Risk factors for differentiated thyroid carcinoma (DTC) are poorly understood, but serum TSH levels, thyroid nodularity, and presence of autoimmunity are well-recognized factors that modulate DTC prevalence. TSH stimulates proliferation of both normal and neoplastic follicular cells. Consequently, thyroid-stimulating immunoglobulins (TSI), because of its TSH-like action, should induce DTC progression in patients with Graves’ disease (GD). The study objective was to compare the prevalence of incidental DTC in patients undergoing thyroidectomy for benign thyroid disease.
MethodsThe pathology reports of 372 patients with preoperative diagnosis of euthyroid multinodular goiter (EMG) or hyperthyroidism were reviewed. Scintigraphy results and serum TSI levels were used to diagnosed either GD or hyperactive MG (HMG) to hyperthyroid subjects. Prevalence of DTC in each category was calculated using a Chi-square test.
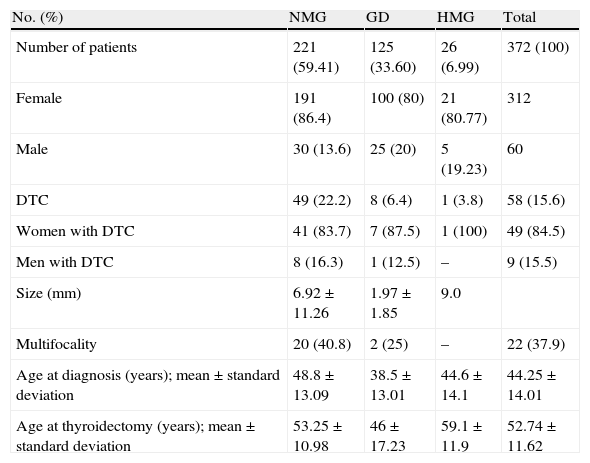
ResultsEMG, GD, and HMG were diagnosed in 221, 125, and 26 patients. There were 58 DTCs, distributed as follows [n (%)]: EMG, 49 (22.2%); GD, 8 (6.4%), and HMG, 1 (3.8%). Difference in prevalence of incidental DTC between the groups was statistically significant (p<0.001). After adjustment for age, patients with EMG had a greater DTC prevalence than GD patients, with an OR of 4.17 (p<0.001). Tumor size (mm, mean±SD) was 6.92±11.26, 1.97±1.85, and 9.0 for EMG, GD and HMG respectively (p=0.017).
ConclusionsIncidental DTC was less prevalent in GD as compared to EMG irrespective of age. This finding may suggest a predisposition to develop DTC in patients with thyroid nodular disease and/or a potential effect of autoimmunity to protect against development of neoplastic disease.
Entre los factores moduladores de prevalencia de carcinoma diferenciado de tiroides (CDT) destacan la concentración plasmática de TSH, la nodularidad tiroidea y la asociación con la autoinmunidad. La TSH estimula la proliferación de células foliculares normales y neoplásicas. Los anticuerpos contra el receptor de TSH (TSI), por su acción TSH-like, deberían estimular el crecimiento del CDT. El objetivo fue comparar la prevalencia de CDT incidental en pacientes tiroidectomizados por enfermedad benigna.
Pacientes y métodosSe estudió la anatomía patológica de 372 pacientes con diagnósticos prequirúrgicos de bocio multinodular normofuncionante (BMN) o hipertiroidismo. La gammagrafía, y/o presencia de TSI diferenció entre bocio multinodular hiperfuncionante (BMH) y enfermedad de Graves (EG). Se comparó la prevalencia de CDT en cada categoría (χ2).
ResultadosSe encontraron 221 sujetos con BMN, 125 con EG y 26 con BMH. Se hallaron 58 CDT con la siguiente distribución: BMN, 49 (22,2%); EG, 8 (6,4%) y BMH, 1 (3,8%). La diferencia de prevalencia de CDT entre los grupos fue estadísticamente significativa (p<0,001). Ajustando por edad, el BMN tiene mayor prevalencia de CDT respecto a EG, con OR de 4,17 (p<0,001). El tamaño (mm) tumoral (media±DE) fue: 6,92±11,26; 1,97±1,85 y 9,0 en BMN, EG y BMH respectivamente (p=0,017).
