One of the objectives of the Spanish Society of Arteriosclerosis is to contribute to better knowledge of vascular disease, its prevention and treatment. It is well known that cardiovascular diseases are the leading cause of death in our country and entail a high degree of disability and health care costs. Arteriosclerosis is a multifactorial disease and therefore its prevention requires a global approach that takes into account the different risk factors with which it is associated. Therefore, this document summarises the current level of knowledge and includes recommendations and procedures to be followed in patients with established cardiovascular disease or at high vascular risk. Specifically, this document reviews the main symptoms and signs to be evaluated during the clinical visit, the laboratory and imaging procedures to be routinely requested or requested for those in special situations. It also includes vascular risk estimation, the diagnostic criteria of the different entities that are cardiovascular risk factors, and makes general and specific recommendations for the treatment of the different cardiovascular risk factors and their final objectives. Finally, the document includes aspects that are not usually referenced in the literature, such as the organization of a vascular risk consultation.
La Sociedad Española de Arteriosclerosis tiene entre sus objetivos contribuir al mayor y mejor conocimiento de la enfermedad vascular, su prevención y su tratamiento. Es de sobra conocido que las enfermedades cardiovasculares son la primera causa de muerte en nuestro país y conllevan además un elevado grado de discapacidad y gasto sanitario. La arteriosclerosis es una enfermedad de causa multifactorial y es por ello que su prevención exige un abordaje global que contemple los distintos factores de riesgo con los que se asocia. Así, este documento resume el nivel actual de conocimientos e integra recomendaciones y procedimientos a seguir ante el paciente que presenta enfermedad cardiovascular establecida o se encuentra con elevado riesgo vascular. En concreto, este documento revisa los principales síntomas y signos a evaluar durante la visita clínica, los procedimientos de laboratorio y de imagen a solicitar de forma rutinaria o aquellos en situaciones especiales; igualmente, incluye la estimación del riesgo vascular, los criterios diagnósticos de las distintas entidades que son factores de riesgo cardiovascular, plantea recomendaciones generales y específicas para el tratamiento de los distintos factores de riesgo cardiovascular y sus objetivos finales. Por último, el documento recoge aspectos habitualmente poco referenciados en la literatura como son la organización de una consulta de riesgo vascular.
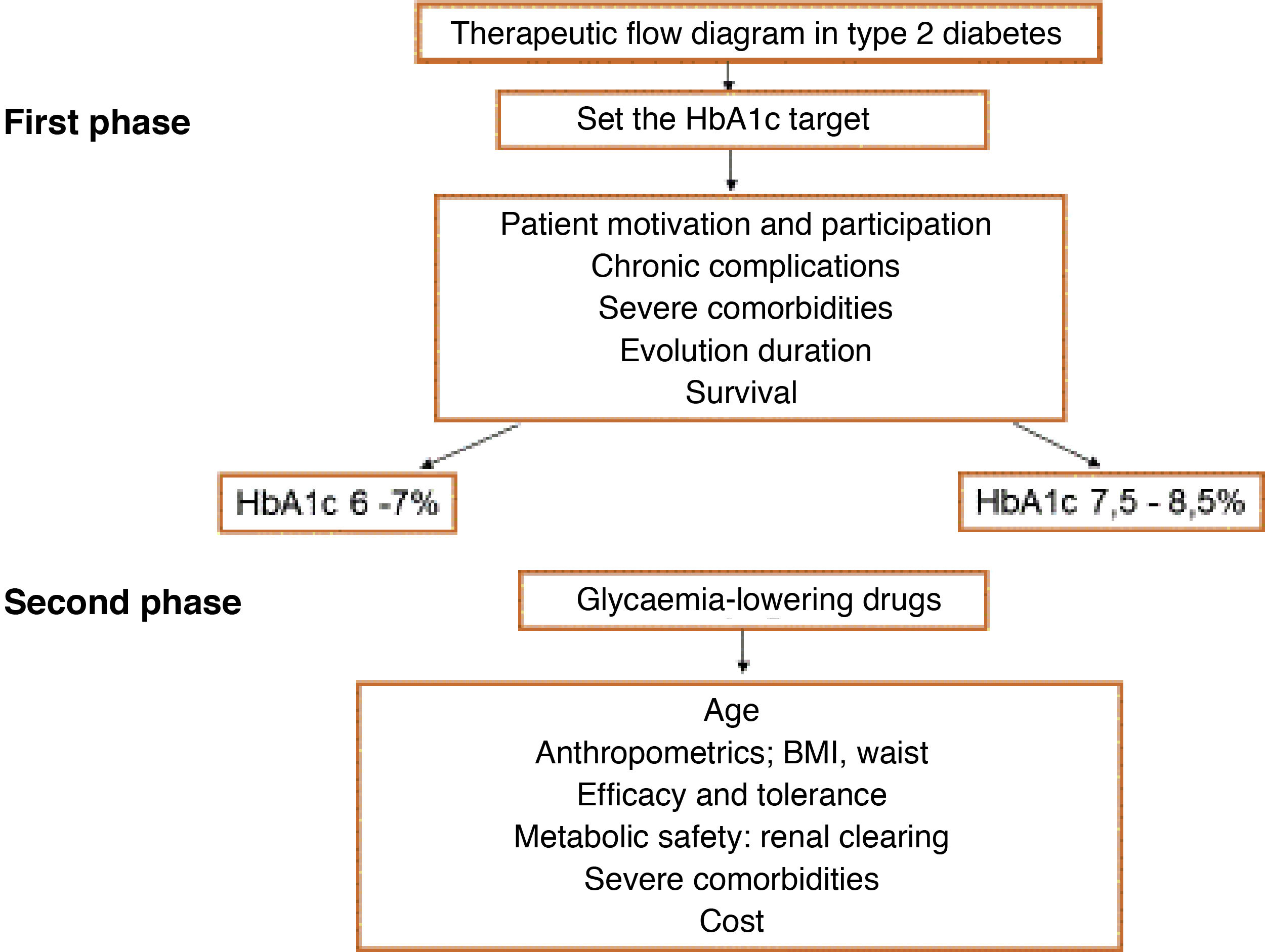
Medicine is a science that changes constantly. In recent years we have seen continuous progress in the diagnosis and treatment of cardiovascular (CV) disease and its risk factors, making it necessary to continuously update the therapeutic guides.
In the last decades the advance in the diagnosis and pharmacological treatment of arterial hypertension (AHT), hypercholesterolaemia and type 2 diabetes mellitus (DM2) has been spectacular. Although this has led to a greater degree of control, this is still a long way from optimum levels, and it is overshadowed by groups of patients with low adherence and medical groups with a high level of therapeutic inertia. Moreover, smoking is now less prevalent in certain subgroups of the population, and this is largely explained by the restrictions on consumption which came into force in recent years. However, expectations in other areas of cardiovascular prevention are not so positive; the increasing prevalence of metabolic syndrome (MS), obesity and diabetes mellitus (DM) at ever earlier ages indicates that there is still a long way to go, together with an opportunity to improve the prevention of atherosclerotic cardiovascular disease (ACVD), or at least to delay its appearance.
One of the aims of the Spanish Arteriosclerosis Society (SEA) is to contribute to improving the knowledge and control of cardiovascular risk factors (CVRF) in our country, especially dyslipidaemia, through its network of Lipid Units. This involves encouraging research and training activities. ACVD is multifactorial, so that it requires a strategy which aims to control all of the CVRF, including dyslipidaemia. The SEA has therefore decided to prepare these Standards for the Comprehensive Control of Cardiovascular Risk as a means of bringing together the scientific evidence and national and international recommendations about the main risk factors. We are sure that this document will both clarify and update diagnostic procedures, showing the actual utility or value for research purposes of a range of biochemical or imaging tests. It classifies vascular risk and treatments for the same, in terms of lifestyle and most especially diet, as well as pharmacological treatments.
As the first version of these Standards states, it was created with the intention that it would be regularly revised and updated. Changes have therefore been made based on new forms of evidence in connection with the diagnosis, clinical evaluation and treatment of different risk factors, as well as those contained in recently published Clinical Practice Guides. This document still has the same aim: to be of use for all of the clinicians who in one way or another treat patients who are at vascular risk, from primary care to hospitalization, in primary or secondary prevention, and in general, all of the members of the societies who belong to the Spanish Interdisciplinary Committee for Vascular Prevention (CEIPV). This document is also for professionals undergoing training, and not exclusively those in medical professions, and in particular for those doing basic research who are interested in the process of arteriosclerosis.
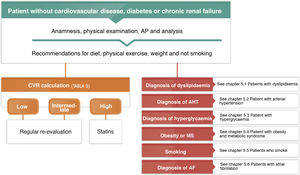
Anamnesis, examination and complementary tests in the surgeryA conventional clinical history and the ordered recording of patient signs and symptoms is the normal procedure by which the pathology is diagnosed. In this section we will centre on underlining the most important characteristics involved in this process. Table 1 shows the items which should be covered in a visit that includes the exploration of cardiovascular risk (CVR).
Requirements, anamnesis, examination and complementary tests for estimating vascular risk.
| Indispensable | Recommendable | |
|---|---|---|
| Anamnesis | – AF of early onset ACVD or CVRF | – Edinburgh questionnaire 1 (Annex 1) |
| – PH of ACVD (territory, form of presentation, date or age at event) | – Erectile dysfunction questionnaire (SQUED)2 (Annex 2) | |
| – PH of CVRF | – Fargenström smokers’ test4 (Annex 3) | |
| – Alcohol consumption and smoking | ||
| – Treatments, including treatment of CVRF: diabetes, AHT, dyslipidaemia | ||
| – Symptoms per apparatus (cardiological, intermittent claudication, erectile dysfunction) | ||
| Physical examination | – Anthropometry: weight, height, BMI, abdominal perimeter | – Test for corneal opacity and tonsillar hypertrophy |
| – Measure arterial pressure | – Funduscopic. | |
| – Central and peripheral pulses and vascular murmurs | ||
| – Cardiac examination | ||
| –Abdominal examination: hepatomegaly and splenomegaly | ||
| – Xanthomas, corneal arch | ||
| Complementary tests | – ECG | – OBPM or SBPM |
| – Lipid profile (TCh, HDL-c, triglycerides, non-HDL and LDL-cholesterol) | – Genetic tests for specific diagnoses | |
| – Apo B | – Apo E genotype | |
| – Hepatic profile (bilirubin, ALT, AST, GGT, FAL) | – Abdominal ultrasound scan. | |
| – Glycaemia, Na, K, Ca, uric acid | – Monofilament test | |
| – HbA1c | – Apo A1 | |
| – eGFR and Albuminuria | – Lipoparticles | |
| – TSH | ||
| – CPK | ||
| – Lp(a) | ||
| Diet and physical activity questionnaires | – General evaluation of adherence to diet and exercise | – Mediterranean diet score MEDAS5 (Annex 4) |
| – IPAQ6 Exercise questionnaire (Annex 5) | ||
| Study of subclinical cardiovascular disease | – AAI | |
| – Carotid and femoral ultrasound scan | ||
| – CAC |
AAI: ankle-arm index; AHT: arterial hypertension; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ACVD: cardiovascular disease; AP: arterial pressure; Apo A1: apolipoprotein A1; Apo B: apolipoprotein B; Apo E: apolipoprotein E; AST: aspartate aminotransferase; BMI: body mass index; Ca: calcium; CAC: coronary calcium; CPK: creatinine-phosphokinase; CVRF: cardiovascular risk factors; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; FH: family history; GGT: gamma glutamyltransferase; HbA1c: glycosylated haemoglobin; HDL-c: high density lipoprotein colesterol; K: potassium; Lp(a): lipoprotein (a); Na: sodium; OBPM: outpatient blood pressure measurement; PH: personal history; SBPM: self-blood pressure measurement; TSH: thyroid stimulating hormone.
It is necessary to know first and second degree family history regarding prevalent diseases that are associated with ACVD as well as risk factors, above all in cases where there is a suspicion of familial hypercholesterolaemia (FH) or early onset ACVD. Family histories are of greatest value when they correspond to first-degree relations (father, mother, children or siblings), together with those at early ages, under 55 years in men and 65 years in women.
Personal historyAs well as the conventional items in a personal history (PH) (allergies and surgical operations, etc.) specific questions should be asked about a history of ACVD and the most important CVRF (DM, AHT, dyslipidaemia, smoking and obesity). If any of these are present the age of onset should be recorded together with any treatments, regardless of their indication. Adverse reactions to or intolerance of medication and the existence of pregnancy or the possibility of the same should also be known. The degree and chronology of CVRF should be quantified (number of cigarettes per day and years smoking, maximum levels of LDL cholesterol (LDL-c), glycosylated haemoglobin (HbA1c) and systolic arterial blood pressure (SAP) or weight). Likewise, the presence of systemic diseases involving low grade inflammation should be recorded, such as psoriasis, human immunodeficiency virus (HIV), rheumatoid arthritis or systemic erythematosus lupus, as well as any neoplasias, as they or their treatment increase vascular risk. Any history of AHT or gestational DM should also be recorded for women, together with polycystic ovary syndrome, the date menopause started and any hormonal treatments.
Current and systemic anamnesisThe reason for visiting should be examined, as visits to the Vascular Risk unit are usually due to a lack of control of one or more CVRF. The patient should be asked about the symptoms associated with ischemic events in the three most important territories that may have gone unnoticed or have yet to be diagnosed: transitory neurological deficits, thoracic pain with effort, palpitations, dyspnoea or intermittent claudication. Questioning should also cover the cardinal symptoms of DM, cephalalgia or dizziness associated with higher arterial blood pressure (AP), symptoms associated with processes that cause secondary AHT (Table 2) and asymptomatic raised lipid levels that are exceptionally associated with xanthomas. If the patient has received the relevant instructions, it would be advisable to record their outpatient self-measurements of AP.
Symptoms and signs which suggest secondary AHT.
| – Paroxysmal raised AP or established AHT with added crises, and the classic triad of cephalalgia, sweating and palpitations (pheochromocytoma). |
| – Rapid progressive worsening of pre-existing AHT. |
| – Presence of arterial murmurs with suspicion of vasculorrenal AHT. |
| – Snoring and hypersomnia (sleep apnoea). |
| – Prostatic symptoms (obstructive IRC). |
| – Muscle cramps, weakness (hypopotassaemia due to hyperaldosteronism). |
| – Oedemas, asthenia, tenesmus and pollakiuria (renal disease). |
| – Central obesity, full moon face, ecchymosis, stretchmarks (Cushing syndrome). |
| – Drugs or drugs of abuse (alcohol, NSAID, cocaine, amphetamine, liquorice, topical corticoids, etc.). |
| – No family history of AHT. |
| –AHT appears in young subjects (< 35 years). |
| – Resistant/refractory AHT: requiring more than three or five drugs, respectively, to be controlled. |
| – Paradoxical response to beta-blockers. |
The patient’s weight, height and abdominal perimeter should be recorded, and their body mass index (BMI) should be calculated. AP should be measured according to the recommendations contained in Table 3. Basic cardiac and circulatory examination is obligatory, especially in the presence of heart murmurs and depending on the magnitude of the arterial pulses; the interpretation of findings will depend on the context: the absence of pulses in the feet may indicate an elderly patient with claudication and peripheral arterial disease (PAD), while pulse asymmetry in a young hypertensive patient may indicate aortic restriction. The findings of hepatomegaly and/or splenomegaly should be recorded. The presence of xanthomas and their morphology and location are often prime diagnostic factors. Indicative examples of this are that tendon xanthomas suggest FH, tuberous-eruptive ones indicate chilomicronaemia and striated xanthomas on the palms are characteristic of dysbetalipoproteinaemia. The presence of hard xanthomas adhered to bone surfaces suggests cerebrotendineous xanthomatosis.
Recommendations for the measurement of AP and the diagnosis of AHT.
| Measuring AP |
| – Patient sitting for 3−5 min before starting AP measurements. |
| – Measure at least twice, in sitting position and without crossing the legs, separated by 1−2 min, and take additional measurements if the first two are very different. Recording the average of the last two measurements. |
| – Take more measurements if the patient has arrhythmia (atrial fibrillation, for example). |
| – Use a standardized sleeve: 12−13 cm wide and 35 cm long, but have a longer one available in case of arm circumference >32 cm. |
| – The sleeve must be located at the height of the heart; the back of the hand and arm should be supported on a table to prevent isometric muscle contraction affecting AP. |
| – Bilateral measurement is recommended (simultaneous measurement of AP in both arms on the first visit, especially in high vascular risk patients. In subsequent visits use the arm with the highest AP levels as the reference: a difference between the two arms greater than > 10−15 mmHg is associated with increased CVR. When there is a major difference (≥20 mmHg) stenosis of the subclavian artery should be suspected. |
| – After five minutes resting in supine decubitus, measure AP and then again after 1 and 3 min standing: this should be done in the first visit or when there is a clinical suspicion of orthostatic hypotension, especially in patients who are elderly or diabetic, or neurological patients with neurodegenerative disorders. If the fall in SAP/DAP is ≥20/10 mmHg, it is advisable to also measure AP after five minutes in supine decubitus to rule out associated supine AHT. |
| The diagnosis of AHT |
| – The diagnosis of AHT (especially grade 1 AHT) is established after checking AP in two or more measurements taken in one, two or more occasions separated by several weeks. We recommend confirming the diagnosis by outpatient measurements of AP: SBPM or OBPM during 24 h. |
Blood analysis is an important part of the evaluation of vascular risk and the diagnosis of dyslipidaemia. The optimum conditions for sample extraction, processing and evaluation have been published in the form of consensus documents by the European arteriosclerosis and laboratory medicine societies,11 and these may be consulted in Appendix A Annex 7.
