using the SCORE scale. Grade of recommendation I, level of evidence C.
- -
CAC scoring or detection of carotid or femoral artery plaques may be useful as vascular risk modifiers in patients presenting low or moderate risk. Grade of recommendation IIa, level of evidence B.
Statins prevent vascular diseases, including stroke31–35, decreasing overall and cardiovascular mortality rates in both sexes36 and all ages37. According to the reduction in LDL-C, statins may be characterised as follows38:
- -
High-intensity statin treatment (reduction ≥ 50%): atorvastatin 40−80 mg, rosuvastatin 20−40 mg.
- -
Moderate-intensity statin treatment (reduction of 30%–49%): atorvastatin 10−20 mg, rosuvastatin 5−10 mg, simvastatin 20−40 mg, pravastatin 40−80 mg, lovastatin 40−80 mg, fluvastatin 80 mg, pitavastatin 1−4 mg.
- -
Low-intensity statin treatment (reduction < 30%): simvastatin 10 mg, pravastatin 10−20 mg, lovastatin 20 mg, and fluvastatin 20−40 mg.
Furthermore, statins increase HDL-C by 1%–10% and reduce triglycerides by 10%–20%. Statins are safe and rarely cause severe adverse effects. They have consistently been associated with muscular adverse effects (myalgia and in rare cases myopathies/rhabdomyolysis), diabetes, and a possible increase in the risk of haemorrhagic stoke39.
Statins in primary stroke preventionDifferent analyses observe that statins achieve reductions of 15%–20% in stroke risk, due to a decreased risk of ischaemic stroke40–44. Although they do not reduce the risk of fatal stroke41–44, they do slow the progression of carotid atherosclerosis45. A meta-analysis of different trials with statins showed that a 1-mmol/L (39-mg/dL) reduction in LDL-C is associated with a 21.2% reduction in stroke risk41.
Regarding the intensity of statin therapy, the Treating to New Targets (TNT) trial compared atorvastatin dosed at 80 mg/day and 10 mg/day in patients with ischaemic heart disease, observing a 25% reduction in the relative risk of stroke with the more intensive treatment46. These data are confirmed in various meta-analyses comparing high-intensity statin therapy with other statin treatments, reporting reductions of 14%–18% in the relative risk of stroke32,47,48 and 16% for ischaemic stroke32 with high intensity statins.
Statins in secondary stroke preventionThe Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) clinical trial compared atorvastatin 80 mg/day vs placebo in the prevention of recurrent stroke after a non-cardioembolic stroke or TIA49. Atorvastatin 80 mg/day decreased the risk of stroke by 16%, despite a significant increase in the risk of haemorrhagic stroke. It also decreased the risk of TIA (26%), major coronary events (35%), major cardiovascular events (20%), and arterial revascularisation (45%).
A subanalysis of the SPARCL trial50 revealed greater benefits with atorvastatin 80 mg/day in cases of carotid stenosis, reducing the risk of stroke by 33% and of major coronary events by 43%. No relevant differences were observed with regard to the aetiology of the stroke or TIA that led to study inclusion51, although larger reductions in absolute risk vs placebo were observed in patients with atherothrombotic ischaemic stroke than in those with lacunar and cryptogenic strokes.
Trials with low- or moderate-intensity statins do not show a reduction in the risk of stroke. The Japan Statin Treatment Against Recurrent Stroke (J-STARS) study52 assessed the effect of pravastatin 10 mg/day vs placebo after a non-cardioembolic ischaemic stroke, revealing no significant differences in the primary endpoint (stroke or TIA) or in cardiovascular events. However, pravastatin was associated with a 67% reduction in the relative risk of atherothrombotic strokes. A subanalysis of the Heart Protection Study (HPS) trial, including 3280 patients with history of cerebrovascular disease, reported that moderate-intensity statins (simvastatin 20−40 mg/day) did not significantly decrease stroke risk compared to placebo, although they did decrease the risk of major cardiovascular events53.
A meta-analysis evaluating different lipid-lowering drugs in the prevention of recurrent strokes and vascular diseases included 8 trials, 5 of which used statins (one with atorvastatin 80 mg [SPARCL], 2 with simvastatin 40 mg [HPS, FASTER], and 2 with pravastatin 40 mg [CARE, LIPID]). The meta-analysis showed reductions of 22% in the relative risk of ischaemic strokes and 23% in the relative risk of major cardiovascular events, with the relative risk of haemorrhagic strokes significantly increasing by 72%54.
