Advanced hybrid closed-loop (AHCL) systems have demonstrated improved glycemic control in individuals with Type 1 Diabetes Mellitus. The aim of this study is to compare patient satisfaction among three available AHCL systems (Medtronic Minimed780 G, Roche Diabeloop DBLG1, and Tandem t:slim X2 Control IQ) after six months of treatment and to determine if it is related to glycemic control.
MethodsThe data of 75 individuals were analyzed, including 15 using the DBLG1 system, 9 using Control IQ, and 51 using MM780 G. Patient satisfaction was assessed using the Diabetes Treatment Satisfaction Questionnaire for Diabetes Mellitus (DTSQc), a validated instrument.
ResultsAll systems demonstrated treatment satisfaction. The DBLG-1 system scored 14 (−15–21) points, while Control IQ scored 21 (9–24) and M780 G scored 19 (11–24) (p = 0.004). The multivariate analysis revealed that the DBLG-1 system is associated with a lower DTSQc score (OR 0.19, p = 0.019) independent of glycemic control, sex, age, duration of diabetes, duration as an insulin pump user, and daily insulin dose.
ConclusionAHCL systems are satisfactory treatments for users, with potential variations observed between each system regardless of the achieved glycemic control.
Los sistemas avanzados de asa cerrada híbrida (AHCL) han demostrado mejorar el control glucémico en personas con Diabetes Mellitus Tipo 1. El objetivo de este estudio es comparar la satisfacción del paciente entre tres sistemas AHCL disponibles (Medtronic Minimed780 G, Roche Diabeloop DBLG1, y Tandem t:slim X2 Control IQ) tras seis meses de tratamiento y conocer si se relaciona con el control glucémico.
MétodosSe analizaron los datos de 75 individuos (15 con sistema DBLG1, 9 con Control IQ y 51 con MM780 G) midiendo la satisfacción mediante un cuestionario validado para tratamiento en Diabetes Mellitus (DTSQc).
ResultadosTodos los sistemas mostraron satisfacción con el tratamiento. El sistema DBLG-1 obtuvo 14 (−15 – 21) puntos, mientras que Control IQ 21 (9–24) y MM780 G 19 (11–24) (p = 0,004). El análisis multivariante mostró que el sistema DBLG-1 se asocia con menor puntuación en DTSQc OR 0,75 (p = 0,019) independientemente del control glucémico, sexo, edad, años de evolución de la diabetes, tiempo como usuario de sistema de infusión continuo subcutáneo de insulina, y dosis diaria de insulina.
ConclusiónLos sistemas de AHCL son tratamientos satisfactorios para los usuarios, pudiendo haber diferencias entre cada sistema independientemente del buen control glucémico alcanzado.
Advanced hybrid closed-loop (AHCL) systems are the latest advance in automation aimed at achieving optimal blood glucose control in people with type 1 diabetes (DM1).
Currently in Spain, the National Health System allows funded access to several AHCL systems approved for adults: the Tandem t:slim X2 Control IQ™ system (Tandem Inc., San Diego, California, USA); the Minimed™ 780 G system (Minimed Medtronic, Northridge, California, USA); the Diabeloop Generation 1 (DBLG1®) system (Grenoble, France); and the CamAPS FX app (CamDiab, Cambridge, UK). These systems have consistently been shown to provide great benefits in blood glucose control and quality of life1,2 in DM1 compared to multiple dose insulin (MDI) therapy or insulin infusion systems with predictive "low-glucose suspend" system.3–5 However, there are no studies available comparing the three systems in adults, nor are there studies focusing on users' perceptions of these systems.
The aim of this study was to compare patient satisfaction with treatment and blood glucose control among users of Minimed 780 G (MM780 G), Tandem Control IQ and Diabeloop DBLG1® at six months after starting the therapy.
Material and methodsObservational study with retrospective follow-up of single-centre data conducted from September 2021 to October 2022. We selected 75 people over 18 years of age with DM1 under follow-up in a tertiary hospital in Madrid (Spain) with MM780 G, Control IQ or DBLG1® systems.
