One of the main goals of prescribing physical activity for people with type 2 diabetes is to reduce hyperglycaemia, as it is a risk factor for the development of chronic complications. As less time spent each day in sedentary behaviour would lead to higher glucose consumption by skeletal muscle tissue, this could have significant positive effects on blood glucose control parameters. For this reason, the aim of this study was to analyse the information from different protocols for breaking sedentary behaviour and the association with blood glucose control parameters in patients with type 2 diabetes.
Material and methodsA systematic search was carried out for randomised controlled studies on this topic published in the scientific literature. The following databases were considered: PubMed, Cochrane, EBSCO, WoS, ScienceDirect and Medline.
Results24 studies were identified and analysed using the COVIDENCE platform. Seven articles were selected for the final analysis, comprising 138 patients. The results show that breaks in sedentary behaviour with light physical activity in people with type 2 diabetes are effective in reducing insulin resistance, the area under the glucose curve, fasting and postprandial blood glucose, and blood glucose variability.
ConclusionsAcute interruption of sedentary behaviour, through light-intensity and short-duration exercise, can improve blood glucose indicators in patients with type 2 diabetes, including short term blood glucose variability.
Uno de los principales objetivos de la prescripción de actividad física para personas con diabetes de tipo 2 es reducir la hiperglicemia, ya que es un factor de riesgo para el desarrollo de las complicaciones crónicas. Puesto que un menor tiempo diario en conducta sedentaria implica un mayor consumo de glucosa por parte del tejido muscular esquelético, esto puede tener efectos positivos agudos sobre los parámetros de control glicémico. De acuerdo con lo anterior, el objetivo de este estudio fue analizar la información de distintos protocolos de quiebre en la conducta sedentaria y su asociación con parámetros de control glicémico en pacientes diabéticos tipo 2.
Material y métodoSe realizó una búsqueda sistemática de los estudios controlados aleatorizados sobre este tema que se encuentran publicados en la literatura científica. Para ello se consideraron las siguientes bases de datos: PubMed, Cochrane, EBSCO, WoS, ScienceDirect y Medline.
ResultadosSe identificaron 24 estudios sobre el tema, los cuales se analizaron con la plataforma COVIDENCE. Para el estudio final se seleccionaron 7 artículos que suman un total de 138 pacientes. Los resultados muestran que los quiebres en la conducta sedentaria, a través de actividad física ligera, son eficaces en disminuir la resistencia insulínica, el área bajo la curva de glucosa, las glicemias de ayuna y postprandial, y la variabilidad glicémica.
ConclusionesEl quiebre agudo en la conducta sedentaria, a través de ejercicio de intensidad liviana y duración breve, es capaz de mejorar los indicadores de control glicémico en personas con diabetes tipo 2, incluyendo la variabilidad glicémica medida a corto plazo.
The World Health Organization (WHO) 2020 guidelines stipulate that adults should undertake at least 150 min, ideally 300 min of moderate-intensity physical activity per week, as this variable is key to achieving minutes of moderate-intensity physical activity per week, as this variable is key to achieving both primary and secondary prevention of people’s health. The WHO defines physical activity as “any bodily movement produced by skeletal muscles that requires energy expenditure”. These recommendations proposed by the WHO are primarily aimed at preventing the onset of cardiovascular diseases, obesity, type 2 diabetes mellitus (T2DM) and some forms of cancer.1 However, despite the known benefits of achieving the physical activity targets proposed by international bodies, their attainment continues to pose a significant challenge, particularly for the prevention and treatment of T2DM.2,3 This challenge is particularly complex in patients with T2DM and middle-aged people living with overweight and older adults, who are more likely to be inactive and/or sedentary and have a lower exercise tolerance than healthy individuals.4,5 Prescribing moderate-to-vigorous intensity physical activity (intensity greater than 3 MET) in these subjects is usually not very feasible to achieve and, in many cases, unattainable.6
Moreover, population studies have found that more than half of people manifest high sedentary behaviour (SB) during a typical day, understanding SB to comprise all activities - while awake - that require energy expenditure of less than 1.5 MET, such as: watching TV or sitting in front of the computer, reclining or lying down.7 It has specifically been shown that people with T2DM spend approximately 64% of their awake time in SB.8 This lifestyle is a global trend of particular concern since research suggests that long periods of SB are very harmful to health, irrespective of whether or not physical activity recommendations are achieved.9
Since 2016, the American Diabetes Association has proposed breaks in sedentary behaviour (BSB) as part of a healthy lifestyle for people with T2DM. BSB are defined as SB interrupted by a short bout of light physical activity (between 1.5 and 3.0 MET). This type of physical activity includes, but is not limited to, walking, going up and down stairs or doing squats. The ADA recommends interrupting sedentary behaviour with at least 3-min bouts of light physical activity every 30 min for people with diabetes mellitus.10 The ADA’s proposal is based on the powerful beneficial effects of BSB on cardiorespiratory fitness; skeletal muscle contraction-mediated glucose uptake and insulin response.11
There is a growing trend to focus exclusively on intentional and structured moderate-to-vigorous physical exercise. Yet this approach has clear limitations in people with morbidities.12 In this context, replacing prolonged time in SB with brief and light-standing activities could be more effective strategies for behaviour change, particularly if performed in the workplace. This is where people often spend much of their time in SB. BSB implemented in this way could be a useful tool to introduce people to a more active lifestyle, enabling them to achieve the appropriate physical activity recommendations for their health later.