ConclusionesLa prevalencia de CDT incidental es menor en EG que en BMN, siendo el resultado independiente de la edad. Este hallazgo puede indicar una predisposición hacia el desarrollo de CDT en pacientes con enfermedad nodular tiroidea y/o que la reacción autoinmunitaria puede resultar un factor protector contra el desarrollo de enfermedad neoplásica.
The conditions which predispose to the development of differentiated thyroid cancer (DTC) are not well known, but factors such as autoimmunity, circulating thyroid-stimulating hormone (TSH) levels, and the presence of nodules appear to modulate its prevalence.
The potential association of autoimmunity and DTC development is an open issue which has been the subject of extensive research.1–6 Several authors have analyzed the potential association of DTC with both Graves disease (GD) and Hashimoto thyroiditis and reported conflicting results.1,2,7 While some studies appear to suggest that autoimmunity is a risk factor for DTC development,1,7 other data suggest a potential protective role against DTC.2
It has also been suggested that a linear relationship exists between serum TSH levels and the frequency of DTC in patients with thyroid nodules.7–9 In this regard, the presence of thyroid-stimulating immunoglobulins (TSIs) should promote an increased prevalence of DTC in patients with GD, due to their TSH-like action.
Finally, other authors have emphasized that patients with thyroid nodules have a greater risk of developing DTC than those with no nodules.10–14
Considering these three potential modulators of DTC frequency, a study was designed to compare the prevalence of incidental DTC in surgical specimens from patients undergoing total thyroidectomy for benign thyroid disease at our center. The categories to be analyzed included Graves’ disease (GD), normal functioning multinodular goiter (NMG), and hyperfunctioning multinodular goiter (HMG).
Materials and methodsStudy populationInformation was collected from 372 patients undergoing total thyroidectomy at our center over an 11 year period (October 1998–September 2008). Presurgical diagnoses were GD, NMG, or HMG.
Presurgical diagnoses were based on clinical examination and biochemical, immunological, scintigraphic, and ultrasound techniques. The diagnostic criteria for hyperthyroidism included the finding of low serum TSH levels with normal or elevated circulating thyroxine (T4) levels and, sometimes, elevated triiodothyronine (T3) levels. The differential diagnosis of hyperthyroidism was based on the result of scintigraphy: diffuse high uptake for GD and unifocal or multifocal high uptake for HMG. In most cases, the diagnosis of GD was also supported by the finding of high TSI levels. In selected patients, information provided by Doppler ultrasound was also available. The thyroid glands from patients with GD usually showed an increased Doppler flow. Patients with goiter and nodules in ultrasound examination associated with TSH and thyroid hormone levels within the normal range and low uptake in nodular areas with well preserved parenchyma in scintigraphy were included in the NMG group.
The pathological report of all surgical specimens was reviewed and the occurrence of incidental thyroid carcinoma was recorded, special attention being paid to tumor size and DTC multifocality. Pathological information confirmed clinical diagnosis.
Exclusion criteria included prior treatment with radiotherapy or radioactive iodine. This was a retrospective study, and iodine consumption could not therefore be recorded.
MethodsFree T3, free T4, and TSH levels were tested by an electrochemiluminescence immunoassay using a Modular Analytics E170 analyzer (Roche), and anti-TSH receptor antibodies (TSI) were tested using Riazen TSH-R Ab radioimmunoassay kits (ZenTech). Reference ranges were 0.38–4.7μU/mL (TSH), 9.1–23.9pmol/L (free T4), 2.3–5.3pmol/L (free T3), and positive anti-TSH receptor antibodies (TSI) >14U/L, 9–14U/L being considered the limit range.