We included patients with a diagnosis of DM1 with at least one year since disease onset, on previous treatment with MDI, open-loop continuous insulin infusion systems or insulin infusion with predictive "low-glucose suspend" system. For indicating the closed-loop system, we used the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) guidelines, which recommend its use in motivated, technologically proficient individuals with DM1 who demonstrate basic skills in counting carbohydrate rations and blood glucose impact on fat and protein.6,7 The Diabetes Treatment Satisfaction Questionnaire change version (DTSQc)8 was administered to all patients six months after AHCL system placement. A negative result to the survey outcome variable was considered an unsatisfactory change, a 0 result was considered neutral and a positive result was considered satisfactory after the change of treatment. In order to avoid confusion, the numerical values of the items referring to hyperglycaemia and hypoglycaemia were reversed to maintain consistency between positive data and higher satisfaction and negative data and lower satisfaction. Blood glucose control data were obtained from continuous glucose monitoring and glycosylated haemoglobin (HbA1c) sensors at the time of the survey. In addition, glucometer and HbA1c control data prior to AHCL system placement were collected retrospectively for each patient. We compared the survey results six months after starting the treatment and the blood glucose results from the systems with each other and before and after AHCL system placement. Glycosylated haemoglobin was determined routinely by liquid chromatography (ADAMS A1c, HA-8180 V ARKRAY®).
Statistical analysisStatistical analysis was performed using STATA 17.0 BE-Basic Edition software (Lakeway Drive, College Station, Texas, USA) and R, version 4.3.0.
The categorical variables are expressed as numbers or percentages of the sample. Quantitative variables were tested for normal distribution using both statistical tests (Saphiro-Wilk) and graphs (normal probability plot).
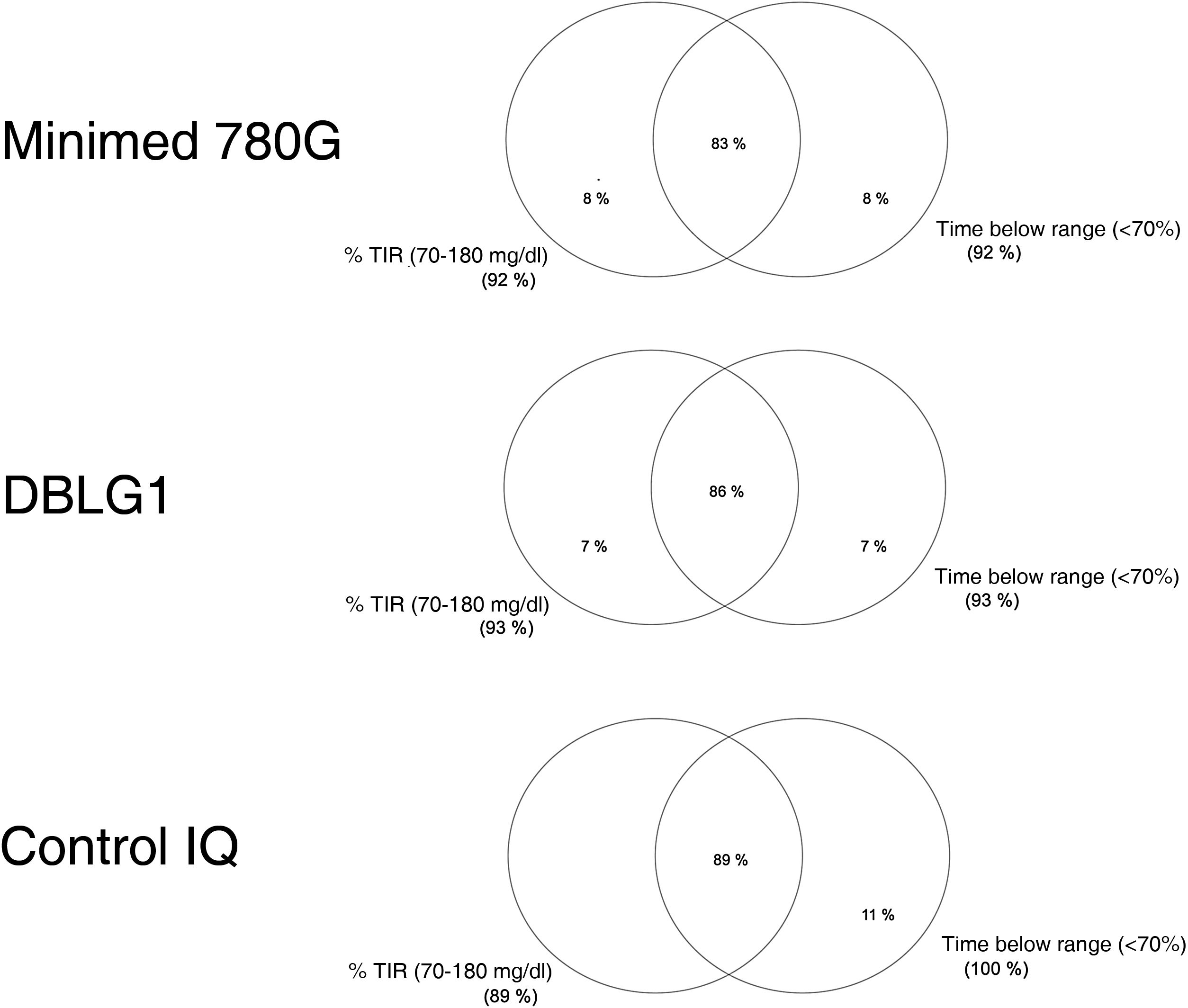
Normally distributed continuous variables are presented as mean and standard deviation (SD) while non-normally distributed continuous variables are shown as median and range. We calculated a blood glucose control variable known as "optimal control" defined as time in range (70−180 mg/dl) (TIR) >70% and time below range or in hypoglycaemia (<70 mg/dl) (TBR) <4%, as per the recommendations.9 Venn diagrams were made for the "optimal control" variable with the different AHCL systems.