Moreover, it is now known that it is not only the degree of exposure to hyperglycaemia - traditionally measured by long-term HbA1c (glycated haemoglobin) - that contributes to the pathogenesis of diabetes complications, but also glycaemic variability (GV), which evaluates short-term daily variations in blood glucose levels (within a single day or over consecutive days).13 GV in T2DM is associated with a higher risk of complications, particularly macrovascular complications,14 by increasing oxidative stress, pro-inflammatory markers and advanced glycation end products.15–17 As such, it is important not only to identify changes in the classic blood glucose indicators, such as the incremental area under the curve (iAUC) for glucose, fasting blood glucose and post-prandial blood glucose, or the dawn phenomenon (DP), but also GV and its indicators, such as time in range, time in hyperglycaemia, glucose standard deviation (SD), the coefficient of variation (CV) of glucose, the mean amplitude of glucose excursion (MAGE) and continuous overall net glycaemic action (CONGA).16,17
In light of the above, it is extremely important to understand the evidence on the benefits of BSB, evaluated by robust physiological parameters of blood glucose control, to help prevent long-term complications in people with T2DM. As a result, the aim of this study was to analyse the short-term effects of different BSB regimens on the physiological parameters of blood glucose control in patients with T2DM.
Material and methodsSearch strategyThis article is a systematic review. Articles were searched for in the PubMed, ScienceDirect, Cochrane, WoS, MEDLINE and EBSCO bibliographic databases. The search limits were: studies published between 2011 and 2021 (June), conducted in humans aged 18 years or over.
The search strategy included the following MeSH terms: (glycaemic variability) AND (glucose control) AND (type 2 diabetes) AND (sedentary behaviour OR breaking sitting) AND (continuous glucose monitoring) AND (humans). Articles in English, Spanish, Portuguese and German were considered for the study.
Article selectionArticles on randomised crossover trials (RCTs) were included. Literature review articles, letters to the editor, case studies and expert opinions were not considered. The population evaluated was: subjects aged 18 years or over with T2DM undergoing short-term GV monitoring with continuous glucose monitoring devices. Two researchers initially reviewed the article abstracts. Those articles whose abstracts met the inclusion criteria were read entirely to determine their eligibility.
Data extractionThe data were extracted by an author who was not involved in the study selection. The information extracted from the chosen studies included descriptive information, analytical methods and results (Table 1).
Effect of break in sedentary behaviour in people with diabetes mellitus.