Analysis should include a complete lipid profile (total cholesterol (TCh), triglycerides (TGS), HDL cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c) [estimated using Friedewald’s formula or by the direct method] and calculation of non-HDL cholesterol if TGS levels are raised). If the necessary facilities are available, it is useful to determine Apolipoprotein B (Apo B), because this may aid screening for dysbetalipoproteinaemia and differentiate it from the more common form, which is combined familial hyperlipidaemia.12 Raised lipoprotein (a) [Lp(a)] plays a relevant role in the increased vascular risk shown by some patients with FH, and in subjects with early onset or recurring ischemic disease, in spite of their good control of the other CVRF. Determination of the levels of Lp(a) is advisable at least in the first analytical sample from the patient, especially in cases of early onset atherosclerosis.
The first visit by the patient should include a request for a conventional haemogram and biochemical blood tests which include the glycaemic profile (fasting glycaemia, HbA1c), renal and hepatic functioning, as well as levels of creatinine-phosphokinase (CPK), Na, K, Ca, uric acid and thyroid-stimulating hormone (TSH). For the urine sample, which preferentially should be given on waking in the morning, the urine albuminuria/creatinine coefficient should be requested. It is necessary to measure protein in urine to rule out nephrotic syndrome. Given that the risk of hepatic toxicity due to treatment is exceptional,13 no systematic monitoring of transaminases during treatment is recommended, except when the dose is increased14 (EAS/ESC 2019). A resting electrocardiogram (ECG) will offer valuable information in patients who are evaluated for AHT.
Anamnesis, examination and complementary tests in the surgery: recommendable itemsAnamnesis: the Edinburgh questionnaire and erectile dysfunction questionnaireSmoking should be covered by a specific anamnesis which includes the Fargeström test for smokers (Appendix A Annex 3), (see Patients who are smokers). If there is any suspicion of intermittent claudication, the Edinburgh test as validated for our country (Appendix A Annex 1), makes it possible to further support the clinical diagnosis of PAD.1 The erectile dysfunction evaluation questionnaire (SQUED) is shown in Appendix A Annex 2.
Physical examinationPatients with very low high-density lipoprotein cholesterol (HDL-c) should be specifically examined for the presence of corneal opacity (lecithin-cholesterol acyltransferase [LCAT] deficit) or tonsillar hypertrophy (Tangier disease). Ophthalmoscopy supplies valuable information when examining patients with DM, in cases of primary hyperchylomicronaemia (lipemia retinalis) and in AHT target organ lesions. It is indispensable I grade 3 AHT 3 (SAP ≥ 180 mmHg and/or diastolic arterial pressure (DAP) ≥ 110 mmHg).
Additional complementary testsIf there is a suspicion of FH15 then the clinical and biochemical scale Diagnostic criteria for the clinical diagnosis of HeFH according to MedPed and WHO1 (Appendix A Annex 6) should be applied, prior to confirmation by genetic diagnosis. Massive sequencing procedures and the commercialization of genetic panels for hypercholesterolaemia make it possible to diagnose this condition, differentiating between heterozygotic, compound heterozygotic, double heterozygotic and homozygotic types (as they may overlap in clinical or analytic terms) or the finding of other diseases that may share the phenotype (lysosomal acid lipase deficiency). The apolipoprotein E (Apo E) genotype should be requested when there is a suspicion of dysbetalipoproteinaemia. Although beta quantification (by ultra-centrifuging) may be of interest to confirm dysbetalipoproteinaemia (very low-density lipoprotein cholesterol coefficient (VLDLc)/TGS in mg/dL > 0.3) and to know the composition of lipoproteins in plasma, use of this technique is restricted due to its high cost and complexity. A low concentration of Apo B combined with the presence of hyperlipidaemia gives rise to the suspicion of dysbetalipoproteinaemia. It is recommended that apolipoprotein A1 (Apo A1) be determined in the study of childhood hypercholesterolaemia. An ApoB/ApoA1 level higher than 0.82 has been shown to be more sensitive and specific in the detection of a genetic mutation for FH.16
The SEA understands that it is advisable to measure lipoparticles when there is: a) The suspicion of a lack of fit between the lipid concentration and the number of particles. This often occurs in DM, obesity and MS; b) Early onset or recurring ACVD, without CVRF that would explain this; c) Rare or complex lipid disorders, such as extreme concentrations of HDL-c, and d) Clinical situations where it is impossible to apply classic analytical techniques, such as when there are very low concentrations of LDLc.17
Of the complementary tests, outpatient monitoring of arterial blood pressure (OBPM) during 24 h is especially indicated when there is highly variable discordance between measurements of AP in the surgery and outside the clinical context when nocturnal AHT is suspected (sleep apnoea) and in cases of resistant AHT.18 Standardized self-measurement of AP at home (SBPM) during five to seven days may substitute OBPM, especially during follow-up and if good agreement has been found between them.
Study of subclinical vascular diseaseThe tests described in this section have the sole purpose of re-evaluating the CVR of a subject, as they target patients without established ACVD or symptoms that lead to the suspicion of ACVD. They cover a period within the natural history of the atherosclerotic process during which detectable structural alterations exist in the vessels without any signs and symptoms. This process can by definition only be detected by specific diagnostic tests. CVRF as well as atheromatous disease are systemic, so that finding vascular involvement in one territory will also offer information on the state of the disease in the other territories. The available exploratory techniques should not be invasive, and they should be used to obtain complementary information in the estimation of CVR, to redefine lipid targets and to guide therapeutic decisions.19 It has been suggested that one of these techniques should be used in systematic screening. The following techniques are the ones used the most often to diagnose subclinical ACVD:
The ankle arm indexThe ankle arm index (AAI) is the coefficient of the systolic pressures in the ankle and arm for each lower limb. A value below 0.9 indicates the existence of stenosis greater than 50% between the aorta and the distal arteries of the leg, with a high degree of specificity (90%) and acceptable sensitivity (79%); this makes it possible to identify significant PAD that may develop silently or with poorly defined symptoms. Values ≥1.4 usually indicate the presence of arterial calcification, which is associated with an increased risk of cardiovascular complications and is especially common in diabetic patients. Due to its simplicity the AAI may be applied systematically when evaluating the vascular condition of a patient, on condition that a mini-ultrasound Doppler scanner and 15 min for determination are available.
AAI measurement is not justified in low-risk patients as it is hardly useful in them,20 while it is of maximum usefulness in subjects with the two main risk factors associated with PAD; DM and smoking. In Spanish series, up to 25% of patients with DM2 and without claudication had an AAI < 0.9.21 In patients with long-term DM and a high probability of microangiopathy (who can be identified with the monofilament test), the AAI is not sensitive enough to detect cases of PAD due to the high prevalence of calcification, which masks the measurement.
Atheromatous plaques in the carotid arteryAlthough the quantification of carotid artery intima-media thickness measured by ultrasound imaging has been widely used to evaluate the evolution of the arteriosclerotic process, and has also been used to measure the benefit of treating hypercholesterolaemia, this technique is not currently recommended for the re-evaluation of CVR. This is not the case for the existence of a carotid plaque.19
A carotid plaque is defined as localised thickening greater than 50% of the vessel wall around it, or intima-media thickness greater than 1.5 mm which protrudes into the adjacent opening.22 The presence of plaque is not evaluated alone, as the number of the same is also taken into account, with their size, irregularity and echodensity. These characteristics are associated with the risk of cerebral and coronary cardiovascular complications.
Coronary calciumThoracic computerised axial tomography (CAT) makes it possible to quantify the existence of coronary calcium, which is expressed in Agatston units.23 The presence of coronary calcium indicates an advanced phase of coronary arteriosclerosis, and it is a better predictor of ischemic events than the presence of carotid or femoral plaque.24,25 A score of at least 300 u Agatston higher than the 75 percentile for age, sex and race is the threshold used to define high CVR. This complementary information is used to evaluate the degree to which patients are at risk, especially those with intermediate risk.26 When no calcification is present (Agatston score = 0), the probability of an obstructive coronary lesion is almost zero; while the risk of cardiovascular complications increases with the degree of calcification.26 The score rises inexorably with age, and although repeating the measurement after a few years updates the risk assessment, which corresponds to the latest examination, the changes that are observed rarely modify the preventive or therapeutic attitude.27 The European guides19 consider that coronary calcifications modify risk when they surpass 100 u Agatston. This technique is mainly limited by its cost and, in the past, the risk associated with radiation, although new low radiation fast scans have minimized the latter risk.28 Computed tomographic angiography shows subclinical stenotic coronary disease, and it is able to offer information over and above the classic risk factors, especially in cases of diabetes and patients with a long-term FH.29,30 The information it supplies is independent of the data corresponding to coronary calcium, which still identifies patients at higher risk, even in the absence of stenosis.31
Dietary and physical exercise questionnaires: recommended scalesTo go beyond checking on general information about diet, such as if it is rich in carbohydrates or saturated fats, or whether there are alterations in eating habits, it can be assessed using a simple 14-question questionnaire, the Mediterranean diet adherence screener (MEDAS) (Appendix A Annex 4). This has been validated by the prevention with the Mediterranean diet trial (PREDIMED) and it is associated with the presence of CVRF and with CVR.32 Alcohol consumption should be quantified, and this can be done by recording the amount (volume in mL) of beer, wine and/or liquor consumed per week and calculating the number of grams of alcohol consumed, estimating a score of 6, 12 and 40 degrees, respectively, using the formula [volume in mL x degrees proof x 0.8]/100.
Physical activity during work can be assessed semi-quantitatively (1 = does not work or has a sedentary job; 2 = walks regularly while at work; 3 = walk regularly and lifts weights, and 4 = major physical activity) as well as during leisure time (1 = does not exercise; 2 = walks during at least four hours per week; 3 = walks > four hours per week, and 4 = trains vigorously).33 Lastly, physical activity can be quantified simply by using the International physical activity questionnaire (IPAQ), which has also been validated6 and is available online34 (Appendix A Annex 5).
Indications for special testsSome biomarkers have been widely studied as CVR predictors (homocysteine, A2 lipoprotein-associated phospholipase, thrombogenic factors), although they have not been included in clinical routine as they offer no additional relevant information on the CVR of specific patients. These biomarkers as a whole lack clinical justification, as they do not make it more possible to predict events in comparison with the European Systematic coronary risk evaluation (SCORE).18 The role of the biomarker which has been studied the most of all is more controversial. Reactive protein C is ultrasensitive, and several studies have demonstrated its predictive power for CVR. Determining the level of reactive protein C in epidemiological studies makes it possible to detect patients who may have a residual risk independently of their lipid parameters. However, it has the disadvantage of a high level of intra-individual variability, which hinders its use in clinical practice.35
The relevant complementary tests should be requested if there are suspicious signs or symptoms, or if disease is suspected: ergometry if there is thoracic pain, or imaging tests if secondary AHT is suspected, as well as hormonal tests, etc.
The diagnosis of cardiovascular riskRecording diagnoses in the clinical history: diagnostic criteriaThe clinical history of all patients who are seen in a lipids unit should include a list of standardized diagnoses which includes those shown in Table 4. All of the diagnoses deriving from the diseases they may have should also be added, whether these are cardiovascular or not.8,36–44
Diagnostic criteria.
| Diagnosis | Definition |
|---|---|
| Hypercholesterolaemia | – In secondary prevention (coronary, cerebrovascular or peripheral arterial disease) or if obstructive plaque exists in the carotid or coronary arteries: LDL-c > 55 mg/dL or Non-HDL-c > 85 mg/dL. |
| There is no ideal level for total cholesterol or LDL-c, given that the lower its concentration, the better. Cholesterol is considered to be raised when it reaches levels over which treatment to lower it is recommended. These levels depend on the basal risk of each individual (Table 5). Non-HDL-c would be used in patients with TGS > 400 mg/dL or those with diabetes. | – In type 2 diabetes, con TOL, EVS or with 3 or more risk factors: LDL-c > 55 mg/dL or Non-HDL-c 85 mg/dL. |
| – In type 2 diabetes, without TOL or EVS and with fewer than 3 risk factors: LDL-c > 70 mg/dL or Non-HDL-c > 100 mg/dL. | |
| – In patients with grade 3 CRD, without TOL or EVS: LDL-c > 70 mg/dL or Non-HDL-c > 100 mg/dL. | |
| – In patients with grade 3 CRD, with TOL or EVS: LDL-c > 55 mg/dL or Non-HDL-c > 85 mg/dL. | |
| – In patients with grade 4 or 5 CRD: LDL-c > 55 mg/dL or Non-HDL-c > 85 mg/dL. | |
| – In patients without cardiovascular disease, DM or CRD, LDL-c > 100−116 mg/dL depending on whether their risk if low or moderate, according to SCORE. | |
| Hypertriglyceridaemia | – Desirable TGS level <150 mg/dL |
| – Hypertriglyceridaemia: | |
| – Mild: 150−499 mg/dL | |
| – Moderate 500−000 mg/dL | |
| – Severe: >1000 mg/dL | |
| Mixed hyperlipidaemia | High concentrations of LDL-c or Non-HDL-c, as well as TGS. |
| Familial hypercholesterolaemia | Use the tables in the Diagnostic criteria for the clinical diagnosis of HeFH according to MedPed and WHO1 (Annex 6). |
| Combined familial hyperlipidaemia | ApoB > 120 mg/dL and TGS > 150 mg/dL in at least two members of the family and a family history of premature symptomatic ACVD. No xanthomas. Causes of secondary dyslipidaemia should be excluded. |
| Atherogenic dyslipidaemia | Hypertriglyceridaemia (TGS > 150 mg/dL) and low HDL-c (<40 mg/dL in men and <45 mg/dL in women). Increased numbers of small dense LDL particles |
| Hypoalphalipoproteinaemia | – HDL-c below percentile 10 according to age, race and sex. |
| Hyperlipoproteinaemia (a) | Lp(a) ≥ 50 mg/dL. |
| AHT (measured in the surgery) | – Optimum AP < 120 y <80 mmHg |
| – Normal 120−129 and 80−84 mmHg | |
| – Normal-high 130−139 and/or 85−89 mmHg | |
| – Grade I AHT: SAP 140−159 and/or DAP 90−99 mmHg | |
| – Grade II AHT: SAP 160−179 and/or DAP 100−109 mmHg | |
| – Grade III AHT: SAP ≥ 180 and/or DAP ≥ 110 mmHg | |
| – Isolated systolic AHT: SAP ≥ 140 and DAP < 90 mmHg | |
| Diagnosis is established after checking AP levels using at least two measurements in two or more visits separated by several weeks. | |
| When the SAP and the DAP are in different categories then the highest category will be applied. | |
| Isolated systolic AHT is classified in grades (1, 2 or 3) according to the level of systolic AP. | |
| Diabetes | Glycaemia after fasting for at least 8 h ≥ 126 mg/dL (7.0 mmol/L),* or |
| Glycaemia at 2 h after an OGTT of 75 g ≥ 200 mg/dL (11.1 mmol/L)*, or | |
| HbA1C ≥ 6.5 % (48 mmol/mol), or | |
| A patient with classic symptoms of hyperglycaemia with glycaemia ≥ 200 mg/dL independently of their fasting status (11.1 mmol/L).* | |
| *In the absence of unmistakable hyperglycaemia the results should be repeated, with a second analysis. | |
| Prediabetes | The presence of: |
| – Altered fasting glycaemia: fasting glycaemia from 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L), or | |
| – Glucose intolerance: glycaemia 2 h after OGTT of 75 g, from 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L), or | |
| – HbA1c from 5.7 to 6.4 % (39–47 mmol/mol). | |
| Obesity | BMI ≥ 30.0 kg/m2 |
| Grade I obesity 30.0−34.9 kg/m2 | |
| Grade II obesity 35.0−39.9 kg/m2 | |
| Grade III obesity ≥ 40 kg/m2 | |
| Overweight | BMI ≥ 25.0 kg/m2 to <30.0 kg/m2 |
| Grade I 25.1−27.5 kg/m2 | |
| Grade II 27.6−30.0 kg/m2 | |
| Normal weight | BMI 18.50−24.9 kg/m2 |
| Low weight | BMI < 18.5 kg/m2 |
| – Extremely thin < 16.0 kg/m2 | |
| – Moderately thin 16.0−16.9 kg/m2 | |
| – Slightly thin 17.0−18.4 kg/m2 | |
| Metabolic syndrome | 3 of the following 5 criteria have to be fulfilled: |
| 1. High abdominal perimeter (≥94 cm in men and ≥80 cm in European women). | |
| 2. TGS ≥ 150 mg/dL (1.7 mmol/L) or under treatment with TGS lowering drugs. | |
| 3. HDL-c < 40 mg/dL (1.0 mmol/L) in men or <50 mg/dL (1.3 mmol/L) in women, or under treatment with HDL-c raising drugs. | |
| 4. AP ≥ 130/85 mmHg or under treatment with drugs to reduce AP. | |
| 5. Fasting glycaemia ≥ 100 mg/dL or under treatment with antidiabetic drugs. | |
| Current smoker42 | An individual who has smoked at least one cigarette in the past six months. The following may be differentiated under this heading: |
| – Daily smoker: someone who has smoked at least one cigarette per day during the past six months. | |
| – Occasional smoker: someone who has smoked at least one cigarette per day. | |
| Quantification of cigarette consumption (packs per year index): (No. of cigarettes smoked per day x No. of years as a smoker)/20. | |
| Ex-smoker | Someone who having smoked before has abstained completely for at least the last six months. |
| Never smoked | Someone who has never smoked or who has smoked fewer than 100 cigarettes in their whole life. |
| Passive smoker | Someone who does not smoke, but who habitually breathes others’ tobacco smoke or second-hand or ambient smoke. |
| Target organ lesion | –Arterial rigidity: Pulse pressure (in the elderly) ≥ 60 mmHg or Pulse Wave Velocity > 10 m/s. |
| – Left ventricular hypertrophy: | |
| - in the ECG (Sokolow–Lyon Index > 3.5 mV; RaVL > 1.1 mV; voltage product by Cornell duration > 2440 mV × ms), or | |
| - Echocardiographic imaging (left ventricular mass > 115 g/m2 in men or > 95 g/m2 in women per body surface area). | |
| – Microalbumnuria (30–300 mg/24 h.) or albuminuria/creatinine coefficient (30–300 mg/g) or macroalbuminuria (>300 mg/24 h.) | |
| Subclinical vascular disease | Presence of: |
| – AAI < 0.9 (some authors consider a value > 1.4 to also be pathological), or | |
| – At least one plaque in the epicardial coronary, carotid or femoral artery, or | |
| –CAC quantification: Agatston ≥ 300 units |
| GFR (ml/min/1.73 m2) in CRD | Grade | GFR | Definition |
| G1 | ≥ 90 | Normal | |
| G2 | 60−89 | Slight fall in GFR | |
| G3a | 45−59 | Slight to moderate fall in the GFR | |
| G3b | 30−44 | Moderate fall in the GFR | |
| G4 | 15−29 | Severe fall in the GFR | |
| G5 | <15 | Renal failure (predialysis) | |
| Albuminuria categories (albuminuria/ creatinine coefficient in mg/g) in CRD | A1 | <30 | Normal |
| A2 | 30−300 | Moderately raised | |
| A3 | >300 | Very high |
AAI: ankle-arm index; ACVD: atherosclerotic cardiovascular disease; AHT: arterial hypertension; AP: arterial pressure; Apo B: apolipoprotein B; BMI: body mass index; CAC: coronary calcium; CRD: chronic renal disease; DAP: diastolic arterial pressure; DM: diabetes mellitus; ECG: electrocardiogram; GFR: glomerular filtration rate; HbA1c: glycosylated haemoglobin; HDL: high density lipoproteins; HDL-c: HDL cholesterol; LDL: low density lipoproteins; LDL-c: LDL cholesterol; Non-HDL-c: non-HDL cholesterol; OGTT: oral glucose tolerance test; RaVL: R wave voltage in the aVL lead; SAP: systolic arterial pressure; SVD: subclinical vascular disease; TOL: target organ lesions; TGS: triglycerides.