Differences between groups were studied using ANOVA and Kruskal-Wallis tests in those with normal and non-normal distribution respectively. For the study of categorical variables, the chi-square test was used. To study patient satisfaction with the treatment change, a negative binomial regression model was performed using the DTSQc results as the dependent variable, and previous treatment, current AHCL system, blood glucose control, HbA1c, gender, age, time since disease onset and time on continuous subcutaneous insulin infusion system as covariates.
The ethics and clinical research committee of the Hospital Universitario de la Princesa approved this study (Study number: 2022–4997 - 17/222).
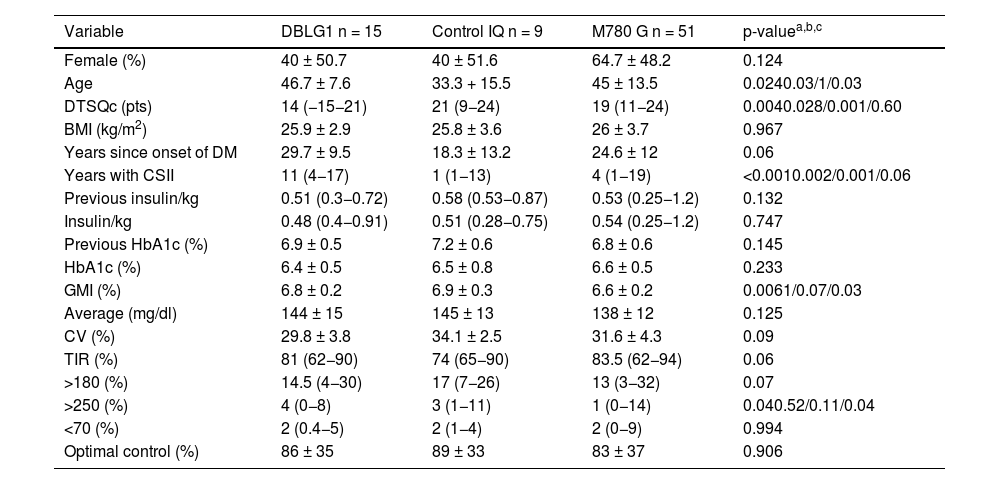
ResultsSeventy-five people with a mean age of 43.7 ± 14.4 years were included, 43 of whom (56.6%) were women. Time since onset of diabetes was 24.8 ± 11.9 years. The baseline characteristics of the sample distributed by each type of system are shown in Table 1.
Characteristics of the sample categorised and compared by each AHCL system six months after the start of treatment.