EzetimibeEzetimibe inhibits the intestinal absorption of cholesterol, decreasing the level of LDL-C by 15%–22%. This level decreases a further 15%–20% when ezetimibe is combined with statins. This combination reduces the risk of stroke and myocardial infarction55.
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT)56 assessed the effects of simvastatin 40 mg + ezetimibe 10 mg vs simvastatin 40 mg alone after acute coronary syndrome, observing that the additional reduction in LDL-C with the combination therapy was associated with a significant decrease (6%) in the primary endpoint (cardiovascular mortality, non-fatal myocardial infarction, unstable angina requiring hospitalisation, coronary revascularisation, and non-fatal stroke) and in the risk of ischaemic stroke (21%).
Regarding the effectiveness of ezetimibe in secondary prevention, a secondary analysis of the IMPROVE-IT trial57 observed that simvastatin 40 mg + ezetimibe 10 mg was more effective in patients with history of stroke than in patients without history of stroke, decreasing the relative risk of stroke by 48%.
Proprotein convertase subtilisin/kexin type 9 inhibitorsPCSK9 inhibitors are monoclonal antibodies that target the PCSK9 protein, which participates in the catabolism of the LDL-C receptor, thus increasing the abundance of these receptors on the cell surface. These drugs decrease LDL-C levels by up to 60%. Two PCSK9 inhibitors are currently available: evolocumab and alirocumab.
The Further cardiovascular OUtcomes Research with PSCK9 Inhibition in subjects with Elevated Risk (FOURIER) trial58 compared evolocumab against placebo in 27 564 patients with history of cardiovascular disease (non-haemorrhagic stroke in 19.4%) who were under treatment with statins and presented LDL-C levels ≥ 70 mg/dL. Evolocumab significantly reduced the different cardiovascular events assessed, although no reduction in cardiovascular mortality was observed. The researchers observed reductions of 21% in the relative risk of stroke and 25% in the risk of ischaemic stroke, with no significant increase being observed for haemorrhagic stroke. Study participants with history of ischaemic stroke (n = 5337)59 and treated with evolocumab showed a significant reduction of 15% in the relative risk of the primary endpoint (cardiovascular death, myocardial infarction, stroke, unstable angina requiring hospital admission, or coronary revascularisation), and a trend towards reductions in the relative risk of stroke (by 10%) and ischaemic stroke (8%); no increase in the risk of haemorrhagic stroke was observed.
The Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY Outcomes) trial60 evaluated alirocumab in patients with acute coronary syndrome under treatment with statins and presenting LDL-C ≥ 70 mg/dL, observing significant reductions in cardiovascular events, with a decrease of 27% in the relative risk of ischaemic stroke. In this trial, no differences were observed between the outcomes reported in patients with and without history of cerebrovascular disease61, although better results were observed in patients with no history of stroke, who showed a reduction of 38% in the relative risk of stroke, compared to 10% in the group of patients with history of stroke (944 cases).
A meta-analysis of 39 clinical trials with PCSK9 inhibitors revealed a 22% reduction in the relative risk of ischaemic stroke62.
FibratesFibrates are able to decrease triglyceride levels by 50%. Furthermore, they achieved reductions below 20% in LDL-C and increase HDL-C levels by 20%. They may decrease the risk of cardiovascular events63,64. However, they do not reduce the risk of stroke in primary64,65 or secondary prevention54,66.
Omega-3 fatty acid supplementationOmega-3 fatty acid (eicosapentaenoic acid [EPA], docosahexaenoic acid) supplementation dosed at 2−4 g/day significantly reduces levels of triglycerides. A Cochrane review of 79 trials did not observe a decrease in the risk of all cardiovascular events or stroke, but reported a decrease of 7% for coronary events67. However, the recently published Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT)68 compared EPA (2 g/12 h) vs placebo in patients with history of cardiovascular disease, diabetes, or other risk factors and baseline triglyceride levels at 135−499 mg/dL under treatment with statins. EPA significantly reduced cardiovascular mortality and cardiovascular events, decreasing the relative risk of stroke by 28%. To date, no subanalysis of patients with history of stroke included in the trial has supported the effectiveness of EPA supplementation in secondary prevention.