Note: Definition adapted from those of the European AHT and Cardiology Societies.
One of the first steps to be taken when evaluating patients with risk factors is to calculate their CVR, as certain decisions depend on the level or value of CVR, such as when to start treatment to bring down hypercholesterolaemia and the therapeutic target set for this.
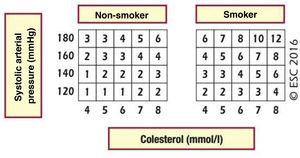
The (absolute) cardiovascular risk is the probability that a certain vascular event will occur within a period of time defined on the basis of the CVRF which correspond to a patient in a certain population group. There is therefore no universal system for calculating CVR. The European cardiovascular prevention guides18 and those for the control of dyslipidaemia,19 to which the SEA adheres through the CEIPV, recommend the use of the SCORE45 system to evaluate CVR using its version for countries with a low CVR and in primary prevention, that is, for individuals who have never had a cardiovascular event. This system calculates the risk of death due to atherosclerosis within a 10-year period, considering the following risk factors: sex, age, smoking, SAP and TCh or non-HDL-c.
Cardiovascular risk calculation systemsThree systems for estimating CVR have been the most widely used in Spain: one is qualitative-ordinal (from the European Hypertension Guidelines8) and two are quantitative: the Girona COR Registry (REGICOR)46 and the SCORE.45
The AHT guide uses a system that does not quantify CVR numerically, but rather identifies the risk category in question: low, moderate, high or very high.
The REGICOR system is based on a sample of the population in Girona using a mathematical model from the Framingham study that was validated in a set of Spanish samples. Although it is a quantitative system like SCORE, unlike the latter it classifies coronary risk as fatal or non-fatal.
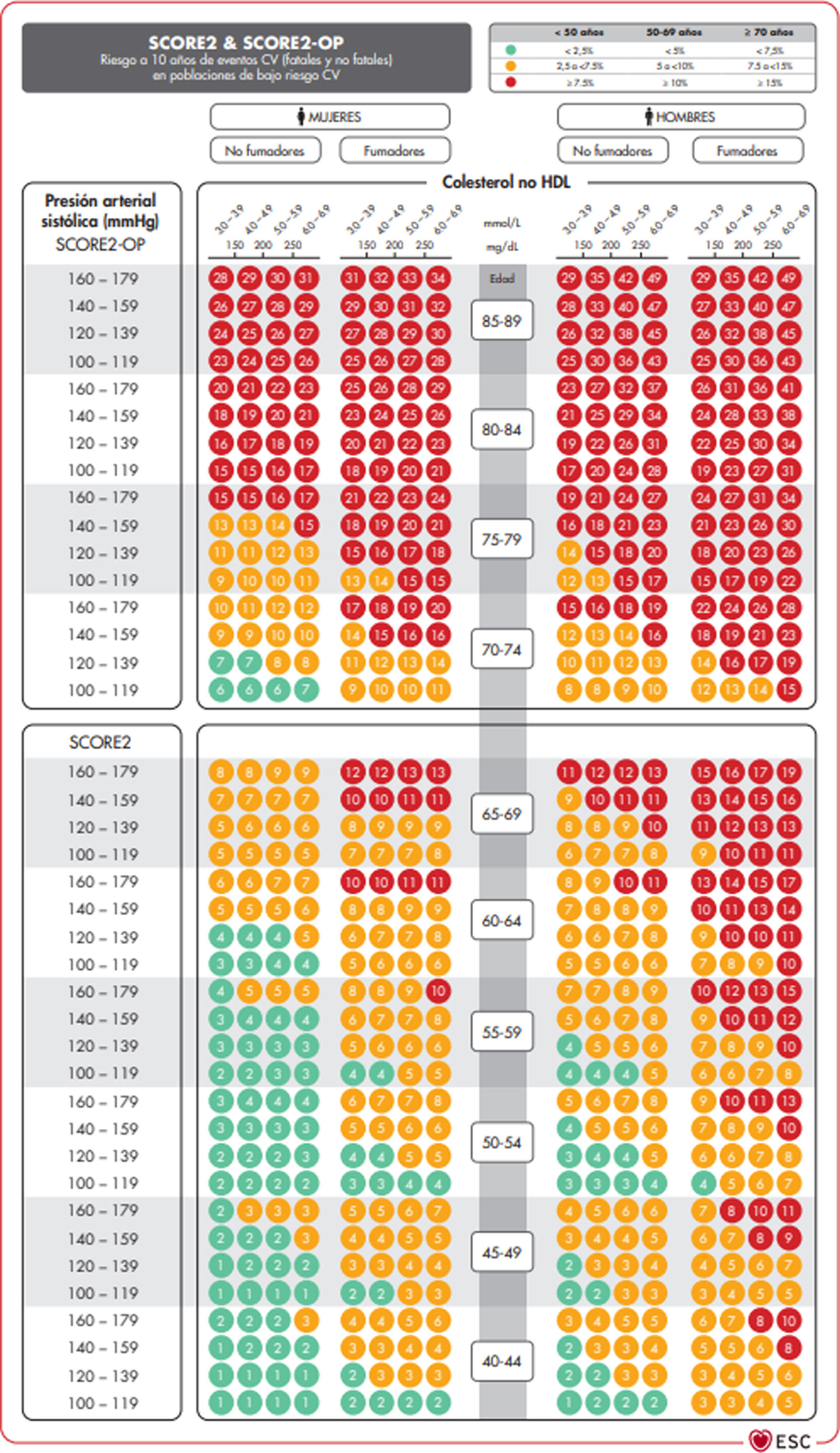
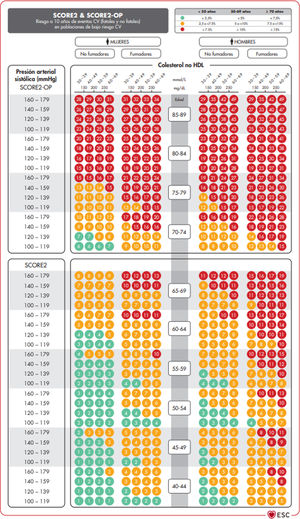
The SCORE and SCORE2 projectWithin the context of the SCORE (Systematic COronary Risk Evaluation)45 project, the risk calculation indexes have recently been updated and published (SCORE2).47,48 This update makes several changes to the original SCORE index: the risk of an event developing has now been added to the calculation of the risk of cardiovascular mortality; analysis was based on the study of 45 cohorts in 43 countries, including 677,684 individuals and 30,121 CV events. The variables used to predict risk are sex, age, smoking (dichotomic) and SAP, while in the new index the lipid parameter used is non-HDL cholesterol. DM is not included as it is considered to be “a priori” a high-risk condition. The risk equation is modulated by the incidence of cardiovascular events in each country, so that the final indices are distributed in four zones: low risk (including Spain); moderate; high and very high; showing a clear East-West gradient. The values are applicable up to the age of 70 years, and specific tables have been developed separately for individuals aged up to 89 years (SCORE2-OP) (Fig. 1).
The SCORE2 and SCORE2-OP systems for countries with low cardiovascular risk.
CV: Cardiovascular; SCORE2: Systematic Coronary Risk Estimation 2; SCORE2-OP: Systematic Coronary Risk Estimation 2-Older Persons.
Visseren et al.18 Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. Material translated with their permission.
Based on these new SCORE2 and SCORE2-OP indices, cardiovascular risk at 10 years is distributed in three categories in three age bands:
Comprehensive calculation of cardiovascular riskThe general calculation of CVR should include a comprehensive assessment of the patient that does not centre exclusively on the level of risk calculated using SCORE. It should include factors that modify risk, data on lesions to target organs and the presence of ACVD (Table 5).8,14,18
Estimation of cardiovascular risk.
| Without TOL,1 SVD1 or other risk-modulating factors2 | With TOL,1 SVD1 or other risk-modulating factors2 | Arterial pressure ≥ 180/110 mmHg or LDL-c ≥ 190 mg/dL (especially with familial hypercholesterolaemia) | |
|---|---|---|---|
| – No ACVD, DM or CRD | Estimated according to SCORE | Increase the category obtained with SCORE by one step | High |
| – Grade 3 CRD or | High | Very high | Very high |
| – Type 1 or 2 DM3 | |||
| –Clinical cardiovascular disease or equivalent4 | Very high | Very high | Very high |
| – Grade 4 or 5 CRD | |||
| – DM with 3 or more risk factors; type 1 with more than 20 years evolution | |||
| 1Go to Table 4: Diagnostic criteria. | |||
| 2The increase in risk depends on the number and intensity of modulating factors. In general, several of them are necessary or they have to be extremely severe to raise the risk category to the same level as would the presence of SVD or TOL: | |||
| – Obesity or a sedentary lifestyle | |||
| – Individuals in a situation of social exclusion | |||
| – Glucose intolerance or altered fasting glycaemia. | |||
| – Raised TGS, Apo B, Lp(a) | |||
| – A family history of early onset ACVD. | |||
| – Diseases that lead to an increase in inflammatory-metabolic stress: autoimmune diseases, sleep apnoea/hypopnea syndrome, metabolic syndrome, systemic lupus erythematosus, psoriasis, cancer, HIV. | |||
| – Severe psychiatric illnesses | |||
| – Non-alcoholic fatty liver | |||
| 3Patients with type 1 diabetes under the age of 35 years, or type 2 under the age of 50 years, and with less than 10 years evolution, may have moderate cardiovascular risk. | |||
| 4The following conditions are considered to be ACVD or equivalent: | |||
| – Established clinical ACVD: | |||
| – Coronary event (myocardial infarct, acute coronary syndrome, stable angina, revascularization procedure). | |||
| – Cerebrovascular event: ictus or AIT. | |||
| – Symptomatic PAD | |||
| – Abdominal aorta aneurism | |||
| – Heart failure (HF) independent of FE. | |||
| – ACVD shown by imaging techniques, i.e., the presence of significant atheromatous plaque: | |||
| – Shown by angiography or coronary CAT (multivessel disease with obstruction > 50% of two pericardial arteries) | |||
| – Shown by carotid or femoral ultrasound scan (stenosis > 50%). | |||
ACVD: atherosclerotic cardiovascular disease; AHT: arterial hypertension; Apo B: apolipoprotein B; CRD: chronic renal disease; CVRF: cardiovascular risk factors; DM: diabetes mellitus; EF: ejection fraction; LDL-c: low density lipoprotein cholesterol; PAD: peripheral arterial disease; SVD: subclinical vascular disease; TGS: triglycerides; TIA: transitory ischemic attack; TOL: target organ lesion; Lp(a): lipoprotein (a).
It is advisable to follow the strategy of the European guides for cardiovascular prevention and dyslipidaemia control, as well as those for AHT, which classify subjects in four risk categories: low, moderate, high and very high.
Some situations lead to the classification of risk as high or very high: grade 3 AHT, hypercholesterolaemia with LDL-c > 190 mg/dL, DM, a target organ lesion, stage 3 or higher chronic renal disease (CRD), or established ACVD. We use the SCORE system in all other situations. If we use the SCORE2 scale, we will use the cut-off points shown in the previous section. The presence of risk modifiers means that the risk category should be raised to the next level, if risk values are close to those of the same.