| Author/year (reference) | n (age, years) | Regimen/condition | Design | Blood glucose monitoring method | Washout period | Outcome-Blood glucose control parameters |
|---|---|---|---|---|---|---|
| Dempsey et al.,21 2016 | 24 (62 + 6) | 7 h total SB regimen. Three regimens: | RCT | Venous blood samples every 30 min | 6–14 days | Compared to the SIT regimen, both the SRA and LW regimens significantly attenuated iAUC (7 h): glucose and insulin p < 0.001 |
| 1: SIT (control) prolonged sitting | BSB with LW or SRA regimens significantly attenuated post-prandial glucose and insulin | |||||
| 2: LW: sitting + 3-min bouts of light walking (3.2 km/h) every 30 min of SB | ||||||
| 3: SRA: sitting+3 min of simple resistance activities every 30 min of SB | ||||||
| Dempsey et al.,20 2017 | 24 (62 + 6) | 7 h total SB regimen. Three regimens: | RCT | CGM | 6–14 days | Compared to the SIT regimen, both LW and SRA significantly reduced 22-h glucose levels, p < 0.001 |
| 1: SIT (control) prolonged sitting | Average nocturnal and waking glucose levels significantly decreased in the LW and SR regimens compared to the SIT regimen (p < 0.001) | |||||
| 2: LW: sitting + 3-min bouts of light walking (3.2 km/h) every 30 min of SB | 7-h BSB with LW or SRA regimen significantly reduced 22-h hyperglycaemia and iAUC | |||||
| 3: SRA: sitting+3 min of simple resistance activities every 30 min of SB | Glucose SD, CONGA-1 and MAGE significantly decreased with LW and SRA regimens compared to the SIT regimen. However, there were no significant differences in CV% in the three regimens | |||||
| No episodes of hypoglycaemia were identified in any of the regimens | ||||||
| Duvivier et al.,19 2017 | 19 (63 + 9) | Three regimens | RCT | CGM | 10 days | iAUC for 24-h glucose was significantly lower during the SIT Less regimen than Sitting (p = 0.002), falling by 36%. However, the Sit Less and Exercise values were similar (p = 0.499) |
| 1: Sitting: 14 h | The 24-h time in hyperglycaemia fell from 211 min/day in Sitting to 118 min/day in Sit Less (p = 0.002). In Exercise, the 24-h time in hyperglycaemia was 152 min | |||||
| 2: Exercise: replace 1.1 h/day of SB with exercise (intensity 5.9 MET) | ||||||
| 3: SIT Less: 17,502 steps/day with 4.7 h/day of SB replaced by walking and standing | ||||||
| Dempsey et al.,18 2018 | 24 (62 + 6) | 4 h SB each regimen | RCT | Venous blood samples every 1 h | NR | The results suggest that greater metabolic benefits are derived from BSB versus the control regimen as insulin resistance increases |
| 1: SB: 4 h | The impact of the SB regimen on the tAUC for glucose versus BSB was greater by 1.23 mmol/h/l for every 1 mmol/l more fasting glucose (p < 0.001) | |||||
| 2: BSB every 20 min (3.2 km/h) for 2 min (in overweight/obese adults) | ||||||
| 3: BSB every 30 min (3.2 km/h) for 3 min (in diabetics) | ||||||
| Paing et al.,24 2019 | 12 (60 + 3) | 7 h SB each regimen. BSB: 3 min of light-intensity exercise-walking | Incomplete block RCT | CGM | 5 days | Fasting glucose and duration of DP were lower for regimen 3 than regimen 2 (p = 0.041) and regimen 1 (p = 0.004) |
| 1: BSB every 60 min | The CV of nocturnal GV was lower in regimen 3 compared to regimen 2 (p < 0.03) and regimen 1 (p < 0.02) | |||||
| 2: BSB every 30 min | ||||||
| 3: BSB every 15 min | ||||||
| Paing et al.,22 2019 | 12 (60 + 11) | 7 h SB each regimen. BSB: 3 min of light-intensity exercise-walking | Incomplete block RCT | CGM | 5 days | The AUC for glucose (21 h) was lower in regimen 3 than in regimen 1 (p < 0.001) and regimen 2 (p = 0.002) |
| 1: BSB every 60 min | Post-breakfast: the iAUC for glucose fell in regimen 3 (p < 0.04) versus regimen 1 | |||||
| 2: BSB every 30 min | Post-lunch: the iAUC for glucose was significantly lower in regimen 3 (p < 0.03) and regimen 2 (p < 0.05) than regimen 1 | |||||
| 3: BSB every 15 min | ||||||
| Homer et al.,23 2021 | 23 (62 + 8) | Three regimens. | RCT | Venous blood samples every 30 min | 6-14 days | Glucose and insulin 7-h iAUCnet were attenuated significantly during SRA6 compared to the SIT regimen (p < 0.05) |
| 1: SIT: 7-h uninterrupted SB | Glucose and insulin 7-h iAUCnet were not attenuated significantly during SRA3 compared to the SIT regimen | |||||
| 2: SRA3: SB +3 min resistance exercise every 30 min | In diabetic adults, the 6-min BSB every 60 min significantly reduced (p < 0.05) post-prandial glucose and insulin | |||||
| 3: SRA6: SB +6 min resistance exercise every 60 min |
AUC: area under the curve for glucose; BSB: break in sedentary behaviour; CGM: continuous glucose monitoring; CONGA-1: continuous overall net glycaemic action; CV: coefficient of variation; CV%: percentage coefficient of variation; glucose SD: standard deviation for glucose; iAUC: incremental area under the curve for glucose; iAUCnet: net incremental area under the curve for glucose; LW: light walking; MAGE: mean amplitude of glucose excursion; MET: metabolic equivalent of task (unit of measure of metabolic rate, equal to 3.5 ml O2/kg ⋅ min or 1 kcal/kg/h); RCT: randomised crossover trial; SB: sedentary behaviour; SIT: sitting; SIT Less: sitting less; SRA: simple resistance activities.
