The diagnosis of Lyme borreliosis (LB) is based on the epidemiological history, clinical manifestations and microbiological findings in the early disseminated and late phases of the disease. Related to this fact, microbiological diagnostic techniques have recently appeared. Far from facilitating the diagnosis and the clinical-therapeutic management of LB patients, they are generating confusion. Herein, experts and representatives of Spanish Scientific Societies [Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Spanish Society of Neurology (SEN), Spanish Society of Immunology (SEI), Spanish Society of Pediatric Infectology (SEIP), Spanish Society of Rheumatology (SER), and Spanish Academy of Dermatology and Venereology (AEDV)] exposed the executive summary after reviewing the epidemiology, clinical spectrum, available diagnostic techniques for the diagnosis of Borrelia burgdorferi infection, therapeutic and prevention options of LB. By consensus, recommendations for microbiological diagnosis are offered together with those supporting the therapeutic management and prophylaxis of infection.
El diagnóstico de la borreliosis de Lyme (BL) se basa en la historia epidemiológica, las manifestaciones clínicas y los hallazgos microbiológicos de las etapas temprana diseminada y tardía de la enfermedad. En relación a este hecho, han aparecido recientemente técnicas diagnósticas microbiológicas que, lejos de facilitar el diagnóstico y el manejo clínico-terapéutico de los pacientes con BL, están generando confusión. Por ello, los expertos y representantes de las sociedades científicas españolas [Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), Sociedad Española de Neurología (SEN), Sociedad Española de Inmunología (SEI), Sociedad Española de Infectología Pediátrica (SEIP), Sociedad Española de Reumatología (SER) y Academia Española de Dermatología y Venereología (AEDV)] han presentado el documento de síntesis tras revisar la epidemiología, el espectro clínico, las técnicas disponibles para el diagnóstico de la infección por Borrelia burgdorferi, así como las opciones terapéuticas y preventivas de BL. De manera consensuada, se ofrecen recomendaciones para el diagnóstico microbiológico, así como recomendaciones que respaldan el manejo terapéutico y la profilaxis de la infección.
Lyme borreliosis (LB) is a complex multisystemic process predominantly distributed in the northern hemisphere, transmitted by hard-ticks of the Ixodes ricinus complex and caused by Borreliaburgdorferi sensu lato genospecies (hereafter, B. burgdorferi). In Europe, I. ricinus is the main vector [see Figures 1−3 in supplementary material (SM)]. LB has been classified in different stages (early localized, early disseminated and late) that usually correlate with phisiopathological patterns and correspond with a broad, but defined, clinical spectrum (SM-Table 1).
Diagnosis of LB may be easy in tick-bitten patients developing typical clinical manifestations, like erythema migrans (EM) (SM-Figure 4), in a LB endemic area. In absence of EM, other suggestive manifestations (meningorradiculitis, facial nerve paralysis…) may be caused by other agents/processes and a microbiological confirmation is required. Complicating the diagnosis, many patients do not remember tick-bite or it goes unnoticed. In addition, clinical reports of LB include nonspecific manifestations (prolonged asthenia, arthro-myalgia, lack of concentration, etc.) that, taken away from the appropriate clinical-epidemiological environment, can lead to misdiagnosis.
For many years, and this is still the case for most Public Network Centres in Spain, microbiological criteria recommended by Health Agencies/Scientific Societies (such as CDC, ESCMID, IDSA), have been applied. But in recent years, ‘other non-validated techniques’ have appeared, leading to the diagnosis in patients without clear clinical-epidemiological criteria, and being a source of confusion. Other difficulty is to distinguish an active from a past infection, and the high prevalence of antibodies against B. burgdorferi found in endemic areas. Literature also includes B. burgdorferi-infected patients without typical immunitary response.
For the above-mentioned reasons, and taking into account that is very frequent that we are consulted by patients diagnosed of LB without meeting clinical–epidemiological and microbiological criteria and sometimes subjected to prolonged non-scientific-evidence-based treatments, SEIMC considered the need of a Consensus Document with other Scientific Societies, such as SEN, SEIP, SER, SEI and AEDV, for the diagnosis and management of LB.