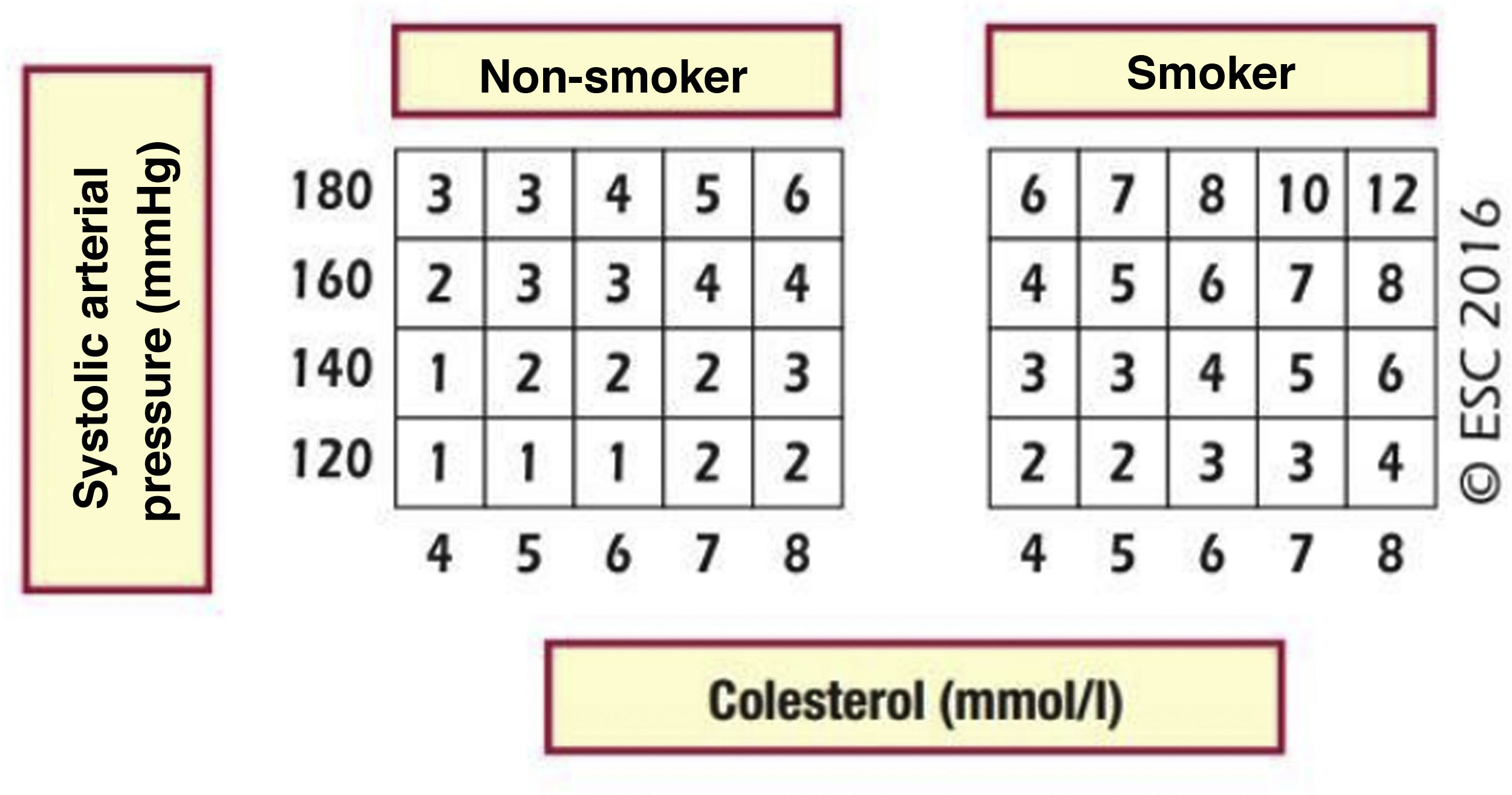
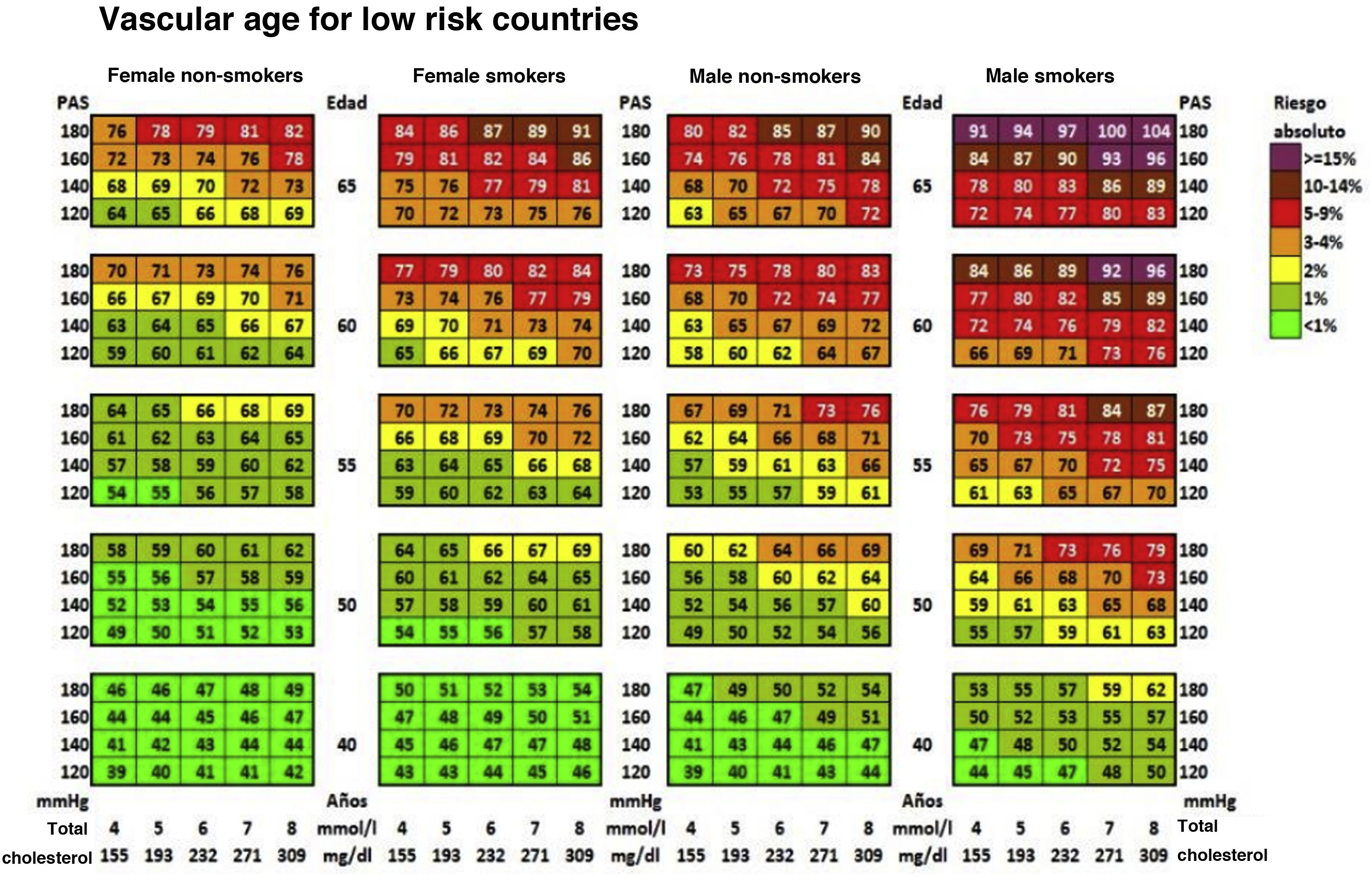
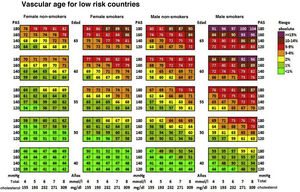
Cardiovascular age and relative riskRelative risk (Fig. 2) and vascular age49 (Fig. 3) can be calculated in young adults with major elevation of several CVRF (Fig. 3). Information a patient of their vascular age is a means of communicating their level of CVR that is easier to comprehend than the mathematical value of absolute risk. Patients should be aware of their level of risk so that they can adopt therapeutic lifestyle measures and pharmacological ones too, if applicable.
Relative risk table.
SAP: systolic arterial pressure.
Piepoli MF, Hoes AW, Agewall S et al., 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Heart Journal 2016; 37 (29): 2315–2381 doi:10.1093/eurheartj/ehw106.
Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. Material was translated with their permission.
Vascular age table according to SCORE for countries with low cardiovascular risk.
SAP: Systolic arterial pressure; SCORE: Systematic Coronary Risk Estimation.
Cuende et al.49 Translated and reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology.
The vascular age table derived from SCORE offers information about absolute risk and vascular age. No calibration is required for the calculation of vascular age, so that it can be applied to any general population, without differentiating between territories.
Vascular age and relative risk can be applied to any age, and they are of more clinical utility for subjects who are not at high risk.
The speed of vascular aging50 is derived from the concept of vascular age, which compares vascular and chronological ages.
Several specific tools have been developed for patients with FH who cannot be subjected to the usual CVR calculation tables. One such tool is based on the follow-up data of the SAFEHEART51 Spanish cohort. This equation takes several factors into account, such as age, smoking, LDL-c level while under treatment, BMI, AP and the levels of Lp(a). It makes it possible to distinguish differences in the risk of this highly specific population which is so important in our work. The SEA registry offers another instrument for classifying vascular risk in patients with FH who are being treated with statins. It is based on the presence of other CVRF (male sex, obesity, AHT and diabetes), together with maximum levels of LDL-c and a positive genetic test for FH.52 Lastly, a tool has been developed to calculate risk in patients with the FH phenotype: the SIDIAP-FH tool has greater predictive power in primary as well as in secondary prevention.53
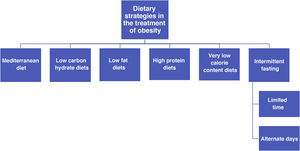
General recommendations for the control of cardiovascular riskGeneral recommendations for the population to reduce cardiovascular riskThe SEA Nutrition and Lifestyle workgroup has a detailed consensus document54 which offers useful ranked evidence, and it can be used by healthcare professionals to help their patients, based on the concept that health recommendations should be the same as those for the control of any risk factor and for the primary and secondary prevention of ACVD. This document summarises the accumulated evidence for lifestyle factors such as diet and physical exercise.54 It also places special emphasis on foods and above all the most important eating patterns for cardiovascular prevention, rather than the consumption of isolated ingredients. This concept of an eating pattern has taken root in recent years as a means of examining the relationship between nutrition and health. It is also an educational tool for the population, modifying the traditional paradigm so that basic nutritional units are no longer nutrients themselves (such as fatty acids) but rather the foods that contain them (oils, nuts, red meat and dairy products, etc.), as their variants contain a multitude of components that are able to interact in a synergic or in a conflicting way on metabolic routes which are decisive for cardiovascular health. The main recommendations of this document are shown in this guide.54
The different types of healthy diets have many ingredients in common. Some of these are recommendable, such as fruit, vegetables, nuts, pulses and fish, while others should be restricted. The latter include certain foods that are rich in saturated fats, those with added sugar, abundant salt or foods which have been processed. There is strong evidence that vegetable-based diets which are low in saturated fatty acids, cholesterol and sodium, and which have a high content in fibre, potassium and unsaturated fatty acids are beneficial and reduce the expression of CVRF. The Mediterranean diet stands out here, as do the dietary approaches to stop hypertension (DASH) diet, a vegan-vegetarian diet and the Alternative Healthy Diet Index, all of which are vegetable-based with abundant complex carbon hydrates. The data from large cohort studies and, in the case of the Mediterranean diet, the PREDIMED randomized clinical study, indicate that adherence to these dietary patterns confers a clear cardiovascular benefit.55 However, the efficacy of a low-fat diet is currently doubted due to its lack of potential for cardiovascular protection. Of the edible fats, virgin olive oil is the cooking fat that is the most effective in preventing ACVD.56 The nutritional intervention during approximately five years in the PREDIMED study showed that the participants who were assigned to the Mediterranean diet supplements by extra virgin olive oil or nuts experienced an average fall of 30% in major cardiovascular events,55 as well as other beneficial effects including a fall in the risk of DM2 and atrial fibrillation (AF).57 It has to be pointed out that the majority of modern margarines are free of trans fatty acids and supply n-6 and n-3 polyunsaturated fatty acids.
The consumption of fish or shellfish at least three times a week, two of them in the form of blue fish, reduces CVR. Encouraging this form of consumption is therefore an important part of the lifestyle changes involved in preventing ACVD. Replacing meat as the main course of meals could lead to a benefit that is far from insignificant. Even so, the above-mentioned document underlines that due to its high content in sea pollutants, large blue fish species should not be eaten by children and women of fertile age. These include red tuna, swordfish, shark and mackerel, as they contain more pollutants than smaller species. The evidence respecting meat indicate that consuming white or lean meat (without any visible fat), three or four times a week does not increase CVR. This is not the case for processed meats (bacon, sausages or cold meats) which contain harmful additives such as salt and nitrates, which increase total mortality and the development of DM2 and ACVD.
Regarding dairy products, it is advisable to consume at least two portions every day (milk, fermented milk, cheese or yogurt, etc.), due to their important nutritional role in calcium metabolism and richness in proteins which are of high biological quality. Although restricting whole-milk dairy products does not seem to be a suitable strategy to reduce CVR, it is not advisable to regularly consume dairy products with added sugar. The consumption of concentrated dairy fat such as butter and cream should be reduced for cardiovascular prevention. In the last decade conflicting recommendations have been made about the consumption of eggs and health, largely because of a lack of evidence. However, current scientific evidence suggests that egg consumption is not harmful within the context of a healthy diet. The healthy general population as well as individuals with CVRF, previous ACVD or DM2, may consume up to one egg per day without worrying about their cardio-metabolic health. Nor do there seem to be sufficient arguments to restrict egg consumption in DM2 patients to reduce their CVR or improve metabolic control, although some series limit egg consumption to a maximum of three per week.58
Whole-grain pulses and cereals are seeds that contain multiple healthy nutrients, and frequent consumption of them is associated with a reduction in risk factors and ACVD. It is recommendable to consume a portion of pulses at least four times a week to promote cardiovascular health and reduce cholesterolaemia. The recommended consumption of whole-grain cereals stands at approximately four portions per day, including bread with all meals, pasta two or three times a week and rice two or three times a week. Based on current evidence, it is recommended that vegetables and fruit should be consumed in four to five portions per day, as they reduce total as well as cardiovascular mortality. Furthermore, the beneficial effect of fruit and vegetables is dose-dependent, and it is also more evident in terms of cerebrovascular disease than it is for coronary disease. The consumption of tubers (potatoes above all) is not associated with any increase in CVR unless they are fried in oils that are not recommended and served with salt.
The frequent consumption of nuts is associated with a fall in ACVD and coronary disease above all, together with mortality due to any cause.55 Consuming a handful (equivalent to a 30 g portion) of nuts frequently (every day or at least three times a week) is highly recommendable to control cholesterol and for general health. It is recommended that fruit should be consumed raw and unpeeled (neither toasted nor salted) if possible, as the majority of antioxidants are contained in the skin. Nuts should be eaten during the day rather than after dinner as a dessert at night because of their satiating effect and to prevent weight gain. The nuts which are recommended for consumption include hazel nuts, walnuts, almonds, pistachios, cashews, macadamias and pine kernels, etc. Although peanuts are not really the fruit of a tree, but rather pulses, their general composition and high unsaturated fatty acid content makes them similar to other nuts, in nutritional terms as well as their biological effects.
Cacao beans contain abundant nutrients and consuming their chief derivative, chocolate, improves risk factors and is associated with reduced ACVD, cerebrovascular accidents (CVA) and DM2. Some information indicates that it has cholesterol-lowering and anti-hypertensive effects. It helps to overcome insulin resistance, so that ≥ 70% sugar-free black chocolate can be consumed as part of a healthy diet. It is advisable to consume chocolate during the day rather than at night after dinner, when its satiating effect cannot be counterbalanced by eating less in the next meal.
Sweetened drinks are a part of the usual diet of a great many people, and they may account for up to 20% of their daily calorie intake, favouring increased ACVD, obesity and DM2. It would be very important to replace drinks of this type with water, to reduce calorie consumption and the risk of these pathologies and their complications. If a patient does not accept this substitution, drinks with artificial sweeteners may be tried, as there is as yet no first-level scientific evidence that they are innocuous. The consumption of any type of alcoholic drink increases HDL-c, and drinking moderate amounts (of fermented rather than distilled drinks), in comparison with abstention or excessive consumption, is associated with reduced ACVD and cardiovascular mortality. Alcoholic drinks may be consumed in moderation and always with meals, and as part of a healthy diet such as the Mediterranean diet. Recommendations differ for men and women, as the latter are more sensitive to the effects of alcohol. Alcohol consumption should not be encouraged in habitual non-drinkers. Coffee (normal as well as decaffeinated) and tea are rich in polyphenols, and there is evidence that habitual consumption of these drinks is associated with reduced ACVD.
There are many functional foods (nutraceuticals) which have the purpose of reducing CVR, chiefly by reducing cholesterolaemia. The efficacy of vegetable sterols and preparations including soluble fibre, at intestinal level, has been widely demonstrated. The monacolin which is present in red rice yeast also reduces cholesterol, as it has the same chemical structure as the active form of lovastatin. There is also consistent evidence that omega-3 fatty acids at pharmacological doses reduce triglycerides in plasma.
Excessive salt consumption is associated with ACVD and mortality due to cardiometabolic causes. A diet that is low in salt should be recommended (<5 g/day) for the population in general, although it is even more justified for hypertensive patients and their families. It should be remembered that the sodium content of foods should be multiplied by 2.5 to calculate the total amount of salt. For this target it is especially effective to restrict the intake of high- salt foodstuffs, such as precooked meals, tinned foods, salted foods, processed meat and carbonated drinks. An alternative to salt consists of using lemon juice, garlic or aromatic herbs.
According to the World Health Organization (WHO), physical activity is defined as any bodily movement originated by the skeletal muscles and which consumes energy. When it is performed regularly and over time, physical activity protects against CVR and improves its risk factors. It should be adapted to suit the particular needs of each individual, based on the principle that a little is better than nothing, while considering that it includes activities such as those involved in work, active forms of transportation, housework and leisure activities. Although physical exercise is in itself a form of activity, it is undertaken in a way that is planned, structured, repetitive and goal-oriented, with the purpose of improving or maintaining fitness. All types of physical activity should be performed in moderation and without rushing, rather than in an intense and concentrated way.
It is reasonable to believe, as recent evidence shows, that there is no single standard model of healthy diet, but rather that the biological response to diet varies between individuals, especially due to differences in their genome and microbiome. It will be a challenge for the scientific community in the coming years to develop personalized precision diets, as well as those based on other sciences such as chronobiology, in which each individual will select the diet that is most beneficial for them personally.59 Finally, one of the most complex problems which arise in the relationship between people and their diets consists of adherence. This depends on a very wide range of factors, such as those pertaining to the patient, their family, the medical team guiding them and the healthcare system itself. It is therefore fundamental to implement strategies to achieve adherence.
Table 6, which is contained in the said document,54 shows the frequency, manner and amounts in which foods are consumed, in a practical way.
Frequency in the form and amount foods are consumed.
| Frequency of consumption | Daily | Three times a week at most | Unadvisable or occasional |
|---|---|---|---|
| Edible fats | Olive oil, preferentially virgin | Margarine | Frying with seed-based oils |
| Eggs | Whole eggs are not considered unadvisable | Patients with diabetes | |
| Fisha | Blue or white | Shellfish | Salted or smoked fish. |
| Meatb | Fowl and rabbit | Lean red meat | Processed and cold meats |
| Dairy products | Semi-skimmed or skimmed milk and yogurt (sugar-free). | Whole milk and yogurt (sugar-free) | Butter, cream |
| Fresh cheeses | Cured cheeses | Cured cheese in hypertensive patients | |
| Pulses and cereals | Whole grain cereals, pulses | Rice, pasta | Refined flour cereals |
| Nuts and peanuts | Raw (30–45 g) | Toasted | Salted |
| Chocolate | Black with ≥ 70% cacao | Black with cacao < 70% | Chocolate with milk and white chocolate |
| Coffee and tea | Unrestricted tea. Up to five cups a day of coffee, without sugar. | ||
| Fruit, greens, starches | 4−5 portions combining different types of fruit and greens | Foods high in starch (potatoes) | Commercial fruit juices and crisps |
| Alcoholic drinks | Restrict to 30 g alcohol in men who are drinkers and y 15 g in women. Preferentially fermented drinks (wine, beer) with meals. | Not advisable for non-drinkers | |
| Products with added sugar | Avoid all foods with added sugar | ||
| Food preparationc | Preferably boiled, grilled or lightly fried. | Foods fried in virgin olive oil | Avoid smoked and processed foods and foods fried in refined oil |
| Salt | From 2.5 to 4 g per day | Salted foods |
Published with the editor’s permission. Original source: Pérez-Jiménez et al.54
One way of evaluating adherence to the Mediterranean diet is to use the MEDAS questionnaire (Appendix A Annex 4).