| Variable | DBLG1 n = 15 | Control IQ n = 9 | M780 G n = 51 | p-valuea,b,c |
|---|---|---|---|---|
| Female (%) | 40 ± 50.7 | 40 ± 51.6 | 64.7 ± 48.2 | 0.124 |
| Age | 46.7 ± 7.6 | 33.3 + 15.5 | 45 ± 13.5 | 0.0240.03/1/0.03 |
| DTSQc (pts) | 14 (−15−21) | 21 (9−24) | 19 (11−24) | 0.0040.028/0.001/0.60 |
| BMI (kg/m2) | 25.9 ± 2.9 | 25.8 ± 3.6 | 26 ± 3.7 | 0.967 |
| Years since onset of DM | 29.7 ± 9.5 | 18.3 ± 13.2 | 24.6 ± 12 | 0.06 |
| Years with CSII | 11 (4−17) | 1 (1−13) | 4 (1−19) | <0.0010.002/0.001/0.06 |
| Previous insulin/kg | 0.51 (0.3−0.72) | 0.58 (0.53−0.87) | 0.53 (0.25−1.2) | 0.132 |
| Insulin/kg | 0.48 (0.4−0.91) | 0.51 (0.28−0.75) | 0.54 (0.25−1.2) | 0.747 |
| Previous HbA1c (%) | 6.9 ± 0.5 | 7.2 ± 0.6 | 6.8 ± 0.6 | 0.145 |
| HbA1c (%) | 6.4 ± 0.5 | 6.5 ± 0.8 | 6.6 ± 0.5 | 0.233 |
| GMI (%) | 6.8 ± 0.2 | 6.9 ± 0.3 | 6.6 ± 0.2 | 0.0061/0.07/0.03 |
| Average (mg/dl) | 144 ± 15 | 145 ± 13 | 138 ± 12 | 0.125 |
| CV (%) | 29.8 ± 3.8 | 34.1 ± 2.5 | 31.6 ± 4.3 | 0.09 |
| TIR (%) | 81 (62−90) | 74 (65−90) | 83.5 (62−94) | 0.06 |
| >180 (%) | 14.5 (4−30) | 17 (7−26) | 13 (3−32) | 0.07 |
| >250 (%) | 4 (0−8) | 3 (1−11) | 1 (0−14) | 0.040.52/0.11/0.04 |
| <70 (%) | 2 (0.4−5) | 2 (1−4) | 2 (0−9) | 0.994 |
| Optimal control (%) | 86 ± 35 | 89 ± 33 | 83 ± 37 | 0.906 |
CV: coefficient of variation; GMI: glucose management indicator; CSII: continuous subcutaneous insulin infusion; TIR: time in range (70−180 mg/dl).
The variable "optimal control" is defined as the convergence of time in range (TIR) (70−180 mg/dl) and hypoglycaemia or time below range (TBR) (<70 mg/dl) <4%.