Statistical studyA crude analysis was performed using a Chi-square test to compare DTC prevalence in the groups of patients diagnosed with GD, NMG, and HMG. For means comparisons between the GD and NMG groups, normal distribution was verified using Kolmogorov–Smirnov and Shapiro–Wilk tests, after which a Student's t test, or a Mann–Whitney U test (its non-parametric equivalent) when applicable, was performed. Frequencies were compared using a Chi-square test, or a Fisher's exact test when absolute frequencies were less than five. Odds ratio was subsequently calculated, and unconditional logistic regression was performed to adjust for age and sex. Data are shown as absolute (n) or relative (%) frequencies and as means with their standard deviation (SD).
ResultsA total of 376 patients met the criteria for the review of pathological specimens. Four cases were excluded after the review due to medullary carcinoma (n=3) and anaplastic carcinoma (n=1) being found, which left 372 patients for analysis. Table 1 summarizes patient characteristics.
Characteristics of patients undergoing total thyroidectomy with prior diagnosis of benign thyroid disease.
| No. (%) | NMG | GD | HMG | Total |
| Number of patients | 221 (59.41) | 125 (33.60) | 26 (6.99) | 372 (100) |
| Female | 191 (86.4) | 100 (80) | 21 (80.77) | 312 |
| Male | 30 (13.6) | 25 (20) | 5 (19.23) | 60 |
| DTC | 49 (22.2) | 8 (6.4) | 1 (3.8) | 58 (15.6) |
| Women with DTC | 41 (83.7) | 7 (87.5) | 1 (100) | 49 (84.5) |
| Men with DTC | 8 (16.3) | 1 (12.5) | – | 9 (15.5) |
| Size (mm) | 6.92±11.26 | 1.97±1.85 | 9.0 | |
| Multifocality | 20 (40.8) | 2 (25) | – | 22 (37.9) |
| Age at diagnosis (years); mean±standard deviation | 48.8±13.09 | 38.5±13.01 | 44.6±14.1 | 44.25±14.01 |
| Age at thyroidectomy (years); mean±standard deviation | 53.25±10.98 | 46±17.23 | 59.1±11.9 | 52.74±11.62 |
HMG: hyperfunctioning multinodular goiter; NMG: normal functioning multinodular goiter; DTC: differentiated thyroid carcinoma; GD: Graves disease.
Age at diagnosis was lower in patients with GD as compared to those with NMG (p<0.001). Age at total thyroidectomy was 53.25±10.9; 46±17.2 and 59.1±11.9 years in the NMG, GD, and HMG groups respectively (p<0.001), and was significantly lower in GD as compared to the other thyroid diseases studied.
A total of 58 DTCs were incidentally found in the total sample of surgical specimens removed at total thyroidectomy. Group distribution was: NMG, 49 (22.2%); GD, 8 (6.4%); and HMG, 1 (3.8%). A single patient with HMG was found with DTC, and this group was therefore excluded from analysis. Comparison of the prevalence of incidental DTC between the NMG and GD groups showed a statistically significant difference, with a higher prevalence in the NMG group (p<0.001). After age adjustment, patients with NMG had a higher prevalence of DTC as compared to the GD group, with an odds ratio of 4.17 (p<0.001).
Analysis of the relationship between age at diagnosis of thyroid disease and the occurrence of DTC showed significant differences. Patients with DTC were diagnosed at a mean age (years) of 48.23±11.60, while mean age at diagnosis of those with no DTC was 43.49±14.31; (p=0.04).
The potential influence of sex was also analyzed. As expected, thyroid disease was more common in females. However, no significant differences in incidental DTC rates were found between the NMG and GD groups after sex stratification. DCT rates in females were 83.67% and 14.29% in the NMG and GD groups respectively, while the respective rates in males were 88.89% and 11.11%. Male/female odds ratio was 1.01 (Table 1).
DTC measures collected (in mm) were 6.92±11.26; 1.97±1.85 and 9.0 in the NMG, GD, and HMG groups respectively (p=0.017). Comparison of these data showed statistically significant differences, with a smaller size of incidental DTCs found in the GD as compared to the NMG group. Multifocality was more prevalent in DTCs resected from patients in the NMG group (20, 40.82%) than in those in the GD (2, 25%) and HMG (0, 0%) groups. A statistically significant difference was found for prevalence (p<0.001), but not for multifocality (p=0.27) (Table 1).