After the publication of this trial, the American Heart Association69 concluded that omega-3 fatty acid supplementation is an effective and safe option that may be administered in patients at high risk and not receiving statin treatment.
Lipid-lowering drugs and haemorrhagic strokeThe association between lipid-lowering therapy and haemorrhagic stroke is controversial. Several meta-analyses do not report an increase in the risk of haemorrhagic stroke or a relationship with the extent of the LDL-C reduction32,41,70,71. However, such meta-analyses are heterogeneous, including both primary and secondary prevention studies, with the majority of the latter addressing cardiovascular diseases other than stroke. In patients with history of stroke, the SPARCL trial showed a 67% increase in the relative risk of haemorrhagic stroke49. A subanalysis of that trial72 observed greater risk of haemorrhagic stroke in patients with haemorrhagic stroke as the entry event, older patients, men, patients treated with atorvastatin 80 mg/day, and those presenting high blood pressure values (systolic pressure ≥ 160 mm Hg and/or diastolic pressure ≥ 100 mm Hg). Furthermore, the subanalysis of patients with history of cerebrovascular disease from the HPS trial showed that simvastatin 20−40 mg/day increased the relative risk of haemorrhagic stroke by 86%, compared to placebo53. However, the J-STARS study52 found no increase in the risk of haemorrhagic stroke with pravastatin dosed at 10 mg/day.
Unlike the data observed in other studies54, a recent meta-analysis73 assessing the risk of haemorrhagic stroke in 39 trials of different lipid-lowering drugs did not find a significant increase in the risk of haemorrhagic stroke in the global analysis of all trials; nor did the authors observe an association with the extent of LDL-C reduction. However, separate analysis of the trials addressing the secondary prevention of different vascular diseases identified an increase of 18% in the relative risk of haemorrhagic stroke. This meta-analysis found that for every 1000 lipid-lowering treatments administered, 9.17 ischaemic strokes are prevented and 0.48 haemorrhagic strokes are provoked, amounting to a net reduction of 8.69 strokes/1000 patients treated.