Fifteen subjects were users of the DBLG1 system, previously having used an open-loop continuous subcutaneous insulin infusion (CSII) system. An improvement in HbA1c was observed from 6.9% ± 0.5%–6.4% ± 0.5% (mmol/mol) (p < 0.001). Nine subjects were Control IQ users, all of whom had been previously treated with MDI. Improvement in HbA1c was observed from 7.2% ± 0.6%–6.5% ± 0.8% (p = 0.02). Fifty-one subjects were MM780 G users, 46 of whom had been were previously treated with Minimed 670 G and five with MDI. Previous HbA1c was 6.8% ± 0.6% and current HbA1c was 6.6% ± 0.5% (mmol/mol) (p < 0.001).
The rate of optimal control achieved (grouping of blood glucose targets) was 86% ± 35% in DBLG1, 83% ± 37% in MM780 G and 89% ± 33% in Control IQ™, with no statistically significant differences between them (p = 0.906) (Fig. 1).
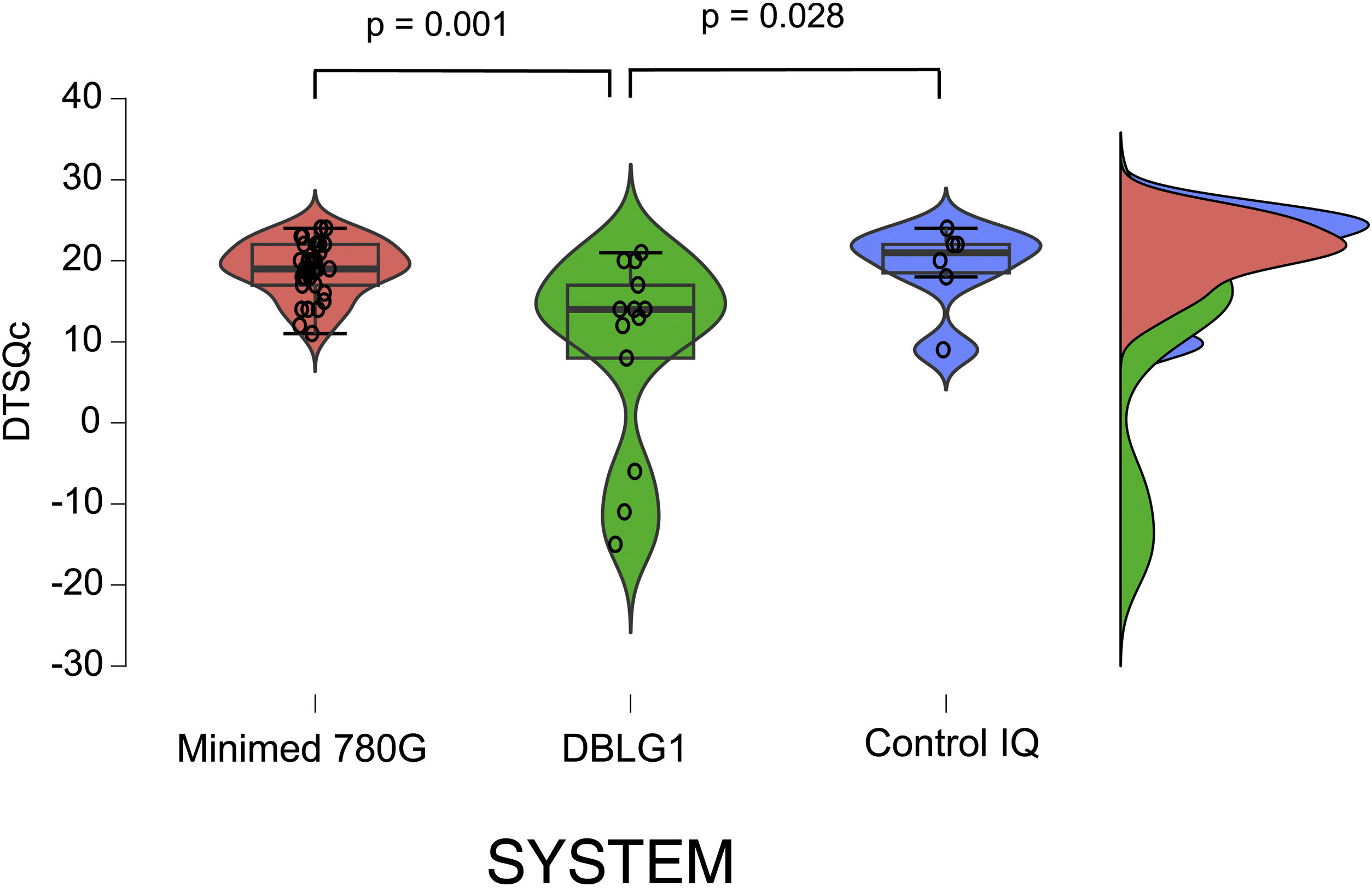
The results of the treatment change satisfaction survey (DTSQc) showed positive data for all three systems. DBLG1 scored 14 (−15−21) points, while Control IQ scored 21 (9−24) and MM780 G 19 (11−24) (p = 0.004). The differences between DBLG1 and the other two systems were also statistically significant when compared separately (Fig. 2). No statistically significant differences were found between Control IQ and MM780 G (p = 0.597).
Results of the DTSQc survey on the different AHCL systems.
All the systems scored positively in the DTSQc survey. Statistically significant differences were found between Minimed 780 G and DBLG1 (p = 0.001) and between DBLG1 and Control IQ (p = 0.028). No differences in score were found between Control IQ and Minimed 780 G (p = 0.60).