Methodology for the evaluation of the documentAn exhaustive bibliographic search was proposed on the state of knowledge of B. burgdorferi infection and LB in PubMed. Bibliographic search with ‘Lyme disease’ or ‘Lyme borreliosis’ or ‘Borrelia burgdorferi’ retrieved more than 15,000 references. Each expert narrowed the field and chose the most relevant ones taking into account previous consensus documents, recommendations of Health Agencies and Scientific Societies and Conference abstracts’ books.
Since available guidelines/consensus documents have been recently published and they can be easily consulted,1–7 we have chosen to use the consensus degree among panelists of this document for final recommendations.
Epidemiological and microbiological aspects of B. burgdorferi infection and clinical manifestations cannot be exposed in this executive summary due to space limitations. Details about these aspects and the complete document can be consulted at supplementary material (Appendix A).
Diagnosis of B. burgdorferi infection and Lyme borreliosisDirect diagnosesThe accurate microbiological diagnosis of B. burgdorferi infection and LB is based on the demonstration of the agent in biological samples by culture and/or visualization. The culture is mainly sensitive in the early localized phase of the disease, in which diagnosis can be made according to clinical-epidemiological aspects. There are different culture media – usually liquid –, with incubations between 30 and 35°C up to 12 weeks and in microaerophilia, such as the Barbour–Stoenner–Kelly (BSK) and its modifications. However, this technique only has a high performance in skin samples, decreasing its sensitivity in sterile fluids, such as CSF or synovial fluid in the early disseminated phase, and even more in late disease stages. There are stains to demonstrate the presence of spirochetes in tissues, although only immunohistochemistry is specific. Therefore, the direct diagnosis is mainly based on molecular biology techniques. Their sensitivity at least overlaps with that of culture methods, and allow us know the involved genospecies. Nevertheless, molecular tests are not standardized, and partial fragment regions (fla, p66, 16S-rRNA, ospA, ospB, VlsE and 5S/23S rRNA) can be used. Using two target genes is recommended. Molecular detection of B. burgdorferi should be also performed with appropriate samples (blood and urine are not suitable). PCR assays are useful in patients with skin manifestations, especially with EM (sensitivity around 70%), with a better profitability (75%) in skin biopsies from achrodermatitis chronica atrophicans (ACA) patients (SM-Figure 5). Synovial fluid is considered a valuable sample for Lyme arthritis (LA) diagnosis (median sensitivity: 77.5%). It decreases up to 22.5% for CSF in neuroborreliosis. Sensitivity is reduced for specimens kept for long periods or paraffin-embedded ones. Samples should be quickly processed under optimized conditions. Negative PCR results do not exclude LB. Specificity of positive results must be confirmed by identification up to genospecies level to reduce contamination risks. We recommend using molecular diagnostic tests with CSF in neuroborreliosis suspicion, with skin biopsies in ACA and with synovial fluids in LA; always in specialized laboratories. (Consensus level: 9/9)
Indirect diagnosesThe most common/accessible techniques are the serological ones to demonstrate antibodies against B. burgdorferi. Negative serological results at an early stage do not necessarily exclude the diagnosis. In patients with EM, to confirm the presence of antibodies is not necessary. Besides, early antimicrobial therapy may abrogate the antibody response. To demonstrate B. burgdorferi infection, serology should be repeated at least four weeks later.
In the early phases, IgM occurs only in half of patients during the first 2–4 weeks (50% are negative to these antibodies). IgM production reaches a peak at 6−8 weeks, and the titre gradually decreases after 3 months. However, there may be patients who remain IgM positive for a long time (up to ten years). In absence of epidemiological and typical signs of LB, the IgM presence may suggest cross-reactions.
For all the above, we do not recommend diagnosing LB based on an isolated positive IgM value, except in early phases, with typical manifestations and always in an adequate epidemiological environment. Thus, support for microbiological diagnosis of LB should preferably be performed by IgG measurement (consensus level 9/9).
The most commonly used serological methods are the enzyme immunoassay (EIA or EIA-based), such as Enzyme Linked Fluorescent Assay (ELFA), ChemiLuminiscence ImmunoAssay (CLIA) and Multiplexed Microbead ImmunoAssay (MMIA), indirect immunofluorescence assay (IFA) and Western-Blot (WB). Sensitivity of commercial EIA-based techniques varies depending on the patient's disease phase. In localized EM without systemic involvement, sensitivity is around 54%. It reaches 81% in neuroborreliosis, 96% in arthritis and 97% in ACA.