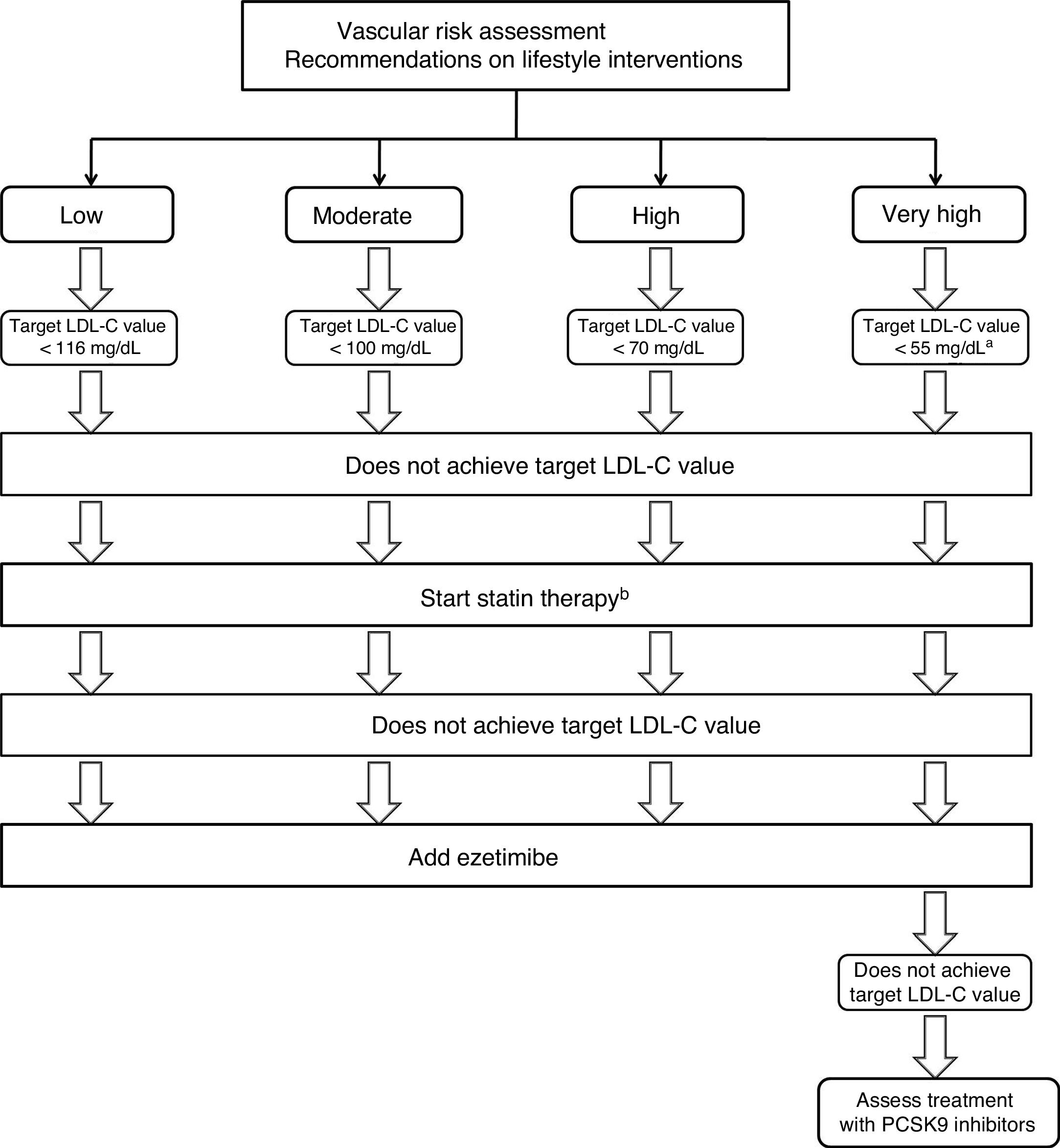
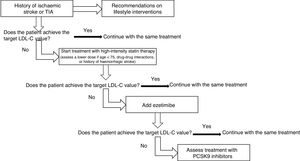
Strategies for the use of lipid-lowering drugs in stroke preventionPrimary preventionThese recommendations are aimed at achieving the target LDL-C values described below, according to the risk group and baseline LDL-C levels. If these objectives are not achieved with lifestyle interventions, statin treatment will be started. In patients with high or very high vascular risk, a 50% reduction in LDL-C levels is recommended. In patients older than 75 years, especially if there is a risk of drug-drug interactions74–76 or history of haemorrhagic stroke, the possibility of administering statins at lower doses will be assessed (Fig. 1).
Therapeutic algorithm for the management of hypercholesterolaemia in primary stroke prevention.
aIn patients with factors increasing the risk of haemorrhagic stroke, a target LDL-C value < 70 mg/dL may be reasonable.
bIn patients presenting high or very high vascular risk, a ≥ 50% reduction in LDL-C level is recommended.
- -
Treatment with statins is recommended in patients not achieving target LDL-C values. Grade of recommendation I, level of evidence A.
- -
In patients older than 75 years, especially if there is a risk of drug-drug interactions or history of haemorrhagic stroke, it is reasonable to start treatment with statins at lower doses. Grade of recommendation IIa, level of evidence C.
- -
If target LDL-C values are not achieved with maximally-tolerated statin therapy, adding ezetimibe is recommended. Grade of recommendation I, level of evidence B.
- -
In patients presenting very high risk, if target LDL-C values are not achieved with maximally-tolerated statin therapy plus ezetimibe, addition of PCSK9 inhibitors should be considered. Grade of recommendation IIb, level of evidence B.
- -
If the vascular risk is high or very high and high levels of triglycerides persist despite statin treatment, it is reasonable to add omega-3 fatty acid supplementation. Grade of recommendation IIa, level of evidence B.