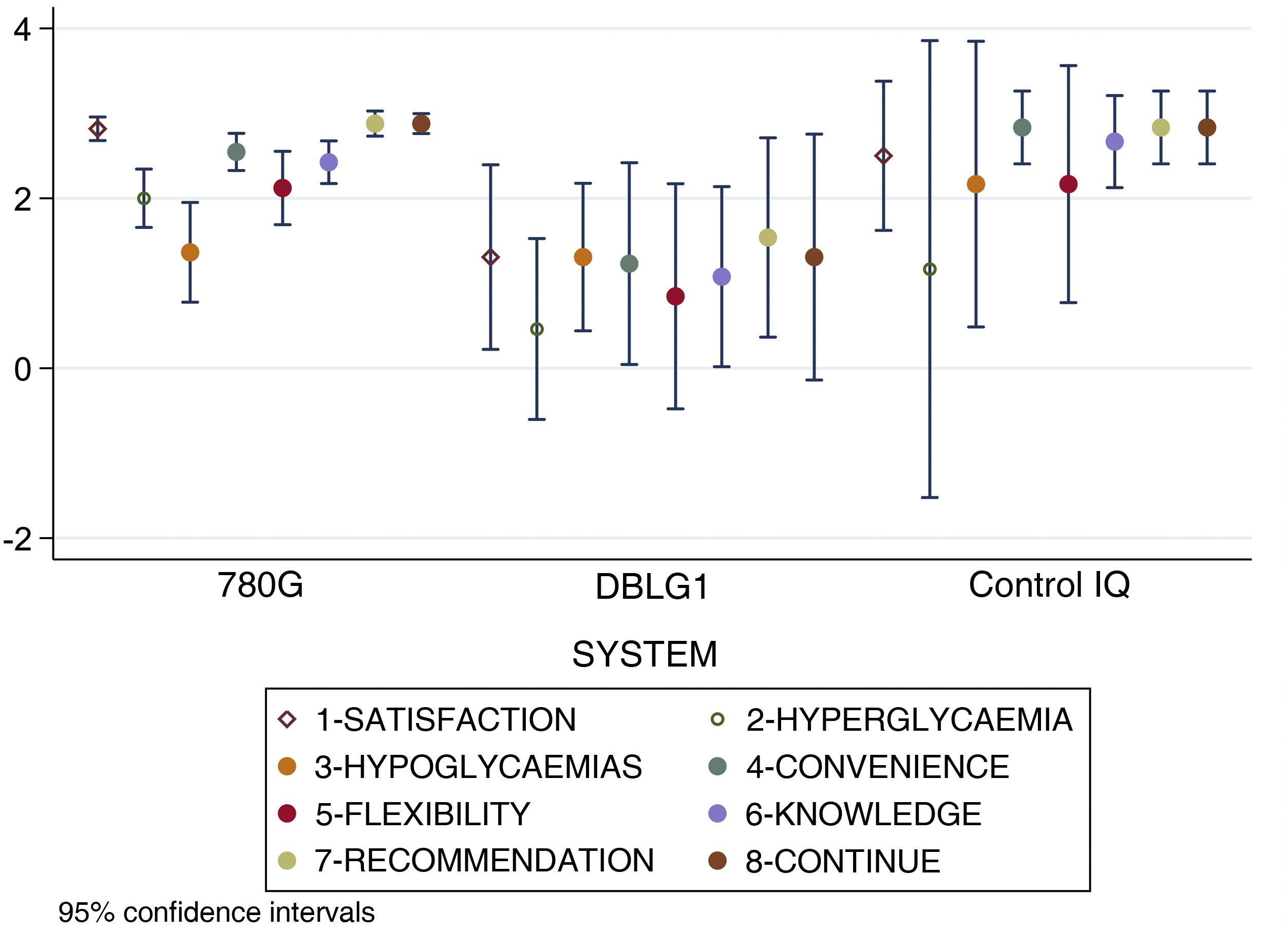
When analysing separately the 8 items that make up the DTSQc questionnaire, statistically significant differences were found in overall satisfaction with the treatment (p < 0.001), control of hyperglycaemia (p = 0.02), convenience (p = 0.002), ease of acquiring knowledge about the system (p = 0.003), recommendation to other users (p < 0.001) and desire to continue with their present system (p = 0.02). No differences were found in control of hypoglycaemia (p = 0.24) or flexibility for adapting to daily life (p = 0.09).
The score results for each item of the DTSQc survey broken down by AHCL system are shown in Fig. 3.
Results for each separate item of the DTSQc survey for the different AHCL systems. Statistically significant differences were found in overall satisfaction with treatment, control of hyperglycaemia, convenience, ease of learning about the system, recommendation to other users and desire to continue with the same system (p < 0.05). No differences were found in hypoglycaemic control and flexibility in adapting to daily life.