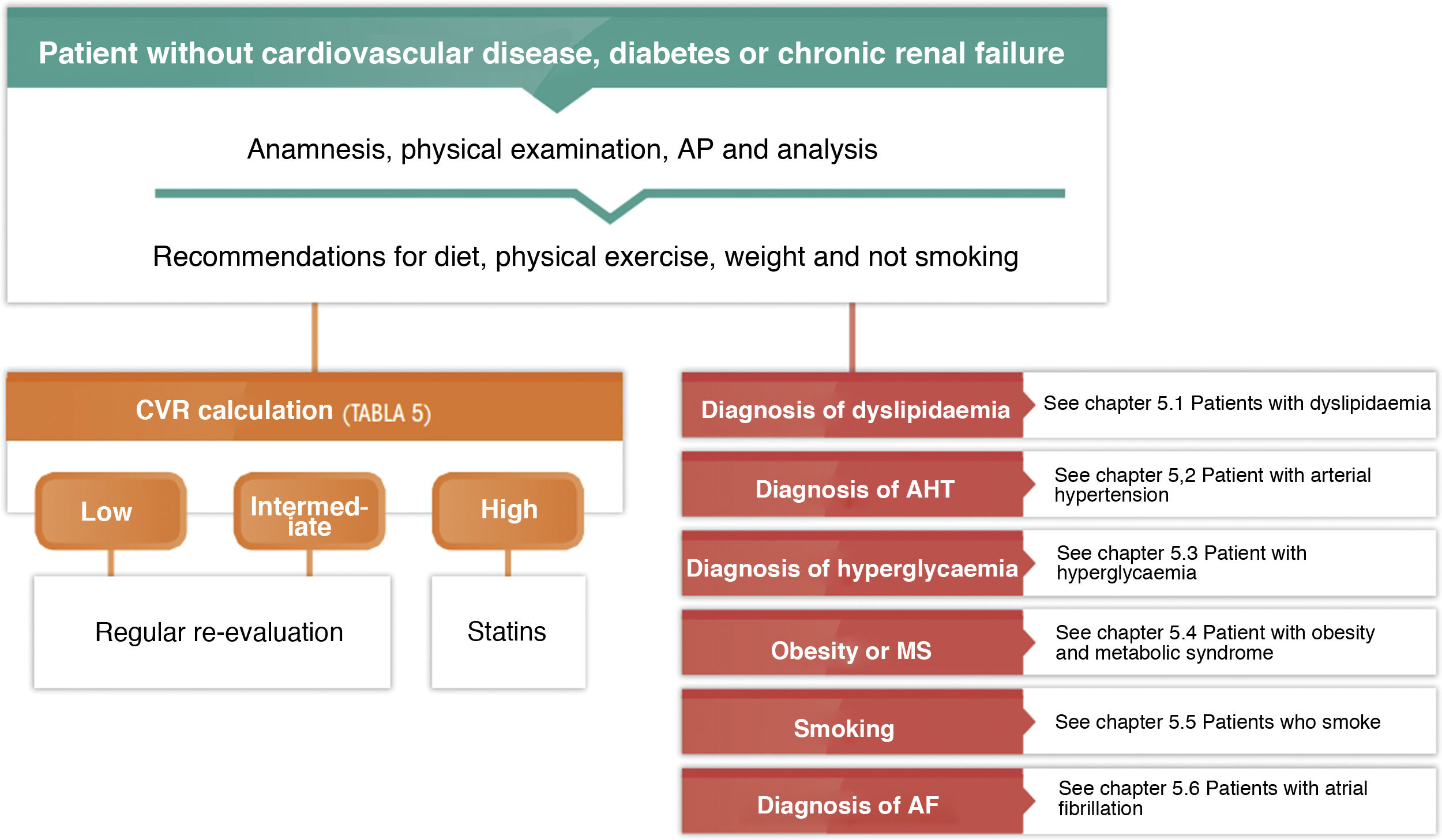
General pharmacological recommendations for patients in primary preventionFig. 4 shows the recommendations for the clinical management of CVR in patients without ACVD, DM or chronic renal failure.
Platelet antiaggregant drugsTreatment with lose doses of acetylsalicylic acid has been shown to reduce the risk of vascular complication, chiefly in middle-aged people, by reducing non-fatal myocardial infarcts and without affecting the risk of ictus or mortality. Nevertheless, some of the benefit of acetylsalicylic acid may be lost because of its adverse effects, above all those connected with its potential to increase haemorrhaging. Due to this the risk-benefit balance of low doses of acetylsalicylic acid has yet to be clearly established in primary prevention.
The US preventive service task force (USPSTF)60 guides recommend commencing low doses of acetylsalicylic acid (100 mg/day or less) for the primary prevention of ACVD in adults aged from 50 to 59 years with a cardiovascular morbimortality risk of at least 10% at 10 years. They must have no increased risk of haemorrhage, have a life expectancy of at least 10 years and be prepared to take this treatment every day for at least 10 years. The decision to start treatment in adults aged from 60 to 69 years with a CVR of at least 10% at 10 years should be made on an individual basis.60
Nevertheless, the 2021 European guides for cardiovascular prevention18 do not systematically recommend antiaggregant treatment for patients without ACVD, due to the increased risk of major bleeding. Thus, several recently published clinical trials of acetylsalicylic acid for primary prevention in diabetic and non-diabetic patients found no clear benefit in using it in the primary prevention of cardiovascular disease,61–63 most especially when any existing CVRF were properly controlled.
Lipid-lowering treatmentStatins have been shown to reduce the number of cardiovascular events in patients without ACVD in numerous clinical trials and meta-analyses,64 even in patients without high levels of cholesterol. Statins reduce the relative risk of ACVD regardless of the basal CVR, although for the treatment to be effective it is important to select patients with a high basal CVR to ensure a greater absolute reduction in CVR (Table 5).
The indications for lipid-lowering treatment in primary prevention are shown in the section on specific therapeutic recommendations.
Vitamin supplementsMany prospective observational studies of cases and controls have observed inverse associations between the intake of vitamins (A, B group, C, D and E) or their concentrations in serum and the risk of ACVD. However, data from prospective studies and clinical trials with intervention giving vitamin and mineral supplements have not demonstrated any cardiovascular benfit.65 The use of vitamin supplements to prevent ACVD is therefore not indicated.
General recommendation in patients with subclinical vascular disease and those in secondary preventionPatients with subclinical vascular disease diagnosed by the presence of plaque in the carotid or femoral artery or by coronary calcium determination, have an intermediate level of risk of cardiovascular complications, between subjects in primary and secondary prevention. Nevertheless, many guides classify these patients as in secondary prevention. Under these circumstances their management would not differ from that of subjects in secondary prevention, although the evidence for the efficacy of antiaggregant treatment is limited. For example, treatment with antiaggregants has not been shown to be effective in patients with a low AAI but without intermittent claudication.66 In any case, their level of risk should be estimated according to the contents of Table 5.
Apart from the treatments indicated to control CVRF, there are a series of treatments for secondary prevention patients, as well as the above-mentioned health and dietary measures (see the General Recommendations for the population to reduce cardiovascular risk), which have been shown to reduce the risk of new cardiovascular events (Table 7).
Pharmacological measures which have been proven to reduce the rate of cardiovascular complications in subjects in secondary prevention.
| Treatment | Potential indications |
|---|---|
| Antiaggregants | – Aspirin at low doses or clopidogrel in patients with coronary, cerebrovascular or peripheral arterial disease |
| – Aspirin plus clopidogrel after TIA or mild ictus.67,68 | |
| – Aspirin and dipyridamole would also be indicated in subjects after ictus or TIA | |
| – Aspirin plus a P2Y12 inhibitor in subjects with acute coronary syndrome or with a stent, maintained over at least 12 months | |
| Lipid-lowering drugs | – Statins in combination or not with ezetimibe to reduce LDL-c to below 55 mg/dL or, at least, to reduce its concentration by 50% |
| – iPCSK9 if appropriate falls are not achieved with the previous treatment to reduce lipids, and according to the criteria of Table 10 | |
| – Fibrates if triglycerides > 200 mg/dL and HDL-c is low, once LDL-c has been controlled using statins in patients at very high CVR | |
| – Omega-3 fatty acids (purified EPA 4 g/day) at high doses in hypertriglyceridaemia > 200 mg/dL which persist in spite of treatment with statins in patients at high CVR.69 | |
| Vitamins or nutritional supplements | – No indication |
| RAS blocker | If ischemic cardiomyopathy and: |
| – FE ≤ 40% or | |
| – Heart failure or | |
| – DM or | |
| –Arterial hypertension | |
| Beta-blockers | – FE ≤ 40% |
| Aldosterone inhibitors | – FE ≤ 40% |
| Neprilisin inhibitors | – Insufficient control of left ventricular dysfunction |
DM: diabetes mellitus; EF: ejection fraction; EPA: eicosapentaenoic acid; iPCSK9: proprotein convertase subtilisin/kexin type 9 inhibitor; LDL-c: LDL cholesterol; P2Y12: adenosine diphosphate chemoreceptor; RAS: renin-angiotensin system; TIA: Transitory ischemic attack.
Acetylsalicylic acid is the platelet antiaggregant which has been studied the most in long-term cardiovascular prevention in patients who have suffered acute myocardial infarct, ischemic ictus or PAD. A meta-analysis of 16 clinical studies which included more than 17,000 patients found that treatment using acetylsalicylic acid significantly reduced the number of major coronary and cerebrovascular cardiovascular events, as well as total mortality.70 Likewise, treatment with acetylsalicylic acid was associated with a significant excess of major bleeding events: nevertheless, the cardiovascular benefits of acetylsalicylic acid are clearly more important than the risk of bleeding.
Although clopidogrel has a similar effect to acetylsalicylic acid in patients with myocardial infarct or ischemic ictus, it may be superior to it in subjects with PAD. The combination of acetylsalicylic acid and clopidogrel in secondary prevention significantly reduces the number of severe cardiovascular events in comparison with acetylsalicylic acid alone, although it also significantly increases the risk of bleeding.
In patients with non-cardioembolic ischemic CVA or AIT, acetylsalicylic acid may be used alone or in combination with dipyridamole, and it is also possible to use clopidogrel alone. In patients with AIT or minor ictus, the benefit of the dual antiaggregant treatment during a maximum of 90 days is greater than the risk of increased bleeding.67,68 Protection arises during the first 21 days, so that this would be the recommendable duration of the dual treatment.71
The standard treatment for a patient who has suffered acute coronary syndrome, with or without the placement of stents, is dual platelet antiaggregation (acetylsalicylic acid with an adenosine diphosphate receptor inhibitor [P2Y12]) during 12 months. The duration of dual antiaggregation may be reduced to from one to three months in patients at high risk of bleeding.
Lipid-lowering drugsNumerous clinical trials and meta-analyses64 have shown that treatment with lipid-lowering drugs (resins, statins, ezetimibe, proprotein convertase subtilisin/kexin 9 inhibitor [iPCSK9]) in patients with established ACVD reduces the number of severe cardiovascular events and mortality.
Guide data19 indicate that patients with established subclinical cardiovascular disease (multi-vessel coronary disease with > 50% obstruction of at least two epicardial arteries shown by coronary CAT or angiography, or the presence of carotid plaques) should be considered to be at very high CVR and treated as if they had already suffered a cardiovascular event. The recommendations for lipid-lowering treatment in these subjects are shown in the section on Specific Therapeutic Recommendations.
Other drugsOmega-3 fatty acid supplements may reduce cardiovascular mortality by diminishing the number of sudden deaths due to cardiac causes in patients with previous coronary disease. It may therefore be reasonable to use these in the secondary prevention of ischemic cardiac pathology, most particularly in patients who do not eat a sufficient amount of fish.72 In patients under secondary prevention or high-risk diabetic patients treated with statins (average LDL-c 75 mg/dL and triglycerides from 150−499 mg/dL), treatment with 4 g EPA reduced the risk of severe cardiovascular events by 25%.69
In patients with ischemic cardiomyopathy and left ventricular dysfunction (left ventricular ejection fraction (LVEF) ≤ 40%), heart failure (HF), DM or AHT, treatment with an angiotensin converting enzyme (ACE) inhibitor would be indicated, or treatment with an angiotensin II receptor blocker in the case of intolerance.
In patients with ischemic cardiomyopathy and left ventricular dysfunction (LVEF ≤ 40%) treatment with beta-blockers and mineralocorticoid receptor antagonists would also be indicated, on condition that there is no contraindication.
Likewise, and in selected cases, if sufficient control of the left ventricular dysfunction is not achieved then sacubitril-valsartan may be used. Sacubitril is a neprilysin inhibitor which increases the activity of the natriuretic peptides. It is combined with an angiotensin II receptor blocker, as this is increased by the effect of the said compound.73
There is no evidence that the fall in homocysteine caused by folic acid or vitamin B12 supplements reduces the risk of ACVD in secondary prevention.
Combining acetylsalicylic acid, a statin and an angiotensin converting enzyme inhibitor in a single tablet aids adherence to the treatment by patients in secondary prevention.74
Intervention on the inflammatory state has recently been studied. Use of an anti-IL-1β monoclonal antibody, canakinumab, significantly reduced the ACVD recurrence rate, showing a benefit in spite of a slight increase in severe and fatal infections.75 Nevertheless, it has not been approved by the North American administration because the CANTOS75 study did not compare it with the maximum therapy, which would include ezetimibe or iPCSK9, and it was associated with an increased risk of severe infections. Colchicine is a drug with anti-inflammatory effects, which at a dose of 0.5 mg per day or every 12 h has been shown to reduce the rate of cardiovascular complications by 32%, without significant differences in side effects.76
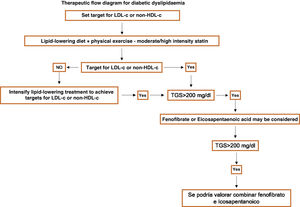
Specific therapeutic recommendationsPatients with dyslipidaemiaPatients with hypercholesterolaemiaAll patients with hypercholesterolaemia should consume a diet low in animal fat, like the Mediterranean diet, according to the dietary recommendations of the SEA (Table 6). The indication for lipid-lowering treatment is based on the concentration of LDL-c and the overall basal CVR level. We recommend the use of lipid-lowering treatment with the aim of achieving at least the LDL-c targets, so that we emphasise the use of high intensity cholesterol lowering therapies which should include statins, according to Table 8.
Lipid-lowering therapies classified according to their cholesterol-lowering intensity.
| Low intensity | Moderate intensity | High intensity | Very high intensity | Extremely high intensity | |
|---|---|---|---|---|---|
| LDL-c <- 30% | LDL-c > 30% < 50% | LDL-c > 50% < 60% | LDL-c > 60% < 80% | LDL-c > 80% < 85% | |
| Oral monotherapy | Simvastatin 10 | Atorvastatin 10−20 | Atorvastatin 40−80 | ||
| Pravastatin 10-2010−20 | Rosuvastatin 5−10 | Rosuvastatin 20−40 | |||
| Lovastatin 10−20 | Simvastatin 20−40 | ||||
| Fluvastatin 40 | Pravastatin 40 | ||||
| Pitavastatin 1 | Lovastatin 40 | ||||
| Ezetimibe 10 | Fluvastatin 80 | ||||
| Pitavastatin 2−4 | |||||
| Combined oral therapy | Simvastatin 10 + Ezetimibe 10 | Atorvastatin 10−20 + Ezetimibe 10 | Atorvastatin 40−80 + Ezetimibe 10 | ||
| Pravastatin 20 + Ezetimibe 10 | Rosuvastatin 5−10 + Ezetimibe 10 | Rosuvastatin 20−40 + Ezetimibe 10 | |||
| Lovastatin 20 + | Simvastatin 20−40 + Ezetimibe 10 | ||||
| Ezetimibe 10 | Pravastatin 40 + Ezetimibe 10 | ||||
| Fluvastatin 40 + Ezetimibe 10 | Lovastatin 40 + | ||||
| Pitavastatin 1 + Ezetimibe 10 | Ezetimibe 10 | ||||
| Fluvastatin 80 + Ezetimibe 10 | |||||
| Pitavastatin 2−4 + Ezetimibe 10 | |||||
| Combined oral + subcutaneous therapy | Alirocumab 75 | Alirocumab 150 | Atorvastatin 40−80 + | ||
| Evolocumab 140 | Alirocumab/Evolocumab | ||||
| Atorvastatin 10−20 + Alirocumab/Evolocumab | Rosuvastatin 20−40 + | ||||
| Rosuvastatin 5−10 + Alirocumab/Evolocumab | Alirocumab/Evolocumab | ||||
| Simvastatin 40 + | Atorvastatin 40−80 + Ezetimibe 10 + | ||||
| Alirocumab/Evolocumab | Alirocumab/Evolocumab | ||||
| Rosuvastatin 20−40 + Ezetimibe 10 + | |||||
| Alirocumab/Evolocumab |
Masana et al.77
Fibrates are usually moderately effective in reducing cholesterol (Table 9).