Serodiagnosis is even more difficult with Borrelia spp. emerged as human pathogens in Europe (e.g. Borrelia miyamotoi), since ELISA/WB designed for LB diagnosis may show cross-reactions. We do not consider IFA as the most appropriate serologic screening technique (Consensus level: 9/9).
WB is used for confirmation of EIA/IFA tests. For its interpretation, qualitative and/or cuantitative criteria have been proposed. Positive bands in WB can indicate past borrelia contact, active/acute/persistent infection, cross-reactivity or be the result of nonspecific monoclonal/polyclonal stimulation of B-lymphocytes in the course of infections by lymphotropic viruses. We recommend serological diagnosis only under LB clinical suspicion and within the appropriate epidemiological environment (Consensus level: 9/9).
The incorporation of the C6 peptide to EIA-techniques has been proposed in America as unique for diagnosis or as second confirmation test ignoring WB. In Europe, unlike in America and due to the great diversity of genospecies, recommended serologic diagnosis of LB consists of an EIA-based technique followed by WB for positive/equivocal cases. Serologic tests must be performed only in clinically suspected LB cases, with the exception of EM, to avoid over-testing and unnecessary costs (Consensus level: 9/9).
Serology results will depend on the disease stage. In early LB, whose only clinical manifestation may be an EM, or in the short-term acute neuroborreliosis, serology can be negative in up to nearly 60% patients. In these cases, with high suspicion of LB and negative serology results, it is advisable to repeat it within 3–4 weeks to check if there is seroconversion (Consensus level: 9/9).
In suspected neuroborreliosis, blood serology could be insufficient, and without other epidemiological or clinical features, CSF analysis should be performed since patients may rarely show IgG antibodies in the CSF in absence of peripheral response. Intrathecal antibody production is highly indicative of neuroborreliosis, and it relies on measuring anti-IgG Borrelia antibodies in CSF and serum. Spirochetal invasion of CNS results in local production of CXCL13, a B-cell attracting chemokine, with the subsequent intrathecal production of specific antibodies.
Patients can reinfect and a serological test is advisable, repeating it 3–4 weeks later to detect any increase in the titre or modifications in WB, with respect to the first infection (Consensus level: 9/9).
There are other techniques [CD57+, ELISPOT, Interferon-γ Test (IFN-γ), IFN-α, Lymphocytic proliferation test, CXCL-13 marker, CCL-19, apolipoprotein B-100, antibody-free chains (kappa/lambda) or the determination of total IgM and albumin] non-approved by scientific agencies as valids for LB diagnosis. For this reason, they are discouraged (Consensus level: 9/9)
Treatment- -
Antimicrobial treatment of LB has not changed in decades, and it depends on the stage/organ/system affected.
- -
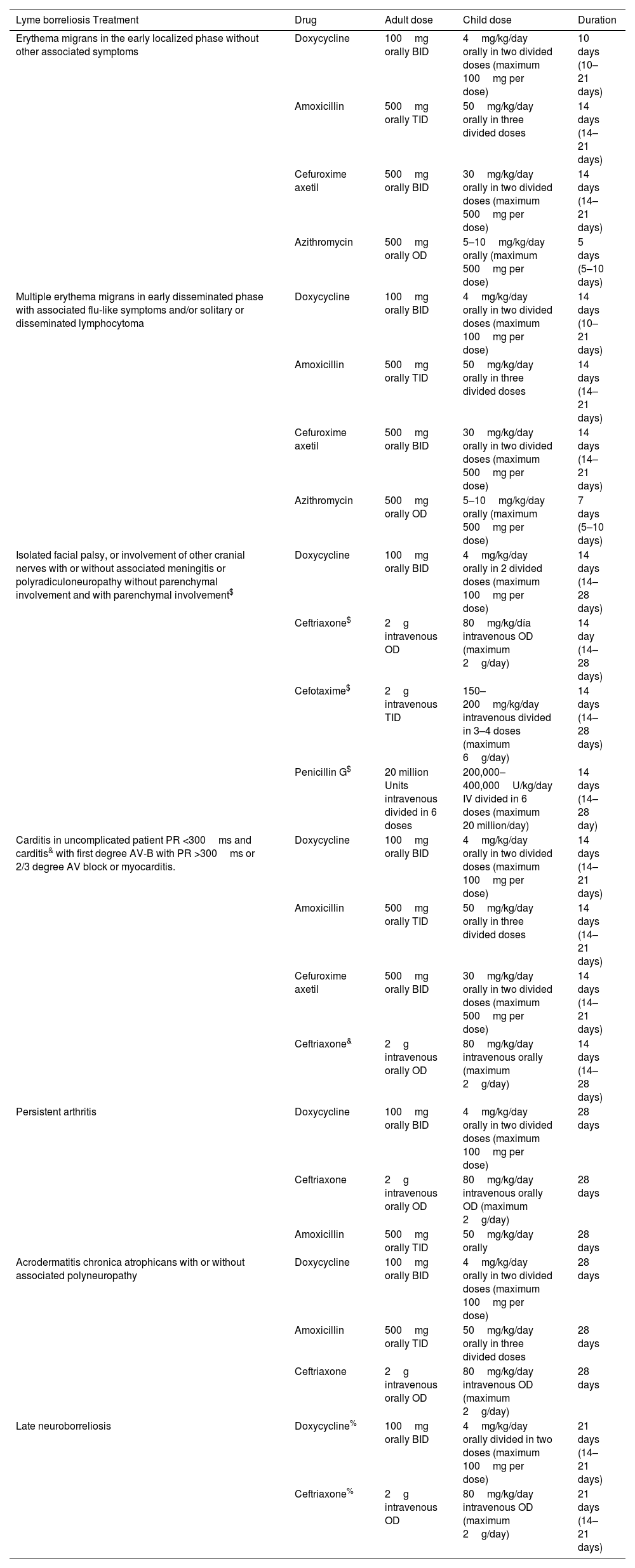
Many recommendations are based on few clinical trials and meta-analysis studies. The choice of the drug will depend on the age, history of allergies, pregnancy, intolerances, or sun exposure given the possibility of photosensitivity with doxycycline (Table 1).