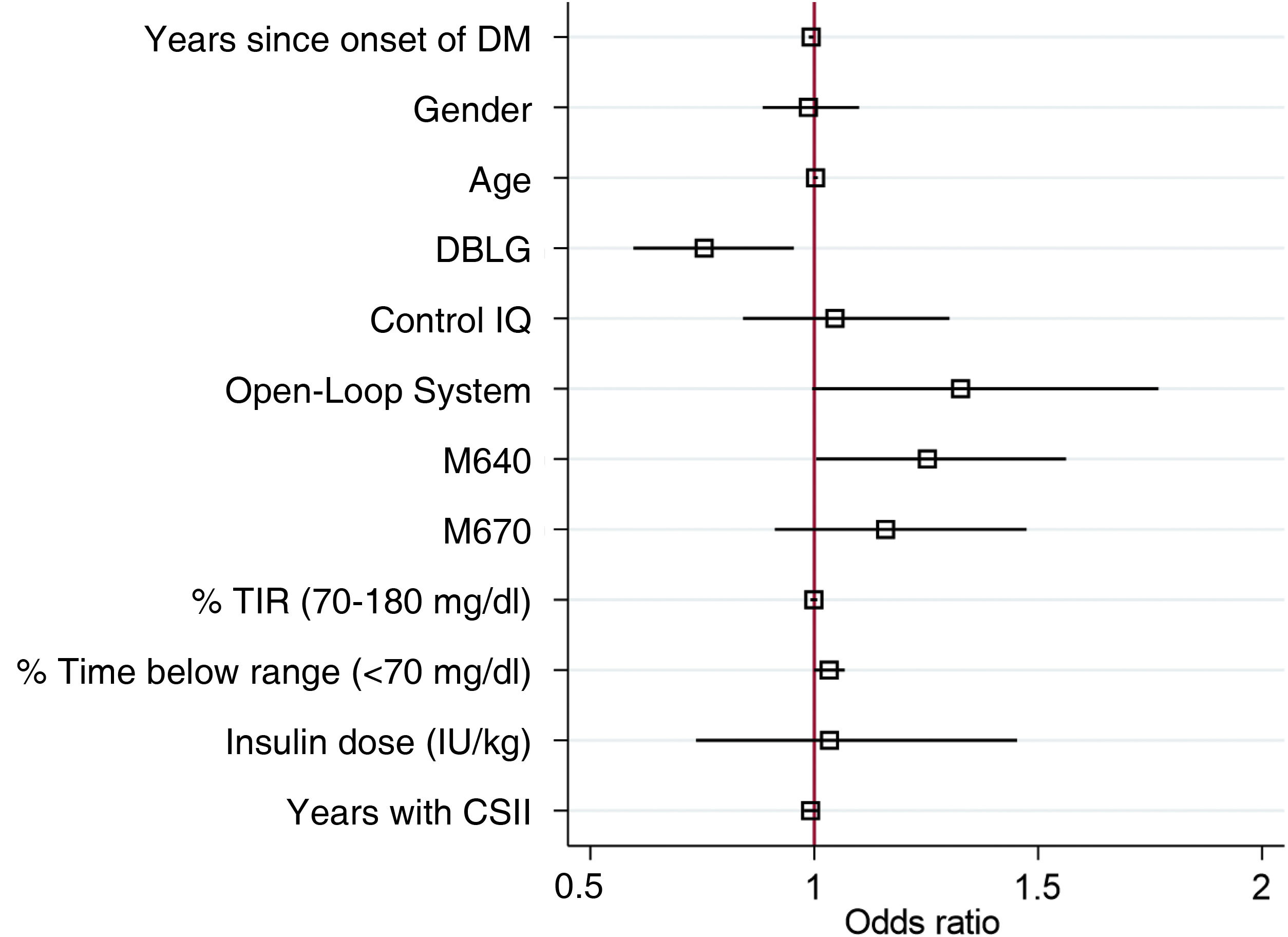
In the multivariate regression model for DTSQc survey score as a function of type of system used adjusted for age, gender, years since disease onset, previous treatment, time as an CSII user (years), time in range, time below range and insulin dose showed that the DBLG1 system (OR 0.75 95% CI [0.59−0.95] p = 0.019), years since disease onset (OR 0.99 95% CI [0.98−0.99] p = 0.011) and prior treatment with Minimed G640 (1.25 95% CI [1.00–1.56] p = 0.046) were factors associated with the DTSQc score independent of all other adjustment variables (Fig. 4).
Results of the DTSQc questionnaire adjusted for the different variables involved. The DBLG1 system (OR 0.75 95% CI [0.59−0.95] p = 0.019), time since disease onset (OR 0.99 95% CI [0.98−0.99] p = 0.011) and prior treatment with Minimed G640 (1.25 95% CI [1.00–1.56] p = 0.046) were shown to be variables which independently affected the DTSQc results.
DM: diabetes mellitus; CSII: continuous subcutaneous insulin infusion; TIR: time in range. In the categorical variables (gender and AHCL system), the references of comparison were being male, Minimed G780 system and previous treatment with multiple doses of insulin, so they are not represented in the graph.
The aim of this study was to compare patient satisfaction with the change in treatment among DBLG1, MM780 G and Control IQ users six months after starting with the new system.