Patients at low to moderate cardiovascular riskLDL-c from 115 to 190 mg/dLThese are patients in primary prevention, without diabetes and preserved renal function, with a CVR below 5% in 10 years according to the SCORE tables, without risk-modulating factors or lesions in target organs (Table 5). The recommended concentration of LDL-c is < 115 mg/dL.
Treatment will be based on therapeutic lifestyle changes, including a Mediterranean-style diet. Functional foods enriched with phytosterols and fibre may be indicated to bring down cholesterol, together with increased physical activity, the cessation of smoking and weight loss if necessary. The prescription of drugs to lower cholesterol levels is not indicated for all patients, and should be considered on an individual basis if a patient has any two of the following factors: age (men > 45 years; women > 50 years); BMI > 30 kg/m2; smoking; AHT; a family history of early onset ACVD; atherogenic dyslipidaemia; MS; or Lp(a) > 50 mg/dL.
Patients at high cardiovascular riskThe therapeutic aim here is to reduce LDL-c < 70 mg/dL, with a fall of at least 50% in the basal values of LDL-c.
The initial treatment will be based on the use of therapeutic lifestyle changes. If levels of LDL-c > 70 mg/dL persist, high intensity cholesterol-lowering treatment is recommended which theoretically guarantee at least a 50% fall in LDL-c (Table 8). The initial treatment should be with statins, and if the associated targets are not achieved, ezetimibe will also be given. This combination should be considered from the start in patients with basal LDL-c levels above 140 mg/dL.
Patients at very high cardiovascular riskThe therapeutic target here is LDL-c < 55 mg/dL and a fall of at least 50% in basal values.
The initial treatment will be based on the application of therapeutic lifestyle changes with simultaneous high intensity cholesterol-lowering treatment which theoretically guarantees at least a 50% fall of LDL-c, making it possible to achieve the target (Table 8). The initial treatment should be with statins, and if the target levels are not achieved, ezetimibe will also be given. This combination should be considered from the first in patients with basal levels of LDL-c higher than 110 mg/dL. The use of iPCSK9 would be recommended according to the indications shown in Table 10.
SEA criteria for the use of iPCSK9.
| Clinical situation | Additional conditioning factors | LDL-c |
|---|---|---|
| Homozygotic familial hypercholesterolaemia | – | >100 |
| Heterozygotic familial hypercholesterolaemia | <4 associated risk factors | >160 |
| ≥4 associated risk factors | >130 | |
| With diabetes | >100 | |
| With arteriosclerotic vascular disease | >70 | |
| Secondary prevention | Stable | >130 |
| Acute coronary syndrome (<1 year) | >100 | |
| Diabetes + one additional risk factor | >100 | |
| More than two uncontrolled additional risk factors | >100 | |
| Lp(a) > 50 mg/dL | >70 | |
| Recurring or non-revascularizable multi-vessel coronary disease | >70 | |
| Isolated symptomatic PAD or polyvascular disease | >70 | |
| Acute coronary syndrome < 1 year + diabetes | >70 | |
| Stage ≥3 IRC + 1 FR | >70 | |
| Primary prevention with very high risk | IRC ≥ 3 b (not in dialysis) + DM | >130 |
Adapted from Ascaso et al.78
A summary of the indications for treatment with lipid-lowering drugs according to the level of risk shown in Table 5 is given in Table 11.
Indications for lipid-lowering treatment according to cardiovascular risk and LDL-c concentration.
| Vascular risk | LDL cholesterol | ||||
|---|---|---|---|---|---|
| 55−70 mg/dL | <70 mg/dL | 70–115 mg/dL | 116–190 mg/dL | >190 mg/dL | |
| Low or moderate | Recommendations for lifestyle changes. No treatment required | Recommendations for lifestyle changes. No treatment required | Lifestyle changes and functional foods. | High risk by definition | |
| Consider lipid-lowering treatment | Lifestyle changes and functional foods. | ||||
| Start high intensity lipid-lowering treatment | |||||
| Objective LDL-c < 115 mg/dL | Objective LDL-c < 70 mg/dL | ||||
| High | Recommendations for lifestyle changes. No treatment required | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. | |
| Start lipid-lowering treatment if targets are not achieved | Start high intensity lipid-lowering treatment | Start high intensity lipid-lowering treatment | |||
| Objective LDL-c < 70 mg/dL | Objective LDL-c < 70 mg/dL | Objective LDL-c < 70 mg/dL | |||
| Very high | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. | Lifestyle changes and functional foods. |
| Consider lipid-lowering treatment | Start lipid-lowering treatment | Start high intensity lipid-lowering treatment | Start high intensity lipid-lowering treatment | Start high intensity lipid-lowering treatment | |
| Objective LDL-c < 55 mg/dL | Objective LDL-c < 55 mg/dL | Objective LDL-c < 55 mg/dL | Objective LDL-c < 55 mg/dL | Objective LDL-c < 55 mg/dL | |
The main aim in patients with atherogenic dyslipidaemia is to achieve therapeutic targets for LDL-c or non-HDL cholesterol according to their level of risk, using treatment with statins or high-intensity therapeutic combinations (Fig. 5). Once these targets have been achieved, in patients in secondary prevention who still have a concentration of triglycerides >200 mg/dL and low HDL-c (<40 mg/dL in men or <50 mg/dL in women) in spite of optimum lipid-lowering treatment, the use of fenofibrate or omega-3 fatty acids may be recommended (ethyl eicosapentaenoic acid at 4 g/day).79
Patients with hypertriglyceridaemiaTriglycerides from 200 to 500 mg/dLFollow the above recommendations for their levels of CVR and LDL-c. According to the recommendations of the European Atherosclerosis Society (EAS), for high-risk patients who still have concentrations of triglycerides > 200 mg/dL after controlling their LDL-c with statins, the use of fenofibrate or omega-3 fatty acids may be considered.19 Likewise, based on the results of the REDUCE-IT study the 2019 EAS/ESC Guides recommend high doses of ethyl eicosapentaenoic acid in combination with statins for patients in secondary prevention or with DM, with an associated risk factor and triglycerides at 150 mg/dL or higher.69
Triglycerides from 500 to 1000 mg/dLFollow the above recommendations for their level of CVR and non-HDL cholesterol levels (applying the same criteria as those for LDL-c plus 30 mg/dL).
If after the application of these recommended therapeutic measures triglyceride levels remain at >500 mg/dL, consider adding fenofibrate and/or omega-3 fatty acids.80
Triglycerides > 1000 mg/dLThe priority is to reduce triglycerides using diet, to prevent pancreatitis by reducing the total amount of fat to less than 30 g per day. In severe and persistent cases medium chain triglyceride (MCT) oil should be considered.
Commence treatment with fenofibrate and/or omega-3 fatty acids (>3 g per day) and consider a combination in the case of insufficient control.
Patients with genetic dyslipidaemiaFamilial hypercholesterolaemiaPatients with FH are considered to be high risk by definition, and they should achieve LDL-c < 70 mg/dL or <55 mg/dL in the case of associated ACVD. As well as diet and a healthy lifestyle, the majority of these patients should receive a combination of powerful statins and ezetimibe.81 Depending on their basal risk, which should be stratified, these patients may be treated with iPCSK9 (Table 10). Early treatment is necessary to prevent the development of cardiovascular complications in the future. As well as conventional lipid-lowering therapy (high-dose statins and ezetimibe), patients with the homozygotic form of the disease may also be given iPCSK9 and, under certain circumstances, lomitapide and/or LDL apheresis.82 The guides suggest that children with FH may commence treatment at from 8 to 10 years old, starting with low doses and increasing them until recommendable levels are reached.14 The recommended LDL-c target level in children is <135 mg/dL.
Combined familial hyperlipidaemiaThere are no specific recommendations for the treatment of this entity. This dyslipidaemia raises CVR and should be treated at first with diet and lifestyle changes; statins are the first drug of choice, as they reduce CVR in patients with concomitant hypertriglyceridaemia; the targets for LDL-c and non-HDL cholesterol are those recommended according to the level of risk. It is often necessary to add fibrate to statin therapy to achieve the therapeutic targets. Fenofribrate is the drug of choice in these cases.
DysbetalipoproteinaemiaThis is a rare form of hyperlipidaemia that is characterized by high levels of intermediate density lipoprotein particles. It leads to severe mixed hyperlipidaemia, the presence of striated xanthomas on the palms and a high risk of early onset ACVD, with especial predilection for peripheral arterial disease. It is usually caused by the combination of an E2/E2 genotype in the Apo E gene and one or several environmental factors (hypothyroidism or obesity, etc.). It should be suspected in any mixed hyperlipidaemia with low levels of Apo B (< 120 mg/dL).83 Treatment here aims to control the coexisting environmental factor, especially obesity, while using statins in combination or not with ezetimibe and/or fibrates. Given the characteristics of this dyslipidaemia, the main aim is to control non-HDL cholesterol.84
Chylomicronaemia syndromeThis consists of an accumulation of chylomicrons in the blood stream due to a lack of lipoprotein lipase (LPL) activity. This leads to insufficient triglyceride catabolism with a severe increase in the concentration of the same.85 There is a group of severe genetically-based chylomicronaemias, the familial chylomicronaemia syndrome,86,87 and another far more common type which is due to the association of less pathogenic genetic variants with aggravating factors, the multifactorial chylomicronaemia syndrome.88 The familial chylomicronaemia syndrome is rare, as it affects 1/1000,000 of the world population. It is due to recessive mutations with loss of function, and it is homozygotic or compound heterozygotic of the LPL, Apo C2, Apo A5, lipase maturation factor (LMF1), protein 1 binding high density lipoproteins anchored in glycosylphosphatidylinositol (GPIHBP1) and glycerol-3-phosphate dehydrogenase 1 (G3PDH1) genes.89,90 Chylomicronaemia appears at first without any aggravating factors, and triglyceride concentrations remain very high chronically, generally > 10 mmol/L (885 mg/dL).91 The most severe complication is acute pancreatitis, which is both more frequent and relapsing the higher the concentration of triglycerides, and it may appear in the first years of life.92 The pancreatitis may develop into chronic pancreatitis with pancreatic insufficiency and pancreoprivic DM. Eruptive xanthomas may also appear on the extension surfaces of the extremities and trunk, together with hepatosplenomegaly and lipemia retinalis.93
Multifactorial chylomicronaemia syndrome is caused by the association of different less pathogenic genetic factors, associated with entities which cause secondary hypertriglycidaemia.94 Although there is a risk of pancreatitis, this is lower than it is in familial chylomicronaemia syndrome due to the lower degree of severity and persistence of the hypertriglyceridaemia.95 The response to diet, physical activity and triglyceride-lowering drugs is usually good. Multifactorial chylomicronaemia syndrome usually appears in adult or middle age.
In both situations treatment is based on a diet which strongly restricts the total amount of fat (<30 g per day), the use of TCM oil, fibrates and omega-3 fatty acids at doses above 3 g per day.
HypoalphalipoproteinaemiaThe most common causes of hypoalphalipoproteinaemia are associated with hypertriglyceridaemia and atherogenic dyslipidaemia. The primary causes are exceptional and include Tangier disease, LCAT deficit and genetic variants at the level of the Apo A1 gene.96 There are no specific treatments for these entities, and these subjects should be treated with the aim of controlling their other CVR factors.97
Patients with arterial hypertensionBefore starting antihypertensive treatment, we recommend appropriate measurement of AP to confirm the diagnosis of AHT (Table 3), especially grade 1 AHT 1: SAP 140−159 mmHg and/or SAP 90−99 mmHg8 (Table 4).
OBPM during 24 h or SBPM are correlated more closely with the prognosis and target organ lesion than clinical measurement, so that we strongly recommend that they be used. As well as its diagnostic value in untreated subjects and the identification of subjects with white coat AHT (high AP in the clinic and normal values in OBPM), it is also a useful instrument for monitoring the effects of treatment, evaluating adherence and detecting subjects with masked AHT (subjects with normal AP in the surgery and high values during OBPM). Table 12 shows the definition of AHT based on clinical, outpatient and self-measured AP in the home.
Definitions of hypertension according to clinical, outpatient and home-measured AP.
| Category | SAP (mmHg) | DAP (mmHg) |
|---|---|---|
| AP in the surgery | ≥ 140 | ≥ 90 |
| Outpatient AP | ||
| Daytime (or when awake) | ≥ 135 | ≥ 85 |
| Nocturnal (or when asleep) | ≥ 120 | ≥ 70 |
| 24 h | ≥ 130 | ≥ 80 |
| AP at home | ≥ 135 | ≥ 85 |
AP: arterial pressure; DAP: diastolic arterial pressure; SAP: systolic arterial pressure.
Adapted from Williams et al.8
The decision to start pharmacological treatment will depend not only on the level of AP but also on the total CVR based on other associated CVRF and the presence of any subclinical lesion in target organs (Table 4). This will predict cardiovascular mortality independently of the SCORE index,8,18 especially in the group at moderate risk, and the decision may also be based on the presence of a ACVD or established renal disease.
The initial treatment should always include lifestyle changes, which are the only opening treatment in subjects with grade 1 AHT and low to moderate risk.98,99 Subjects with grade 1 AHT at high risk or with a target organ lesion should commence pharmacological treatment once the diagnosis of AHT has been confirmed in several visits, preferably by OBPM or SBPM.
Pharmacological treatment should be added from the first in subjects with grade 2 AHT (SAP from 160−179 mmHg and/or DAP of 100−109 mmHg), as well as in subjects with grade 3 AHT. The majority of these patients will require antihypertensive treatment which combines at least two drugs. Approximately 10%–12% of hypertensive patients require treatment with more than 3 antihypertensive drugs.100
The chief benefit of antihypertensive treatment derives from the fall in AP99 regardless of the drug used. Treatment may commence with thiazide diuretics or similar drugs (indapamide, clortalidone), ACE inhibitors or angiotensin A1 receptor antagonists (ARA-II), calcium antagonists or beta-blockers. The presence of the latter as first line drugs has been questioned because of their reduced efficacy in preventing ictus or the possible negative metabolic effects (although the latter may not be applicable to all beta-blockers). The exception to this is in clinical situations where specific indications exist for these agents (coronary disease, HF with a reduced ejection fraction, etc.). They may also be indicated as the first treatment in young hypertensive patients with a hyperkinetic pattern and a tendency to tachycardia, or in women of fertile age in whom renin-angiotensin system (RAS) inhibitors should be used with care, as they are contraindicated in the initial phases of gestation.
A recent meta-analysis has shown that a fall of 5 mmHg in systolic AP reduces major cardiovascular events by approximately 10%, in primary as well as in secondary prevention.101
The therapeutic aim of antihypertensive treatmentThe first goal is to reduce SAP to <140 mmHg in all patients. Based on the results of the SPRINT7,102 study and those of the latest meta-analyses,103 new guides8 state that if antihypertensive treatment is well-tolerated, the therapeutic goal should be SAP of ≥130 mmHg and <140 mmHg in the majority of patients, and a SAP of 120−129 mm Hg in certain specific situations. With respect to DAP, a pressure of < 80 mmHg should be considered for all hypertensive patients, regardless of their previous level, basal risk or comorbidities (Table 13).
Therapeutic targets for clinical SAP in some hypertensive subgroups.
| Clinical conditions | Systolic arterial pressure |
|---|---|
| Age < 65 years | 120 < 130 mmHg |
| Age ≥ 65 years | 130 < 140 mmHga |
| Diabetes | 130 mmHg or lessb |
| Coronary disease | 130 mmHg or less |
| Chronic renal disease | 130 < 140 mmHg |
| Post Ictus/TIA | 120 < 130 mmHg |
TIA: transitory ischemic attack.
Adapted from Williams et al.8
The prevalence of AHT in patients with DM stands at up to 80%, double the level observed in the non-diabetic population of the same age and characteristics. The coexistence of AHT and DM increases the risk of developing ACVD, together with higher incidence of coronary disease, HF, PAD, ictus and cardiovascular mortality.
We recommend starting treatment with a combination of a RAS inhibitor plus a calcium antagonist or thiazide or thiazide-like diuretic, except when the estimated glomerular filtration rate (eGFR) is < 30 mL/min/1.73 m2, in which case a loop diuretic would be indicated. We do not recommend using a combination of two RAS inhibitors.
We recommend starting antihypertensive treatment when the clinical AP stands at ≥140/90 mmHg, and the therapeutic target would be systolic AP of 130 mmHg or less, if it is tolerated, but not below 120 mmHg, with a DAP of < 80 mmHg, but not < 70 mmHg, if there is proteinuria. In subjects aged 65 years or more a therapeutic target for SAP is recommended of from 130 to < 140 mmHg.80,104
AHT and chronic renal diseaseThe prevalence of AHT in these patients stands at from 67%–92%, and it is their most frequent comorbidity. On the other hand, AHT may accelerate the progress of renal damage, as well as increasing the CVR. As is the case for all subjects with AHT, pharmacological treatment must be accompanied by lifestyle changes, with special emphasis on reducing Na intake. Loop diuretics should replace thiazide or thiazide-like diuretics when the eGFR is <30 mL/min/1.73 m2. As the fall in AP reduces the perfusion pressure, a fall of 10%–20% in the eGFR is not uncommon at the start of treatment. Electrolytes should be carefully monitored.
We recommend that treatment should start when AP stands at ≥140/90 mmHg, with a therapeutic target for SAP (in diabetic as well as non-diabetic patients) within the range from 130 to <140 mmHg. In some cases, and on an individual basis, the AP may be reduced by more depending on tolerance and its impact on renal function and electrolytes. In patients with > 1 g/day proteinuria a larger reduction in AP is beneficial.