The protocol for conducting this systematic review was guided by the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) statement and performed using COVIDENCE® software (www.covidence.org).
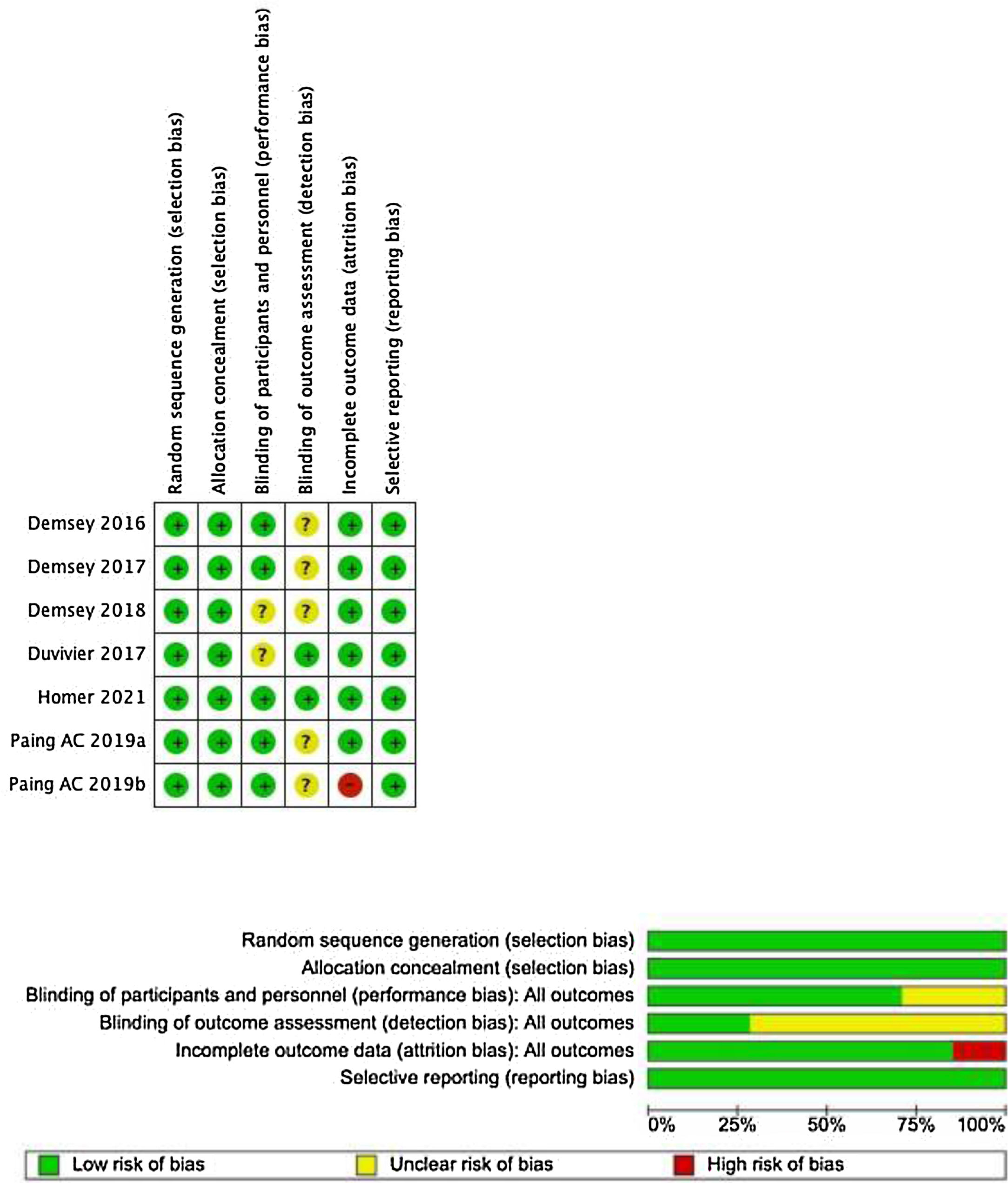
The risk of bias assessment for the studies included was conducted by two reviewers independently for each study, using the RoB 1 tool (Fig. 1). All conflicts between the researchers were resolved by consensus.