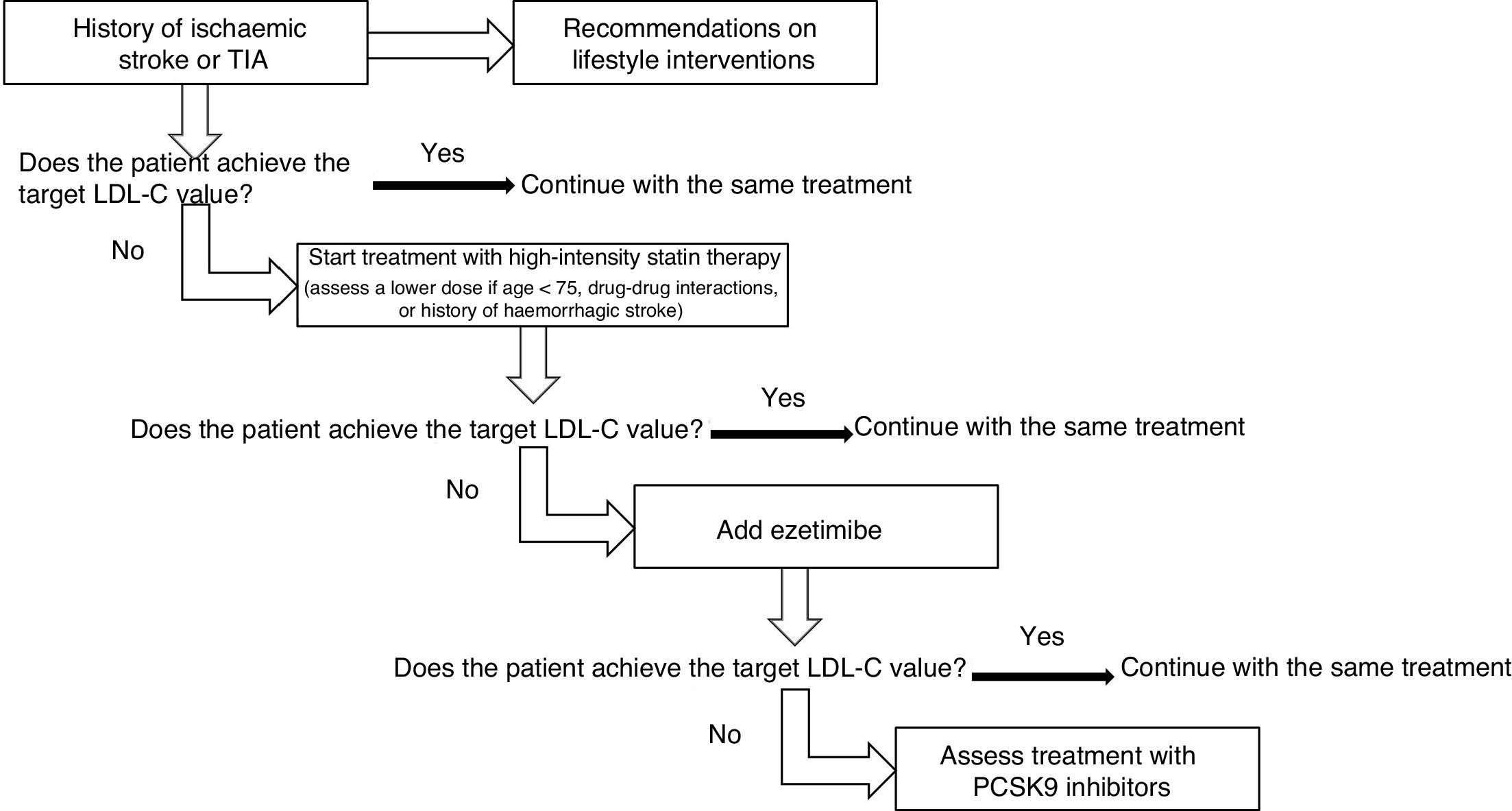
Treatment should be started with high-intensity statins, using lower doses in patients older than 75 years and at risk of drug-drug interactions or with a history of haemorrhagic stroke. If target LDL-C values are not achieved, ezetimibe should be added, followed by PCSK9 inhibitors, if this combination proves insufficient (Fig. 2).
Recommendations- -
Treatment with high-intensity statins is recommended, especially in patients with ischaemic stroke or TIA of atherosclerotic aetiology, or with stroke of other causes, if associated with other atherothrombotic diseases. Grade of recommendation I, level of evidence A.
- -
In patients older than 75 years, especially if there is a risk of drug-drug interactions or history of haemorrhagic stroke, it is reasonable to start treatment with statins at lower doses. Grade of recommendation IIa, level of evidence C.
- -
If target LDL-C values are not achieved with maximally-tolerated statin therapy, it is reasonable to add ezetimibe. Grade of recommendation IIa, level of evidence B.
- -
If target LDL-C values are not achieved with maximally-tolerated statin therapy plus ezetimibe, adding PCSK9 inhibitors should be considered. Grade of recommendation IIa, level of evidence B.
Both primary and secondary prevention should focus on LDL-C levels. In situations in which LDL-C determination may be inaccurate (high triglyceride levels, very low LDL-C levels, diabetic patients), it may be useful to establish objectives based on non-HDL cholesterol or apolipoprotein B cholesterol levels.