Table 1.Treatment of Lyme borreliosis.
Lyme borreliosis Treatment Drug Adult dose Child dose Duration Erythema migrans in the early localized phase without other associated symptoms Doxycycline 100mg orally BID 4mg/kg/day orally in two divided doses (maximum 100mg per dose) 10 days (10–21 days) Amoxicillin 500mg orally TID 50mg/kg/day orally in three divided doses 14 days (14–21 days) Cefuroxime axetil 500mg orally BID 30mg/kg/day orally in two divided doses (maximum 500mg per dose) 14 days (14–21 days) Azithromycin 500mg orally OD 5–10mg/kg/day orally (maximum 500mg per dose) 5 days (5–10 days) Multiple erythema migrans in early disseminated phase with associated flu-like symptoms and/or solitary or disseminated lymphocytoma Doxycycline 100mg orally BID 4mg/kg/day orally in two divided doses (maximum 100mg per dose) 14 days (10–21 days) Amoxicillin 500mg orally TID 50mg/kg/day orally in three divided doses 14 days (14–21 days) Cefuroxime axetil 500mg orally BID 30mg/kg/day orally in two divided doses (maximum 500mg per dose) 14 days (14–21 days) Azithromycin 500mg orally OD 5–10mg/kg/day orally (maximum 500mg per dose) 7 days (5–10 days) Isolated facial palsy, or involvement of other cranial nerves with or without associated meningitis or polyradiculoneuropathy without parenchymal involvement and with parenchymal involvement$ Doxycycline 100mg orally BID 4mg/kg/day orally in 2 divided doses (maximum 100mg per dose) 14 days (14–28 days) Ceftriaxone$ 2g intravenous OD 80mg/kg/día intravenous OD (maximum 2g/day) 14 day (14–28 days) Cefotaxime$ 2g intravenous TID 150–200mg/kg/day intravenous divided in 3–4 doses (maximum 6g/day) 14 days (14–28 days) Penicillin G$ 20 million Units intravenous divided in 6 doses 200,000–400,000U/kg/day IV divided in 6 doses (maximum 20 million/day) 14 days (14–28 day) Carditis in uncomplicated patient PR <300ms and carditis& with first degree AV-B with PR >300ms or 2/3 degree AV block or myocarditis. Doxycycline 100mg orally BID 4mg/kg/day orally in two divided doses (maximum 100mg per dose) 14 days (14–21 days) Amoxicillin 500mg orally TID 50mg/kg/day orally in three divided doses 14 days (14–21 days) Cefuroxime axetil 500mg orally BID 30mg/kg/day orally in two divided doses (maximum 500mg per dose) 14 days (14–21 days) Ceftriaxone& 2g intravenous orally OD 80mg/kg/day intravenous orally (maximum 2g/day) 14 days (14–28 days) Persistent arthritis Doxycycline 100mg orally BID 4mg/kg/day orally in two divided doses (maximum 100mg per dose) 28 days Ceftriaxone 2g intravenous orally OD 80mg/kg/day intravenous orally OD (maximum 2g/day) 28 days Amoxicillin 500mg orally TID 50mg/kg/day orally 28 days Acrodermatitis chronica atrophicans with or without associated polyneuropathy Doxycycline 100mg orally BID 4mg/kg/day orally in two divided doses (maximum 100mg per dose) 28 days Amoxicillin 500mg orally TID 50mg/kg/day orally in three divided doses 28 days Ceftriaxone 2g intravenous orally OD 80mg/kg/day intravenous OD (maximum 2g/day) 28 days Late neuroborreliosis Doxycycline% 100mg orally BID 4mg/kg/day orally divided in two doses (maximum 100mg per dose) 21 days (14–21 days) Ceftriaxone% 2g intravenous OD 80mg/kg/day intravenous OD (maximum 2g/day) 21 days (14–21 days) BID: one doses every 12h; OD: one doses every 24h; TID: one doses every 8h.% In case of coexistence of ACA, 28 days.