Outcome measuresThe articles analysed evaluated the effect of BSB using the following blood glucose control parameters: insulin resistance, iAUC for glucose (incremental area under the curve for glucose), dawn phenomenon (DP) and acute GV measurements: time in hyperglycaemia (post-prandial or 24 h) and hypoglycaemia, CV and glucose SD, CONGA-1 and MAGE.
ResultsOf the 24 articles identified in the literature search, 12 were rejected, and one was added in the secondary search. Subsequently, six were rejected during the full-text review due to their design, topic or intervention, leaving seven articles for analysis, including a total of 138 patients (Fig. 2).
The main characteristics and findings of the studies are summarised in Table 1.
Insulin resistanceDempsey et al.18 found that the higher the underlying degree of insulin resistance, the greater the metabolic benefit from BSB compared to prolonged uninterrupted SB. Moreover, a study conducted by Duvivier et al.19 showed that the “sit less” intervention, defined as 4.7 h a day of sitting replaced by standing and light-intensity walking (as perceived by the patient), significantly reduced (p = 0.015) HOMA2-IR compared to exercise (1.1 h a day of sitting replaced by moderate- to vigorous-intensity cycling) and to SB (14 h sitting/day; just 4,415 steps/day) (p = 0.001). However, unlike the “sit less” intervention, the exercise regimen failed to significantly decrease HOMA2-IR compared to the SB regimen (p = 0.177).
iAUC for glucoseThe iAUC for glucose was evaluated between 7 and 24 h in six of the seven articles. In five studies,18–22 BSB interventions resulted in a significant decrease in the iAUC for glucose compared to the prolonged uninterrupted sitting control regimen, irrespective of the type of physical activity performed. The only exception was the study by Homer et al.,23 which found that glucose iAUC levels did not significantly decrease during the BSB regimen (3 min of resistance-based exercise) compared to prolonged uninterrupted sitting. Worthy of special mention is the study by Dempsey et al.,18 which concluded that the impact of the SB regimen on the total area under the curve (tAUC) for glucose, compared to the BSB intervention, was greater by 1.23 mmol/h/l for every 1 mmol/l more fasting glucose (p < 0.001).
Blood glucoseFasting blood glucoseThe study conducted by Paing et al.24 showed that fasting blood glucose was significantly (p = 0.004) lower with 3-min bouts of BSB for every 15 min of SB the day before the measurement was taken, compared to 3-min bouts of BSB for every 60 min of SB the day before the measurement was taken. Similar results were recorded by Dempsey et al.,20 with 3 min of BSB for every 30 min of SB (p < 0.001).
Time in hyperglycaemia- •
Post-prandial. Both the study by Dempsey et al.20,21 and the study by Homer et al.23 found that BSB with bouts of light aerobic exercise (energy expenditure less than 3 MET) or resistance-based (exercise that uses resistance to achieve increased muscle mass and strength), for 3 min every 30 min, significantly reduced post-prandial blood glucose levels. Moreover, the results obtained by Paing et al.22 show a dose-response relationship, with post-prandial (after breakfast) blood glucose levels progressively decreasing as the frequency of BSB increases.