Different meta-analyses show that the risk of cardiovascular events decreases in line with LDL-C levels34,36,77–79. These results are also observed in stroke prevention, with different lipid-lowering drugs reducing the relative risk of stroke by 23.5% per 1-mmol/L reduction in LDL-C80, which has led to lower target LDL-C values in different vascular risk groups.
In primary prevention, target LDL-C values should be established according to the patient’s vascular risk group, with reductions of 50% being recommended in patients presenting high or very high risk12,32,34,41. In patients with history of such atherosclerotic diseases as ischaemic heart disease or peripheral arterial disease, achieving LDL-C levels below 55 mg/dL decreases the risk of ischaemic stroke. Thus, the IMPROVE-IT trial56 showed that patients treated with simvastatin 40 mg plus ezetimibe 10 mg achieved lower mean LDL-C levels than patients treated with simvastatin plus placebo (53.7 mg/dL vs 69.5 mg/dL) and showed a 21% reduction in the relative risk of ischaemic stroke. The FOURIER trial58 reported that high-intensity statins plus evolocumab achieved greater reductions in LDL-C levels than the combination of statins and placebo (median 30 mg/dL vs 92 mg/dL) and reduced the relative risk of ischaemic stroke by 21%. The ODYSSEY Outcomes trial60 showed that combining alirocumab with high-intensity statins achieved a greater reduction in LDL-C levels than statins plus placebo (40 mg/dL vs 93 mg/dL), with a 27% reduction in the relative risk of ischaemic stroke.
In the prevention of recurrent stroke, the Treat Stroke to Target (TST) trial81 has shown that lower target LDL-C levels are beneficial after an ischaemic stroke or TIA with associated atherothrombotic disease. This study assessed the effectiveness of 2 target LDL-C levels (<70 mg/dL and 90−110 mg/dL) in preventing cardiovascular events. The < 70 mg/dL LDL-C group presented a significant 22% reduction in the relative risk (absolute reduction of 2.4%) in the trial’s primary endpoint (a composite endpoint of ischaemic stroke, myocardial infarction, urgent coronary or carotid revascularisation, or cardiovascular death), with no significant increase (absolute increase of 0.4%) for haemorrhagic stroke. In the study’s French cohort, which was followed up for longer (5.3 years)82, the group assigned a target LDL-C level of < 70 mg/dL presented a 26% reduction in major cardiovascular events. The reduction in the relative risk of the composite of cerebral infarction/haemorrhagic stroke was 28%, with a non-significant increase in the relative risk of cerebral haemorrhage (17%). A post-hoc analysis of the SPARCL trial83 showed that greater reductions and lower LDL-C levels during follow-up significantly decreased the risk of stroke or other cardiovascular events. Thus, compared to patients whose LDL-C levels were not modified, a ≥50% reduction in LDL-C levels was associated with a 35% reduction in the relative risk of stroke and a 37% reduction in the relative risk of ischaemic stroke, with no significant increase in haemorrhagic strokes. However, with smaller reductions in LDL-C levels, the decrease in the rate of strokes and other cardiovascular events was not significant when compared to patients who presented no decrease in LDL-C. This subanalysis of the SPARCL trial, comparing the patients who achieved LDL-C levels <70 mg/dL with those achieving levels ≥100 mg/dL, found that the former group showed significant reductions in the relative risk of stroke (28%), ischaemic stroke (34%), and cardiovascular events (31%), with no increase in haemorrhagic strokes. However, no relevant differences were observed between the groups achieving LDL-C levels of 70−99 mg/dL and LDL-C ≥ 100 mg/dL. Furthermore, the IMPROVE-IT trial57 observed that in patients with history of stroke (n = 682), the additional 17 mg/dL reduction in LDL-C in those who received simvastatin 40 mg + ezetimibe 10 mg over those treated with simvastatin 40 mg only (50−51 mg/dL vs 67−68 mg/dL) decreased the relative risk of ischaemic stroke by 48%, a greater reduction than that observed in patients with no history of stroke (16%). In the FOURIER trial59, patients with history of ischaemic stroke who received evolocumab achieved mean LDL-C levels of 0.7 mmol/L (27 mg/dL), with a significant 15% decrease in the relative risk of cardiovascular events and a trend towards a decrease in the relative risk of stroke (10%) and ischaemic stroke (8%).