- -
We are aware of the controversy with the term ‘chronic Lyme’ and its treatment. Since the panelists do not consider other LB forms than those developed in the text, and reject the term ‘Chronic Lyme Disease’ related to a persistent infection by B. burgdorferi resistant to conventional treatment, we will not make recommendations on this aspect. All members of this consensus are against prolonged treatments with antibiotics and/or their combinations in patients who suffer only unspecific clinical manifestations (asthenia, arthralgia, lack of concentration, etc.) (Consensus level: 9/9).
Most LB patients respond to antimicrobial treatment in a timely manner, depending on the type of clinical manifestation and/or affected organ-system, although in patients under special conditions, such as those undergoing immunosuppressor treatments, anti-TNF, hematological malignancies or in elderly patients, the response may be slow and sometimes patients have to be retreated.
This panel recommends using doxycycline over other therapeutic options, when appropriate, due to the good penetration of this antibiotic into the CNS and other tissues, and the possibility of spirochete dissemination. Unlike the recently published American Guidelines, which consider intravenous beta-lactams the preferential treatment of neuroborreliosis, doxycycline can also be considered as an alternative for the treatment of CNS infections with meningeal involvement. In Europe, doxycycline is considered of choice if there are no parenchymal complications3,5,8,9 (Consensus level: 9/9).
In addition, doxycycline is active and of choice against other tick-borne microorganisms that occasionally can coinfect the patient (Anaplasma phagocytophilum, B. miyamotoi, Rickettsia spp. etc). It is only recommended avoiding doxycycline in pregnancy and lactation, in which the risk/benefit must be always assessed. We agree with the American Academy of Pediatrics and doxycycline can be used for short duration (≤21days) without regarding patient age (Consensus level: 9/9).
Early localized phaseTreatment should be considered according to the severity/persistence of clinical manifestations. Most patients treated with the recommended regimens (Table 1) present a complete resolution of the signs/symtoms in the following 20 days, avoiding the progression to other phases. As in other infectious diseases, some patients present subjective symptoms (headache, musculoskeletal pain, arthralgia or fatigue) that can persist for weeks/months after treatment. Usually, these symptoms spontaneously resolve in the following months, and do not require sustained/repeated antibiotic treatment, as they are not due to the persistence of the infection. However, if other clinical manifestations (e.g.: fever) are observed despite treatment, co-infections with other tick-borne agents should be ruled out (Consensus level: 9/9).