- •
22–24 h. The research conducted by Dempsey et al.20 and by Duvivier et al.19 revealed a fall in the duration of hyperglycaemia in the BSB intervention compared to the SB regimen. The sit-less regimen proposed by Duvivier et al.19 is particularly striking, as the duration of hyperglycaemia over a 24-h time span was almost halved (p = 0.002) compared to uninterrupted sitting.
None of the studies that evaluated hypoglycaemia reported this effect as a result of BSB.19,20
Coefficient of variation, glucose standard deviation, CONGA-1 and MAGEWith regard to the observed effects of BSB on the acute indicators of glycaemic variability, Dempsey et al.18 found that glucose standard deviation, CONGA-1 and MAGE all decreased significantly with 3-min bouts of light aerobic exercise or resistance-based exercise. Contradictory results were recorded for the CV indicator. The study by Paing et al.24 found that the night-time CV fell with 3-min bouts of BSB every 15 min of SB compared to regimens with 3-min bouts of BSB every 30 min (p < 0.03) or 60 min (p < 0.02) of SB. In contrast, Dempsey et al.18 identified no significant differences in the percentage CV between the three regimen types.
Dawn phenomenonThe study conducted by Paing et al.24 reported that 3-min bouts of BSB every 30 min of SB did not significantly reduce (p = 0.370) DP duration. However, DP duration was significantly reduced with 3-min bouts of BSB every 15 min compared to bouts every 60 min (p = 0.004) or every 30 min (p = 0.041).
DiscussionDespite the limited number of studies on this subject, the information they provide is comparable, consistent and robust because the study population is homogeneous, with similar age ranges, haemoglobin A1c levels between 7.2% and 7.6%, and none of the subjects was treated with insulin. Moreover, all of the seven studies analysed in this systematic review were randomised crossover trials (RCTs)/controlled clinical trials (CCTs), facilitating robust analysis with small sample sizes. Furthermore, five of the seven studies analysed used continuous glucose monitoring, which provides extremely accurate information about acute GV with BSB.
Almost all of the studies analysed in this systematic review used multiples (6–26; mode: 12) short BSB (2–6 min; mode: 3 min) every 15–60 min (mode: 30 min) of sitting. These publications clearly show that BSB are an effective strategy for improving blood glucose control indicators in people with T2DM. Even the three studies17,20,23 with the shortest cumulative BSB time (21–24 min in total) identified a significant improvement in most blood glucose control indicators evaluated. Moreover, the studies conducted by Paing et al.22,24 suggest that there may be a direct relationship between BSB frequency (light-intensity walking) and blood glucose control (daily and post-prandial) in patients with T2DM.
The only study with a long cumulative BSB time was conducted by Duvivier et al.,19 in which 4.7 h/day of sitting were replaced by 2.5 h of standing plus 2.2 h of light walking. This approach proved to be even more effective in significantly improving blood glucose control parameters in subjects with T2DM than a structured exercise regimen with the same overall energy expenditure (1.1 h of moderate- to vigorous-intensity cycling). Low-intensity and prolonged BSB were found to reduce iAUC for 24-h glucose versus the control (SB alone) to a similar extent as moderate- to vigorous-intensity structured exercise. Still, they resulted in a greater HOMA2-IR reduction than the latter.19
However, it is important to point out that hypoglycaemia was not observed in any studies evaluating its possible occurrence as a result of BSB in patients with T2DM.18,19 This finding is of particular significance regarding the safe prescribing of this type of activity for type 2 diabetes.
In light of the above, current evidence clearly shows that BSB positively affects the various blood glucose control parameters, endorsing the efficacy of this type of physical activity for better blood glucose management. The evidence supports the prescribing of BSB as a key part of treating patients with T2DM and not just as an adjunct or secondary indication to the traditional recommendations of physical activity that currently tend to be prescribed. That is why healthcare professionals should stress to patients with T2DM the importance of “sitting less and moving more”, thereby promoting a behavioural change that is simple yet highly impactful on blood glucose control.
ConclusionThe experimental studies’ findings in this systematic review provide robust evidence that BSB consisting of light physical activity or change of posture, such as walking or standing, is an effective blood glucose control strategy in patients with T2DM. Although further research is required to establish specific BSB recommendations for people with T2DM, this strategy could be the most effective starting point for those patients who are inactive or reluctant to partake in structured physical exercise.
FundingNo sources of funding to declare.
Conflicts of interestThe authors declare that they have no conflicts of interest.