When defining target LDL-C values in patients with history of ischaemic stroke or TIA, the potential for increased risk of haemorrhagic stroke should be taken into account. Although this relationship is unclear, both the SPARCL trial49 and the subgroup of patients with history of cerebrovascular disease from the HPS trial53 presented significantly increased risk of haemorrhagic stroke, which requires us to be cautious when establishing target LDL-C values in these cases, especially in the event of associated factors that increase the risk of haemorrhagic stroke, such as poorly controlled arterial hypertension (systolic pressure ≥ 160 mm Hg and/or diastolic pressure ≥ 100 mm Hg), advanced age, or history of haemorrhagic stroke71.
Ischaemic stroke and TIA of atherothrombotic origin are the only events presenting a clearly established relationship with cholesterol levels, sharing a common origin with other atherothrombotic diseases, such as ischaemic heart disease or peripheral arterial disease. Thus, in the SPARCL51 and J-STARS52 trials, statins achieved greater reductions in the risk of stroke of atherothrombotic origin, although this association was no statistically significant in the former trial. The results of different meta-analyses32,36,78,79 and clinical trials assessing combined treatment with ezetimibe plus statins56 or with PCSK9 inhibitors58,60 have shown that greater reductions and lower LDL-C levels (below 55 mg/dL) are more beneficial in the prevention of atherothrombotic diseases, including ischaemic stroke. In secondary stroke prevention, the results of the TST81 and SPARCL trials83 also show that achieving lower LDL-C levels (<70 mg/dL) is associated with greater efficacy. Patients with ischaemic stroke or TIA of atherothrombotic origin should be classified as presenting very high vascular risk; therefore, a target LDL-C value <55 mg/dL should be recommended, as in other diseases of atherothrombotic origin. However, in the event of factors that increase the risk of haemorrhagic stroke, a target LDL-C level <70 mg/dL may be reasonable.
In ischaemic strokes or TIA of non-atherothrombotic origin, given the unclear association with dyslipidaemia, it is advisable to establish target LDL-C values according to the estimated vascular risk.
In summary, the majority of patients with ischaemic stroke or TIA will be considered to present high or very high risk, although when defining target LDL-C values, the aetiology of stroke or TIA (whether atherothrombotic or otherwise) should be considered, as well as the coexistence of other atherothrombotic diseases, and the particular risk of haemorrhagic stroke.
Recommendations on treatment objectives in stroke preventionPrimary preventionTarget LDL-C values will be stratified according to the vascular risk.
- -
If the risk is low, a target value of < 116 mg/dL may be considered. Grade of recommendation IIb, level of evidence A.
- -
If the risk is moderate, a target value < 100 mg/dL may be reasonable. Grade of recommendation IIa, level of evidence A.
- -
If the risk is high, a target value < 70 mg/dL and a ≥ 50% reduction in LDL-C are recommended. Grade of recommendation I, level of evidence A.
- -
In patients presenting very high risk and history of atherothrombotic disease (ischaemic heart disease, peripheral arterial disease), a target value < 55 mg/dL and a ≥ 50% reduction in LDL-C are recommended. Grade of recommendation I, level of evidence A.
- -
In patients with very high risk but no history of atherothrombotic disease, a target value < 55 mg/dL and a ≥ 50% reduction in LDL-C are recommended. Grade of recommendation I, level of evidence C.
- -
In patients with history of ischaemic stroke or TIA of atherothrombotic origin, target LDL-C values < 55 mg/dL are recommended. Grade of recommendation I, level of evidence B.
- -
In patients with ischaemic stroke or TIA of non-atherothrombotic origin, the same objectives described for primary prevention of stroke are recommended.
- -
In patients with history of ischaemic stroke of atherothrombotic or non-atherothrombotic origin with very high vascular risk and presenting factors associated with increased risk of haemorrhagic stroke, target LDL-C values of < 70 mg/dL may be reasonable. Grade of recommendation IIb, level of evidence B.
The authors have no conflicts of interest to declare.
Please cite this article as: Palacio-Portilla EJ, Roquer J, Amaro S, Arenillas JF, Ayo-Martín O, Castellanos M, et al. Dislipidemias y prevención del ictus: recomendaciones del Grupo de Estudio de Enfermedades Cerebrovasculares de la Sociedad Española de Neurología. Neurología. 2022;37:61–72.