B. burgdorferi infection and LB do not leave permanent immunity and LB may be suffered more than once (very rare). In that case, the patient will be treated under the same recommended guidelines. (Consensus level: 9/9).
Erythema migransThe treatment of choice in this situation is oral doxycycline, amoxicillin or cefuroxime-axetil (Table 1). This panel, if there is no contraindication, preferably recommends using doxycycline for 10–14 days, in children and adults (Consensus level: 9/9).
If a beta-lactam from those specified (equally effective) is chosen, treatment should be prolonged for at least 14 days. Macrolides as first-line drugs are generally discouraged, leaving them as alternative when doxycycline, amoxicillin or cefuroxime cannot be used. If azithromycin is chosen, a 7-day-treatment could be recommended. Ceftriaxone is recommended in pregnant patients (Consensus level: 9/9).
LymphocytomaRecommended treatment for borrelial lymphocytoma (SM-Figure 6) is showed in Table 1.
Early disseminated phaseMultiple erythema migransThe recommended treatment is oral doxycycline for 10–21 days, with the same considerations as those for localized EM. Prolongation of treatment for more than 10 days will be based on accompanying signs and symptoms. Doses/duration of treatment against multiple EM with associated flu-like symptoms and borrelial lymphocytoma are showed in Table 1.
Early neuroborreliosisIn Europe, the new guidelines recommend using oral doxycycline as long as there are no parenchymal complications at the brain or spinal level, or unless clinical manifestations are very severe.
Adjunctive corticosteroids neither improve nor impair the outcome for LB peripheral facial palsy patients treated with doxycycline. In case of parenchymal involvement, an intravenous beta-lactam is recommended (Table 1). Doxycycline is the antibiotic of choice for the remaining neurological manifestations in the early phase, and as alternative, a beta-lactam by intravenous route at the doses/duration specified in Table 1 (Consensus level: 9/9).
CarditisAsymptomatic AV-B with a PR interval <300ms observed with relative frequency in the early stages of the disease does not require antimicrobial treatment different from that of the process itself. Patients with myopericarditis or those with severe/potentially severe involvement should receive intravenous antibiotic treatment at the doses/duration specified (Table 1). This can be simplified to the oral route (doxycycline/amoxicillin/cefuroxime-axetil) once the blockage is resolved and/or clinical improvement occurs until completing 21–28 days. In patients with symptomatic bradycardia that cannot be managed with drugs, temporary pacemakers are recommended (Consensus level: 9/9).
Treatment of other manifestations accompanying the early disseminated phase, such as the possibility of acute arthritis, should be carried out following the scheme in Table 1.
Late phaseArthritisTo prolong treatment with oral doxycycline, amoxicillin or cefuroxime for up to 28 days at the doses specified in Table 1 is recommended. Patients with sustained synovitis refractory to antibiotic treatment may benefit from disease-modifying antirheumatic drugs, such as methotrexate or arthroscopic synovectomies (Consensus level: 9/9).
Acrodermatitis chronica atrophicansTreatment with oral agents is recommended, as showed in Table 1. Doxycycline or amoxicillin for 30 days are recommended. When ACA is accompanied by involvement of the nervous system (usually as axonal polyneuropathy with predominant sensory symptoms), intravenous therapy with ceftriaxone or other beta-lactam should be used (Consensus level: 9/9).
Late neuroborreliosisThis panel, as in the previous sections, recommends doxycycline as the first option, and ceftriaxone as an alternative, depending on the severity of the clinical picture and accompanying manifestations (e.g.: ACA and polyneuropathy), as showed in Table 1. As an adjunct to antimicrobial therapy, accompanying symptoms should be treated. Rehabilitation treatment and psychological support are sometimes needed (Consensus level: 9/9).