DiscussionIncidental DTC after surgery for benign thyroid disease is not an unusual finding,14 despite the fact that DTC frequency in thyroid nodules is less than 5%. However, incidentalomas are fortunately microcarcinomas of very low aggressiveness in most cases.15 The universal trend towards an increased incidence of DTC has also been seen in Spain in recent years. This is due to an increased number of small papillary carcinomas.16,17
The best known risk factor for DCT development is ionizing radiation.13,18 However, DTC occurrence has also been related to other factors such as nodular goiter,13 high circulating TSH levels,7–9 the presence of autoimmune reaction,1,19 female sex,20 and obesity.21
Prior studies have shown an increased prevalence of DTC in patients diagnosed with nodular goiter and a stronger association of DTC with NMG than with HMG.13,14 It is hypothesized that its lower frequency in the latter may be related to the abolition of the trophic effect of TSH on the follicular thyroid cells. In fact, hyperthyroidism is considered as a factor which protects against the development of DTC. Because of this, recent ATA guidelines for the management of DTC do not recommend routine cytological testing in hyperthyroid patients with nodular disease.22 This recommendation must, however, be followed with caution23 because some evidence has led to fine needle aspiration in hyperthyroid patients being recommended, too.24
More information is available about the potential association of hypothyroidism and DTC. In a study of 1500 patients, the prevalence of malignancy increased from 2.8% when TSH levels were less than 0.4mU/L to 29.7% when TSH levels were higher than 5.5mU/L.8 A subsequent study showed that patients diagnosed with DTC in more advanced stages had higher TSH levels.9
It has also been reported that lymphocyte infiltration, which is a characteristic of thyroid autoimmune conditions, protects against the development of DTC, or is at least associated with a good prognosis of the disease.19,25–27 In this regard, it should also be noted that microchimerism, a phenomenon associated with a high percentage of thyroid autoimmune diseases,28,29 is related to a decreased presence of malignant cells, so playing a relevant role as a protective factor in DTC.30 However, other research has suggested that, in addition to serum TSH levels, the presence of positive anti-thyroglobulin antibodies is an independent predictor of malignancy in patients with thyroid nodules, despite the concomitant presence of thyroid autoimmune disease.4,7 Analysis of these studies with conflicting results is difficult because although Hashimoto thyroiditis and GD share the potential effect associated with the modulating role of autoimmune disorder on oncogenesis, their functional presentation differs. While GD causes hyperthyroidism, patients with Hashimoto thyroiditis eventually develop thyroid hypofunction. Thus, studies showing an increased prevalence of DTC in GD stated that this finding may be related to the TSH-like action of TSI antibodies and attributed to these the ability to stimulate the proliferation of neoplastic follicular cells.31
Our results agree with other previous findings.13,14 There is, however, some discrepancy with other studies32 which report a higher incidence of thyroid cancer in GD as compared to NMG. Incidental DTC was only found in a single patient with HMG in our sample, which prevented a comparison of the prevalence between GD and HMG patients. It may be assumed that multicentricity occurs as a consequence of the influence of stimulating or risk factors for the development of DTC. In our series, incidental DTC was multicentric in only two of the eight patients with GD and DTC. This number was not sufficient for an adequate statistical analysis to be made nor for it to be compared with its presence in incidental DTCs found in patients with NMG. However, a higher trend to multifocality was seen in NMG, which agrees with the finding of a greater size and prevalence of DTC in these patients (Table 1).
In conclusion, our results suggest that the prevalence of incidental DTC is lower in GD as compared to NMG and that this result is independent of age. This finding may indicate two trends: a certain predisposition to DTC in patients with nodular disease and/or that the autoimmune reaction may prove to be a factor which protects against the development of neoplastic disease.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Pascual Corrales E, et al. El carcinoma diferenciado incidental de tiroides es menos prevalente en la enfermedad de Graves que en el bocio multinodular. Endocrinol Nutr. 2012;59:169–73.