Initial treatment with a RAS inhibitor is recommended plus a calcium antagonist or a diuretic. We do not recommend the simultaneous use of an ACE inhibitor and an ARA-II.
AHT and stable coronary diseaseAHT is a major risk factor for coronary disease. Numerous clinical trials have shown the benefits of antihypertensive treatment in reducing the incidence of coronary disease in primary as well as in secondary prevention.
Except for certain cases, such as patients over the age of 80 years or frail elderly patients, in general we recommend dual initial therapy with a RAS inhibitor (ACE or ARA-II) plus a beta-blocker or calcium antagonist. Nevertheless, other combinations may be used, such as a dihydropyridine calcium antagonist plus a beta-blocker. As a second step, if the AHT is not brought under control then the patient should be treated with a triple therapy, generally by adding a diuretic to any one of the above combinations.
If a patient has symptomatic angina, we recommend a combination of beta-blockers and a dihydropyridine calcium antagonist.
The therapeutic goal would be a SAP of 130 mmHg or less if tolerated, although not <120 mmHg, and a DAP of <80 mmHg, but not <70 mmHg. The therapeutic target in subjects aged 65 or more may be a SAP from 130−140 mmHg.
AHT and heart failure or left ventricular hypertrophy75% of patients with chronic HF have a history of AHT. We recommend antihypertensive treatment when clinical AP is ≥140/90 mmHg, in patients with HF and a reduced EF as well as those with a preserved EF and HF. In patients with HF and a reduced EF we recommend treatment with a RAS inhibitor (ACE or ARA-II), a beta-blocker and a diuretic, associated or not with a mineralocorticoid receptor antagonist. If the previous treatment is unable to bring AHT under control then a dihydropyridine calcium antagonist could be added.
In patients with HF and a preserved EF, no specific drug has been proved to be effective in terms of morbimortality apart from controlling the AHT. However, in the case of left ventricular hypertrophy (LVH), which is very common in these patients, we recommend the use of a RAS inhibitor in combination with a calcium antagonist or a diuretic. For patients with LVH we recommend a therapeutic SAP target of from 120 to 130 mmHg.
AHT and ictusIctus is a major cause of death, disability and dementia, and it is independently associated with the increase in major cardiovascular events in older people of both sexes.105 Given that ictus refers to a heterogeneous group in terms of its causes and underlying haemodynamics, controlling AHT in these patients is complex and a true challenge. The therapeutic target is AP < 140/90 mmHg, although a target SAP of from 120 to 130 mmHg may be suitable in many cases. A recent meta-analysis106 confirmed the benefit of reducing AP to below 130/80 mmHg in the secondary prevention of ictus.
Resistant AHT and refractory AHTSeveral reasons exist for the poor control of AHT in clinical practice, and these should be taken into account before labelling a patient as having resistant hypertension (Fig. 6)107.
Resistant hypertension accounts for approximately 10%–12% of treated cases of hypertension.100 AHT is considered to be resistant when it has been impossible to reduce AP to <140/90 mmHg in spite of using optimum doses (or the maximum tolerated doses) of three drugs, together with a therapeutic plan that includes a diuretic (typically an ACE inhibitor or ARA-II plus a calcium antagonist and a diuretic).
Poor control of the condition should be confirmed by OBPM (preferably) or by SBPM, and the causes of pseudo-resistance (poor adherence) should be excluded, as well as secondary hypertension.
A new phenotype of refractory hypertension has recently been described, when an AP < 140/90 mmHg is not achieved in spite of use ≥ 5 antihypertensive drugs.108,109 It is not very frequent (1.4%), and it also requires 24 -h OBPM for confirmation. As is the case for resistant hypertension, the causes of pseudo-resistance should be excluded. A recent prospective study110 has shown that patients with refractory hypertension confirmed by 24-h OBPM have a higher risk of major cardiovascular events and mortality, Fig. 7 shows a practical proposal for the clinical management of subjects with resistant or refractory hypertension.111
Monitoring hypertensive patientsAfter starting antihypertensive pharmacological treatment, it is important to follow-up the patient at least once during the first two months, to evaluate the effect on hypertension and detect any possible adverse effects, or to discover any possible poor adherence. Subsequently we recommend a follow-up visit at from three to six months, to verify the control of the hypertension and evaluate adherence.
Poor adherence often causes a lack of control of hypertension. Nurses, community pharmacists and other medical professionals play a fundamental role in assessing and detecting this, and they also greatly help the education, support and long-term follow-up of hypertensive patients. They should therefore be included within the overall strategy to improve the control of AP. Simplifying treatment and reducing the number of tablets will doubtless help to improve adherence and achieve better control of AP over the medium to long terms.
Although subjects with normal to high AP are not treated, lifestyle changes are recommended for them, and they should be regularly followed-up, with at least one visit per year to measure their AP clinically and on an out-patient basis, together with re-evaluation of their CVR.
Patients with hyperglycaemia (prediabetes and diabetes)Classification and diagnostic testsHyperglycaemia is considered to exist when fasting glucose in plasma ≥ 100 mg/dL, and DM is considered when fasting glycaemia in plasma ≥ 126 mg/dL. As well as the fasting glycaemia criterion, prediabetes and DM may be diagnosed when at least one HbA1c criterion is fulfilled, random glycaemia values or plasmatic glycaemia levels at two hours of the oral glucose tolerance test described in Table 14.39
American Diabetes Association (ADA) diagnostic criteria.39
| Diagnosis of prediabetes and diabetes | |||
|---|---|---|---|
| Normal | Prediabetes | Diabetes | |
| HbA1c (%) or | <5.7 | 5.7−6.4 | ≥6.5 |
| Basal plasmatic glycaemia (mg/dL) or | <100 | 100−125 | ≥126 |
| Glycaemia 2 h. OGTT with 75 g (mg/dL) or | <140 | 140−199 | ≥200 |
| Glycaemia at random with hyperglycaemia symptoms (mg/dL) | ≥200 | ||
The OGTT is performed by administering 75 g glucose to an adult or 1.75 g/kg weight (maximum 75 g) in children under standardized conditions. This test is a highly useful means of confirming the diagnosis in asymptomatic high-risk patients (Table 15)41 if fasting glycaemia levels or HbA1c have not been diagnosed.39,41 HbA1c levels must be measured using a standardized method with the DTCC study and Reference 39.
Diabetes screening.41.
| Age ≥ 45 years | Age < 45 years |
|---|---|
| In all cases | Prediabetes (a history of oral intolerance of glucose or altered basal glycaemia or HbA1c ≥ 5.7 |
| A history of gestational diabetes. Test again every three years. | |
| If the BMI ≥ 25 kg/m2, ≥ 23 kg/m2 in Asians, with at least one of the following criteria: | |
| - Waist perimeter: men ≥ 102 cm and women ≥ 88 cm | |
| - Diabetes in first degree family members | |
| - Belonging to an ethnic group at high risk for diabetes (Afro-Americans, Latin Americans, etc.) | |
| - A history of cardiovascular disease | |
| - Arterial hypertension (≥ 140/90 mmHg or under treatment) | |
| - HDL-c < 35 mg/dL, TGS > 250 mg/dL, or both | |
| - Polycystic ovary syndrome | |
| - Sedentary lifestyle | |
| - Other conditions associated with insulin resistance (acantosis nigricans, non-alcoholic fatty liver, combined familial hyperlipidaemia, etc.) |
It is obligatory to screen for DM in asymptomatic subjects at high CVR (Table 15) with the aim of making an early diagnosis.
Control targets in prediabetes and diabetesThe general control targets are shown in Table 16.
Control targets for prediabetes and diabetes.
| 1 Establish and maintain good metabolic control. |
| 2 Prevent the complications of diabetes. |
| 3 Preserve the patient’s life and relieve the symptoms of hyperglycaemia. |
| 4 Enable the patient to achieve good quality of life (personal, family, at work and social). |
| Individualize the HbA1c level |
To gain good control of glycaemia the HbA1c target has to be individualized.39 To set the target level of HbA1c we have to take into account the patient’s degree of motivation, any chronic or severe comorbidities, their age, survival and the duration of evolution (Fig. 8). On young patients without chronic complications or comorbidities, a short duration of evolution and long survival, we will seek to intensify the treatment to achieve 6%–7% HbA1c.39
The overall objectives when treating DM are shown in Table 17.
General objectives when treating a diabetic adult.
| Optimum | Acceptable | Undesirable | |
|---|---|---|---|
| GB mg/dL | 70−130 | 130−179 | ≥ 180 |
| GP 2 h mg/dL | <180 | 180−199 | ≥ 200 |
| HbA1c % | <7.0 | 7.0−7.9 | ≥ 8.0 |
| LDL-c mg/dL* | Variable | Variable | Variable |
| BMI | <25 | 25−26,9 | ≥ 27 |
| AP mmHg | <130/<80 | 130−139/80−89 | ≥ 140/≥ 90 |
| Smoking | DO NOT SMOKE | ||
| BMI | Ideal < 25 kg/m2 in obese patients < 30 kg/m2 | ||
LDL-c depending on risk; CV: very high-risk DM LDL-c < 55 mg/dL, high risk DM < 70 mg/dL, moderate risk DM LDL-c < 115 mg/dL (see Table 23).
BG: basal glycaemia, PG: postprandial glycaemia, AP: arterial pressure.
Appropriate management of DM treatment makes it necessary to not only bring glycaemia under control and seek an individualized level of HbA1c, as we also have to control AP levels, ensure smoking cessation and attempt to achieve optimum weight. This is known as the overall treatment of DM. This treatment will always be both individualized and early.
Treating prediabetes and diabetesDiet and lifestyle changesTreatment should be aimed at achieving all of the overall objectives. Diet is the pillar of the treatment of prediabetes and DM2, together with lifestyle changes and physical activity.19,39,112 As the majority of these patients are obese, dietary treatment is similar to the method described in the section on obese patients.
Modifications to the lifestyle of patients with DM2 are essential to improve not only the control of glycaemia but also the associated comorbidities such as dyslipidaemia, obesity and AHT. Changes towards a healthy lifestyle should include the following aspects: smoking cessation and moderation in alcoholic drinks consumption, intensive weight loss (>5%) in the case of overweight or obesity, nutritional assessment, physical activity and continuous supervision of a change in habits. The intake of carbon hydrates from vegetables, fruit, whole grain cereals, pulses and dairy products should be prioritized over those from other sources.19,112 Processed foods containing added fats, sugars or sodium should be especially restricted. We recommend the consumption of n-3 polyunsaturated fats which are present in fish and especially in blue fish (sardines, salmon, tuna, mackerel and horse mackerel, etc.). To summarise, a Mediterranean-type diet rich in monounsaturated fatty acids (virgin olive oil and nuts) may be useful to achieve the overall control of CVR in these patients.19,112
Different meta-analyses of the effects of lifestyle interventions in individuals with prediabetes found falls of around 50% in the risk of developing DM2.39 The general characteristics of diet and physical exercise in cardiovascular prevention have already been described,19,112 and they support the suitability of a Mediterranean-type diet, with restricted calorie intake if subjects with prediabetes are overweight or obese.113
The general recommendations for physical activity in individuals with prediabetes or DM are shown in Table 18.
General recommendations for physical activity in individuals with prediabetes and diabetes.
| Perform at least 150 min per week of moderate to intense aerobic activity, spread over at least three days of the week. Do not go more than two days without physical activity. |
| Perform 2–3 sessions per week of endurance exercises on non-consecutive days. |
| Reduce the time spent on sedentary activities every day. |
| Older adults should perform flexibility and balance exercises 2–3 times per week. |
In subjects with prediabetes the ADA recommends treatment with metformin, especially in patients with a BMI > 35 kg/m2, age < 60 years and women with a history of gestational DM. Cases should also be considered when fasting glycaemia ≥ 110 mg/dL, HbA1c ≥ 6.1% and predominantly visceral obesity.39
The treatment goals for a patient with DM are: to prevent and delay macrovascular and microvascular complications, and to reduce the high level of cardiovascular morbimortality. The maximum benefit in cardiovascular prevention in DM patients is obtained by simultaneous intervention in all of the CVRF: smoking, dyslipidaemia, AHT and hyperglycaemia.19,39
Centring on pharmacological treatment for hyperglycaemia, once the target level of HbA1c has been decided, choice of the drugs to be used will depend on age, degree and type of obesity of the patient, together with the efficacy and tolerability of the drugs, their cost, and any comorbidities (secondary prevention, HF or diabetic nephropathy) (Fig. 8).
Table 19 shows the main drugs used to treat hyperglycaemia in DM and their indications.
The main drugs used to treat diabetes.
| Drug | Dose | Indication |
|---|---|---|
| Metformin | 850 mg × 3 | DM2 |
| Titrate gradually | Obese DM1 | |
| Gestational diabetes | ||
| DM with heart failure | ||
| Pioglitazone | 30−45 mg/day | DM2 |
| DM2 and fatty liver | ||
| IDPP-4 | Sitagliptin 100 mg/day | DM2 |
| Vidagliptin 50 mg × 2 | ||
| Saxagliptin 5 mg/day | ||
| Linagliptin 5 mg/day | ||
| Alogliptin 25 mg/day | ||
| arGLP-1 | Exenatide 5 μg sc × 2 1st month, 10 μg sc × 2 2nd month | DM2 obese in secondary prevention |
| Liraglutide 0.6 mg/day, 1.2 mg/day 2nd week and if necessary 1.8 mg/day | DM2 when BMI ≥ 30 kg/m2 | |
| Exenatide LAR, 2 mg/week | ||
| Lixisenatide, 10 μg/day 2 weeks, increase to 20 μg/day | ||
| Dulaglutide 0,75 mg/week, increase 1.5 mg/week | ||
| Semaglutide 0.25 mg/week during 4 weeks and raise to 0.5 mg/week Max. 1 mg/week | ||
| SGLT-2 inhibitors | Dapagliflozin 10 mg/day | DM2 |
| Empagliflozin 10 and 25 mg/day | DM2 with HF | |
| Canagliflozin 100 y 300 mg/day | DM2 in secondary prevention | |
| Ertugliflozin 5 y 15 mg/day | DM2 diabetic nephropathy | |
| Meglitinide | Repaglinide 0.5−4 mg before the 3 main meals | DM2 with CRD |
| Nateglinide 120 mg, in the 3 main meals | ||
| Sulfonylurea drugs | Start at low doses and gradually increase the dose | DM2 |
| 2nd or 3rd generation | ||
| Alpha- glucosidase inhibitors | Acarbose or miglitol 50 mg × 3/ day, at the start of meals. Max. 100 mg × 3/day | DM2 |
arGLP-1: GLP-1 receptor agonists.
Table 20 summarises the chief benefits, contraindications, adverse effects and degree of cardiovascular prevention provided by glycaemia-lowering drugs, independently of their main effect.
Benefits, contraindications, chief adverse effects and cardiovascular prevention of the main drugs used to treat diabetes.
| Drug | Benefits | Contraindication | Disadvantages/adverse effects | CV prevention |
|---|---|---|---|---|
| Metformin | ↓ HbA1c 1% | eGFR < 30 mL/min/1.73 m2 | Nausea, vomiting, abdominal pain | Yes39 |
| ↓ weight (1.5−2 kg) | Severe liver failure 24 h. before surgery | Exceptional: vitamin B12 deficiency and lactic acidosis | ||
| ↓ cost | ||||
| No hypoglycaemia | ||||
| Pioglitazone | ↓ HbA1c 0.5−1% | Heart failure | ↑ weight | Yes, in primary prevention |
| ↓ TGS and ↑ HDL-c | ↑ cost | Ictus39 | ||
| Oedemas | ||||
| HF | ||||
| Postmenopausal fractures | ||||
| IDPP-4 | ↓ HbA1c 0.8 % | GFR < 60 mL/min/1.73 m2 adjust dose of sitagliptin 25 and 50 mg/day, alogliptin 6.25 and 12.5 mg/day | ↑ cost | No |
| Weight = | ||||
| No hypoglycaemia | Does not require linagliptin | Skin lesions (bullous pemphigoid) | Saxagliptin ↑ admissions due to heart failure | |
| Do not combine with arGLP-1 | ||||
| arGLP-1 | ↓ HbA1c 1% | Pregnancy | Nausea, vomiting | Yes |
| ↓↓ weight | ↑ cost | Liraglutide and semaglutide in secondary prevention.114,115 Dulaglutide primary and secondary prevention116 | ||
| SGLT-2 inhibitors | No hypoglycaemia | Pregnancy | Urinary infections | Yes |
| ↓ HbA1c 1% | Genital Mycosis | Empagliflozin and Canagliflozin in secondary prevention117,118 | ||
| ↓ weight | Rare orthostatic hypotension, dehydration and euglycaemic cetoacidosis | Empagliflozin mortality117 | ||
| ↓ arterial pressure | Dapagliflozin CV mortality119 | |||
| No hypoglycaemia | Empagliflozin, Canagliflozin, Dapagliflozin Ertugliflozin admission due to heart failure117–119 Canagliflozin and Dapagliflozin preservation of renal function and ↓ progression of diabetic nephropathy120,121 | |||
| Meglitinides | ↓ HbA1c 0.8 % | Pregnancy and breast feeding | Hypoglycaemia | No |
| ↑ Weight | ||||
| Use in chronic renal dis. | ||||
| Sulfonylurea drugs | ↓ HbA1c 1% | Pregnancy and breast feeding | Hypoglycaemia | No |
| ↑ Weight (2−4 kg) | Hospitalized patient | Do not use if CRD, liver failure or heart failure | ||
| alpha- glucosidase inhibitors | ↓ HbA1c 0.5 % | DM1 | Gastrointestinal: flatulence, bloating, pain | No |
| Weight = | Pregnancy and breast feeding | |||
| Gastrointestinal diseases |
Fig. 9 shows the therapeutic guidelines. The first line drug for patients with DM2 is metformin.39 For a second therapeutic step we will select a drug on the basis of whether the patient is in primary or secondary cardiovascular prevention, whether they are obese or have diabetic nephropathy or HF.39 Numerous studies of intervention have shown the capacity of SGLT2 inhibitors117–119 or GLP-1 receptor agonists in cardiovascular prevention114–116 and the progression of diabetic nephropathy in individuals with DM,120,121 and they should be prioritized for use in a second therapeutic step.