Post-Lyme syndrome. Chronic Lyme borreliosisPrescription of an adequate treatment, under recommendations established in the text, allows control the infection with cure for a very high percentage of patients. For patients treated in the early phase, cure usually occurs within three weeks, whereas the response is usually slower in the late-phase. Antibiotic treatment may fail, although this situation is rare, and it is usually due to problems with adherence or absorption of antibiotics rather than to the existence of antibiotic resistance of B. burgdorferi. In patients with the so-called post-Lyme syndrome, there is controversy. Studies showing no effect on such symptoms after prolonging the duration of the antibiotic treatment, repeating it or carrying out cycles of antibiotics, have been performed. This approach is not recommended in any guideline. However, some authors advocate prolonging treatment in persistent symptoms and evidence of coinfection by other tick-borne agents. The issue draws great controversy.
Members of this panel, with current scientific evidence, and since the persistence of B. burgdorferi infection after adequate treatment has not been demonstrated, are positioned not to use prolonged treatments or cycles or combinations of antibiotics in these cases (Consensus level: 9/9).
ProphylaxisProphylaxis of LB is based on pre and post-exposition measures to I. ricinus. Since avoiding tick-bites is the best way to prevent LB, members of this panel assume recommendations showed in Table 2 (Consensus level: 9/9).
General recommendations to prevent Lyme borreliosis.
| • Do not go off the trail when walking in areas where there are ticks. |
| • Use clothes that cover exposed areas of the body (cap, long trousers tucked into the socks, long sleeved shirt into the trousers and appropriate footwear). |
| • Wear light-colored clothing to detect ticks before they attach. |
| • Use tick repellents. |
| • Inspect the body after being in an outdoor area where ticks are abundant. |
| • Remove the tick with tweezers as soon as possible when detected. |
| • Take doxycycline in certain circumstances after tick-bite. |
| • Observe the site of the tick attachment for up to six weeks. |
Previously developed vaccines to prevent LB were only available in USA. Commercial vaccines are not currently available for humans, although new ones are promising.
This panel recommends education programs in schools and recreational/professional associations (hunters, mountaineers...) that instruct in tick-bites prevention, how to recognize them and removing ticks (Consensus level: 9/9).
If a patient is bitten by a tick, we must remove it as soon as possible. After tick removal, skin should be disinfected with povidone iodine or chlorhexidine. Ticks should be kept at −20°C for research in case of the patient becomes ill. This panel recommends not handling ticks and using forceps for ticks’ extraction (SM-Figures 7 and 8) (Consensus level: 9/9).
Doxycycline prophylaxis could be reconsidered in Europe since the administration of a single dose of doxycycline (200mg) within 72h after removing a tick, reduced the relative risk in 67% (95% CI 31–84%) compared to no treatment in people ≥8 years, and no serious adverse events were reported, according to a European open-label, randomized, controlled trial performed in a LB endemic area.10 Anyway, Spain is a sunny area and a reasonable option might be to consider that, if the tick has been manipulated or is engorged or the patient has a high level of anxiety, prophylaxis with doxycycline could be offered (Consensus level: 9/9).
Safety of doxycycline during pregnancy has not been assessed; risks, benefits and uncertainties of doxycycline versus observation should be weighed. Anyway, after suffering from tick bites, it is advisable to instruct patients in the possible signs and symptoms that they may develop and they should observe the tick-bite site for at least six weeks (Consensus level: 9/9).
FundingThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflicts of interest- 1.
José A. Oteo declares no conflict of interest in the writing of this article.
- 2.
Héctor Corominas declares no conflict of interest in the writing of this article.
- 3.
Raquel Escudero declares no conflict of interest in the writing of this article.
- 4.
Fernando Fariñas-Guerrero declares no conflict of interest in the writing of this article.
- 5.
Juan Carlos García-Moncó declares no conflict of interest in the writing of this article.
- 6.
Miguel A, Goenaga declares no conflict of interest in the writing of this article.
- 7.
Sara Guillén declares no conflict of interest in the writing of this article.
- 8.
José M. Mascaró declares no conflict of interest in the writing of this article.
- 9.
Aránzazu Portillo declares no conflict of interest in the writing of this article.
All SM-figures with exception of SM-Figure 5 (A, B) (provided by Dr. Mascaró from Hospital Clinic of Barcelona), have been provided by the Center of Rickettsiosis and Artropod-Borne Diseases (CRETAV) from the Departament of Infectious Diseases of the Hospital Universitario San Pedro-Center of Biomedical Research of La Rioja (CIBIR).