Technological advances such as flash/continuous glucose monitoring have provided considerable improvements in blood glucose control10–12 and quality of life for people with diabetes.13–15 At the same time, sensor-augmented pump (SAP) continuous subcutaneous insulin infusion therapy has also been a step forward in the integration of blood glucose control, with satisfactory results in terms of quality of life and blood glucose control.16–18
AHCL systems are currently spearheading the automation of diabetes management. Available AHCL systems have demonstrated benefits in blood glucose control both in randomised clinical trials4,19,20 and in evidence from real-life over conventional therapy.21 Satisfaction with AHCL systems relative to other therapeutic modalities has been endorsed in recent years1 and, recently, the impact on blood glucose control has also been compared between different AHCL systems.22–25 However, despite the available information, there have been no studies to date comparing patient satisfaction with the different systems.
Our data from comparing the three AHCL systems in adults show that patients are satisfied with the change of treatment regardless of the system used. However, irrespective of the blood glucose control achieved (optimal in most users six months after starting the treatment), there were clear differences between each treatment group. As the American Diabetes Association points out,26 patient satisfaction with diabetes treatment is not always related to perfect blood glucose control. The focus of diabetes care should be on improving the patient's quality of life, addressing not only blood glucose targets, but also other important aspects such as education about the disease, comprehensive medical care, emotional support and empowering the patient to make informed decisions about their own health care.
Given the observational design of the study, our data can provide hypotheses without demonstrating causality. Although the results are adjusted for covariates in regression models, the difference in sample size between the users of the different systems may mean that there are differences that we failed to identify because of our design. Additionally, although the DTSQc is a validated and widely used questionnaire, it may be a generalised survey and not very specific to treatment with AHCL. Randomised clinical trials will be necessary to prospectively study the satisfaction with treatment of people using AHCL systems with specific surveys for these systems.
In summary, treatment with AHCL systems in DM1 is satisfactory for users, but there may be differences between the different systems regardless of the fact that they all provide good blood glucose control.
Ethical considerationsThis study was conducted in accordance with the principles of the Declaration of Helsinki.The ethics and clinical research committee of the Hospital Universitario de la Princesa approved this study (Study number: 2022-4997-17/222). Informed consent was obtained from all individual participants included in the study. In view of the observational nature of the study, all procedures performed were part of routine care.
FundingThis study did not receive any specific funding from public sector agencies, the commercial sector or non-profit organisations.
Authors' contributionsV.N.M. and A.A.M. came up with the concept of study and collaborated in the statistical analysis. V.N.M., M.A.S.N and F.S.V interpreted the data and drafted the manuscript. A.A.M. and M.M. critically reviewed the manuscript. V.N.M. and F.S.V. performed the statistical analysis and interpreted the data. M.L.V and M.M. interpreted the data and critically reviewed the manuscript. A.A.M. is the guarantor of this work and, as such, had full access to all data and assumes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interestAll authors declare that there are no conflicts of interest associated with their contribution to this manuscript.









![Results of the DTSQc questionnaire adjusted for the different variables involved. The DBLG1 system (OR 0.75 95% CI [0.59−0.95] p = 0.019), time since disease onset (OR 0.99 95% CI [0.98−0.99] p = 0.011) and prior treatment with Minimed G640 (1.25 95% CI [1.00–1.56] p = 0.046) were shown to be variables which independently affected the DTSQc results. DM: diabetes mellitus; CSII: continuous subcutaneous insulin infusion; TIR: time in range. In the categorical variables (gender and AHCL system), the references of comparison were being male, Minimed G780 system and previous treatment with multiple doses of insulin, so they are not represented in the graph. Results of the DTSQc questionnaire adjusted for the different variables involved. The DBLG1 system (OR 0.75 95% CI [0.59−0.95] p = 0.019), time since disease onset (OR 0.99 95% CI [0.98−0.99] p = 0.011) and prior treatment with Minimed G640 (1.25 95% CI [1.00–1.56] p = 0.046) were shown to be variables which independently affected the DTSQc results. DM: diabetes mellitus; CSII: continuous subcutaneous insulin infusion; TIR: time in range. In the categorical variables (gender and AHCL system), the references of comparison were being male, Minimed G780 system and previous treatment with multiple doses of insulin, so they are not represented in the graph.](https://static.elsevier.es/multimedia/25300180/0000007000000008/v1_202310300903/S2530018023001348/v1_202310300903/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)