Therapeutic guidelines and scaling in the pharmacological treatment of diabetes attending to the chief comorbidities.
arGLP1: glucagon 1-like peptide receptor agonists; BMI: body mass index; DM2: type 2 diabetes mellitus; HbA1c: glycosylated haemoglobin; iSGLT2: inhibitors of the sodium and glucose cotransporter type 2; IDPP-4: inhibitors of dipeptidyl peptidase 4.
Insulin with a prolonged or basal action will be indicated in DM2 if the triple combination fails (the third therapeutic step, Fig. 9), on condition that arGLP-1 have been tried, or if initially it is clinically clear that there is hyperglycaemia with raised HbA1c (Fig. 9). The initial total daily dose of insulin is 0.1−0.3 U/kg bodyweight, adjusting the dose every three days until the targets are achieved. We should always evaluate whether it is really necessary to increase the dose, as in subjects who are obese or insulin-resistant high doses may lead to an increase in their weight, thereby increasing their insulin-resistance without improving the control of their glycaemia.39
Cardiovascular prevention in diabetesThe treatment and prevention of ACVD in individuals with diabetesCardiovascular prevention in cases of DM requires early, intensive and maintained intervention against all of the CVRF: dyslipidaemia, AP, smoking and abdominal obesity:19,39,112 see Table 21.
Treatment and prevention of cardiovascular disease in individuals with diabetes.
| Objective | Treatment | |
|---|---|---|
| Control of hyperglycaemia | HbA1c < 7%. | Glycaemia-lowering diet |
| –SGLT2 or arGLP-1 | ||
| Arterial hypertension | AP < 140−130/80−90 mmHg If albuminuria, AP < 130/80 mmHg | Reduce salt intake < 3 g/24 h |
| If macroalbuminuria or renal failure, restrict protein consumption to 0.6−0.8 g/kg/24 h | ||
| Reduce alcohol (maximum tolerated 30 g/day) | ||
| Moderate coffee consumption (2 cups/24 h.) | ||
| ACEi or ARA-II | ||
| Smoking | No active or passive smoking | |
| Obesity | BMI < 30 kg/m2 | Strategy based on dietary therapy |
| Dyslipidaemia | Primary: reduce LDL-c according to patient risk (see Table 23) | Statins combined or not with ezetimibe to achieve therapeutic targets, - iPCSK9 in secondary prevention according to SEA recommendations (Table 10). |
| Secondary TGS < 150 mg/dL | Fibrates or omega 3 (EPA) |
Table 22. In general, the individuals with DM2 are considered to be at high cardiovascular risk.19,122 Patients with DM2 and multiple CVRF (dyslipidaemia, AHT, smoking) or target organ lesion (diabetic nephropathy) or who are in secondary prevention have a very high cardiovascular risk.19 The primary goal in cardiovascular prevention is to achieve a level of LDL-c or non-HDL-c as shown in Table 23, depending on the risk classification of the patient.19
Primary targets for LDL-c or non-HDL-c for cardiovascular prevention in individuals with diabetes.19.
| Moderate risk | High risk | Very high risk | |
|---|---|---|---|
| DM2 < 50 years or DM1 < 35 years and less than 10 years evolution and no other CVRF | Those who are not included under moderate or very high risk | DM with ACVD or more than 3 CVRF or target organ involvement | |
| DM1 with more than 20 years evolution | |||
| LDL-c (mg/dL) | <100 | <70 | <55 |
| non-HDL-c (mg/dL) | <130 | <100 | <85 |
| TGS mg/dL | <200 | <200 | <200 |
| HDL-c (mg/dL) (man/woman) | >40/>50 | >40/>50 | >40/>50 |
Diagnostic criteria for the metabolic syndrome.
| Diagnostic criteria for metabolic syndrome. Diagnosis if ≥ 3 criteria | |
|---|---|
| Abdominal obesity | Waist perimeter (above the iliac crests) high according to sex and ethnic group (≥ 94 Caucasian men and ≥ 80 women) |
| Fasting glycaemia (mg/dL) | ≥ 100 mg/dL or specific previous treatment |
| Triglycerides in plasma (mg/dL) | ≥ 150 mg/dL or specific previous treatment |
| HDL cholesterol (mg/dL) | < 40 mg/dL in men or < 50 mg/dL in women or specific treatment |
| Arterial pressure (mmHg) | Systolic ≥ 130 or diastolic ≥ 85 or antihypertensive treatment |
To achieve these goals, it will be necessary to use high intensity statins, usually in combination with ezetimibe. Fig. 5 shows the treatment strategy and pattern for dyslipidaemia in individuals with DM, with the aim of achieving effective cardiovascular prevention. Once the target level of LDL-c or non-HDL-c has been achieved we should seek a secondary goal of TGS < 200 mg/dL in patients with DM and metabolic syndrome.19,41,79
Patients with obesity and metabolic syndromePatients with metabolic syndromeMetabolic syndrome is defined in Table 24.41
Treatment of metabolic syndrome.
| Lifestyle changes | |
|---|---|
| Diet | Mediterranean type (saturated fats < 7% of total calorie intake, cholesterol < 200 mg/day, avoid trans fats) |
| Rich in fibre | |
| Restrict sodium consumption (5−6 g/day) | |
| Foods with a low glycaemic index. Avoid simple sugars. | |
| Increase the intake of fruit, vegetables and whole grain cereals. | |
| Olive oil as the main fat used in preparing food | |
| Avoid processed foods | |
| Physical activity | Moderate-intense |
| 30 min/day (preferable 45−60 min.) | |
| Continuous/intermittent | |
| At least 5 days/week | |
| Adapted to patient age and cardiovascular status. | |
The most important metabolic alterations associated with metabolic syndrome are41:
Dyslipidaemia, fundamentally hypertriglyceridaemia, a fall in HDL-c and the presence of small dense LDL-c particles, with an increase of free fatty acids in plasma. This set of alterations is known as atherogenic dyslipidaemia.
Hyperglycaemia or DMAHTTogether with abdominal obesity these alterations are the established parameters for the diagnosis of metabolic syndrome. Additionally, many other alterations which are not used in diagnosis are of great interest. These include hyperuricaemia or gout, hypercoagulability and fibrinolysis defects which often involve raised levels of plasminogen-1 activator (PAI-1), non-alcoholic hepatic steatosis and hyperandrogenism. The clinical importance of metabolic syndrome is linked to its prevalence, at 20%–40% of the general population and 80%–85% of subjects with DM2. Patients with metabolic syndrome are at high risk of developing ACVD and DM2.
Data from different meta-analyses indicate that individuals with metabolic syndrome have twice the risk of cardiovascular events and an increase of 1.5 times in mortality due to all causes compared to individuals who do not have metabolic syndrome.41 Recent studies have found a 5–10 times increase in the relative risk of developing DM2. Other complications which are no less important in connection with metabolic syndrome are: sleep apnoea, respiratory failure or idiopathic alveolar hypoventilation syndrome and several forms of cancer (breast, uterus, colon, oesophagus, pancreas, kidney and prostrate, etc.).
The treatment of metabolic syndrome should seek to control all of its components (Tables 25 and 26). Table 25 shows the treatments for metabolic syndrome. Treatment centres on lifestyle changes and weight loss, together with the control of atherogenic dyslipidaemia.41,112,123
Treatment of other metabolic syndrome components.
| Treatment | |||
|---|---|---|---|
| Objective | Secondary objectives | Treatment | |
| Dyslipidaemia | LDL-c depending on associated CVRF* | HDL-c mg/dL | Health and dietary measures |
| If hypertriglyceridaemia → non-HDL-c (30 mg/dL above the LDL target) or apoB | >40 H and >50 M | Statins, ezetimibe and/or fibrates if necessary | |
| TGS < 150 mg/dL. | |||
| Hypertension and microalbuminuria | AP < 140/90 mmHg. | Health and dietary measures | |
| NSAID or ARA-II ± other drugs | |||
| Other CVRF | Cease smoking | ||
| Consider antiaggregation in subjects at very high risk (aspirin) | |||
Although MS is not a coronary equivalent it is a risk modulator (Table 5) when assessing risk to set a LDL target.
Classification of individuals according to the body mass index. Definition of thinness and obesity.
| BMI | |
|---|---|
| Underweight | < 18.5 kg/m2 |
| Normal weight | 18.5−24.9 kg/m2 |
| Grade 1 overweight | 25−26.9 kg/m2 |
| Grade 2 overweight | 27−29.9 kg/m2 |
| Grade 1 obesity | 30−34.9 kg/m2 |
| Grade 2 obesity | 35−39.9 kg/m2 |
| Grade 3 or morbid obesity | 40−49.9 kg/m2 |
| Grade 4 or extreme obesity | ≥ 50 kg/m2 |
Obesity is defined as a BMI ≥ 30 kg/m2.124Table 27 shows patients classified according to their BMI.
Indications for very low-calorie diets.
| Indications | Contraindications |
|---|---|
| Patients with a BMI > 35 kg/m2 in which conventional treatment has failed and they also have: | BMI < 30 kg/m2 |
| The need to lose weight quickly. E.g., severe respiratory failure or orthopaedic surgery | Pregnancy breast feeding |
| Severe obesity-associated pathology that responds to weight loss, such as type 2 DM, AHT, dyslipidaemia or SAHS | Severe systemic or organic pathology, except in situations which are clearly aggravated by excess weight, in which individual risk-benefit assessment is recommended |
| Type 1 DM | |
| Psychiatric alterations: eating behaviour disorders, severe depression, psychosis, drug or alcohol addiction | |
| Hydroelectric disorders and orthostatic hypotension | |
| Diseases that cause protein losses: Cushing’s disease, systemic lupus erythematosus, proteinuria, neoplasias, poor absorption, inflammatory intestinal disease, etc. | |
| Acute cardiovascular diseases, cardiac arrhythmia, ictus | |
| Major surgery or trauma in the previous three months |
Obesity is highly prevalent in our country, and it is estimated to affect 14% of the population, while 24% are overweight. Obesity is the main risk factor for developing DM2, and in the case most especially of abdominal obesity it is associated with a high level of CVR.124
Treating obesity is complex and it has to be individualized.124,125 It is based on dietary therapy strategies (Fig. 10),126 lifestyle changes, psychotherapy and drugs (Tables 28 and 29).124,125
Pharmacological treatment of obesity.
| Liraglutide |
| Dosage |
| Saxenda® 6 mg/mL injectable solution in a preloaded pen. |
| Clinical effects |
| 8% weight loss maintained over 3 years. |
| Slowing of the progression from prediabetes to diabetes. |
| Adverse effects |
| Nausea and vomiting |
| Contraindications |
| Pregnancy and breast feeding |
| Multiple endocrine neoplasia (MEN-2) |
| Thyroid medullary carcinoma |
| Advanced renal disease |
| Advanced hepatic disease |
| Orlistat |
| Dosage |
| Orlistat 120 mg/ 2−3 times per day in the main meals (medical prescription required). |
| Orlistat 60 mg/ 2−3 times per day in the main meals (no medical prescription required). |
| Clinical effects |
| ↓ 37% progression to DM2 |
| 120 mg dose/3 times per day ↓ 3.1% initial weight after 1 year |
| ↓ SAP, PAD, total cholesterol, LDL-c |
| Adverse effects |
| 15%–25% gastrointestinal effects |
| May ↓ absorption of liposoluble vitamins |
| Nephrolithiasis due to oxalates (Orlistat ↑ urine oxalate levels and should be used with care in patients with a history of nephrolithiasis due to oxalates) |
| Contraindications |
| Chronic malabsorption syndrome |
| Cholestasis |
| Pregnancy and breast feeding |
| Naltrexone/Bupropion |
| Dosage |
| Naltrexone/Bupropion 8 mg/90 mg prolonged release tablets |
| When started treatment the dose should be raised over 4 weeks: |
| - Week 1: one tablet in the morning |
| - Week 2: one tablet in the morning and one at night |
| - Week 3: two tablets in the morning and one at night |
| - Week 4 and subsequent weeks: two tablets in the morning and two at night |
| We recommend that the last dose be taken in the evening to prevent insomnia if it is taken at dinner time |
| Clinical effects |
| 50% of patients lose ≥ 5% weight |
| Adverse effects |
| Nausea |
| Contraindications |
| Pregnancy and breast feeding |
| Uncontrolled AHT |
| Patients who currently suffer convulsive disorders or who have a history of convulsions |
| Known central nervous system neoplasia |
| Alcohol or benzodiazepine withdrawal syndrome |
| A history of bipolar disorder |
| Any simultaneous treatment that contains bupropion or naltrexone |
| Current or previous diagnosis of bulimia or anorexia nervosa |
| Addiction to prolonged administration opiates or opiate agonists (such as methadone) or with acute opiate withdrawal syndrome. |
| Simultaneous treatment with monoamine oxidase inhibitors (MAOi). At least 14 days should pass between cessation of MAOi administration and the start of treatment with Naltrexone/Bupropion |
| Severe hepatic failure |
| Terminal renal failure |
Surgical treatment of obesity.
| Indications for bariatric surgery | • BMI ≥ 40 kg/m2 |
| • BMI ≥ 35 kg/m2 with one or more severe comorbidities | |
| – The following are considered to be severe comorbidities: | |
| – Type 2 DM | |
| – Arterial hypertension | |
| – Dyslipidaemia | |
| – Respiratory disorders secondary to obesity | |
| – Non-alcoholic fatty liver | |
| – Arthrosis | |
| – Urinary incontinence | |
| Requisites | – Age 18–60 years. In older patients, specific cases should be decided on an individual basis. |
| – Rule out endocrine pathologies. | |
| – A history of morbid obesity during at least five years. | |
| – Failure or insufficient response to medical-nutritional treatment. | |
| – Capacity to adhere to recommendations and lifestyle changes after surgery. | |
| – The patient must understand the treatment they will be subjected to and its long-term consequences. | |
| – Psychiatric evaluation in the case of any relevant history or suspicion of mental illness. | |
| Contraindications | – Severe psychiatric pathology: schizophrenia, major depression, mental retardation. |
| – Psychiatric instability. | |
| – Eating behaviour disorders. BITE test to rule out bulimia | |
| – Abuse of alcohol or other drugs |
The most widely used diets are of 1.200 kcal/day for women and −1700 kcal/day for men. Although lower calorie diets may be prescribed, over the long term they lead to a deficit in minerals and vitamins. As an objective which is possible, the fact of reducing bodyweight by 10% gives rise to a major health benefit, with significant metabolic changes. Moreover, suitable physical exercise for each patient is important, together with psychological support for the modification of eating behaviour.
The pharmacological treatment of obesity should be individualized. The selection of drugs will depend on age, the presence or otherwise of DM and any contraindications (Table 29).
Surgical treatment of obesity is reserved for a group of patients after the therapeutic failure of other more conservative options.127 Bariatric surgery (Table 30) is indicated in cases of morbid obesity or severe obesity with multiple complications that are not controlled by medication, after the failure of other therapeutic strategies. It is an effective treatment that is not free of complications. The indications and contraindications for this treatment are shown in Table 30.
Recommendations on smoking cessation strategies.
| Recommendation | Class | Level |
|---|---|---|
| Any form of tobacco consumption must cease, given that the use of tobacco is intensely and independently associated with the development of cardiovascular disease. | I | A |
| Smokers should be helped to stop smoking, offering them nicotine substitutes and drugs (varenicline and bupropion, alone or combined), when necessary. | IIa | A |
| Smoking cessation is recommendable in spite of the resulting weight gain, given that the latter does not reduce the cardiovascular benefit deriving from cessation. | I | B |
| Passive smoking must be avoided. | I | B |
Adapted from Visseren et al.18
Smoking is a lethal addiction and the first avoidable cause of death, as it doubles the risk of death due to ACVD and multiplies it by 5 in individuals younger than 50 years. Smoking tobacco favours the formation and disintegration of atheromatous plaque. It also causes inflammation, oxidization and dysfunction of the arterial endothelium, predisposing it to arterial spasm, thrombosis and vascular blockage. Smoking tobacco is harmful in all of its forms, proportionally to the amount smoked.128,129 Of all preventive measures, stopping smoking is the most effective in terms of reducing CVR. The benefit of cessation is observed in the first months of abstinence. In Spain the number of smokers fell by 3.13% in the period from 2009 to 2012, and it fell by 4.81% from 2009 to 2017.130 Nevertheless, smoking is still advertised and once the addiction has been acquired it is hard to stop, as according to different reports there will be from 5 to 14 failed attempts before successful cessation.131 All smokers should be urged to stop every time they interact with medical professionals, as this increases the probability of cessation by a patient by more than 50%. An individual is considered to have ceased smoking when 1 year has passed since they smoked their last cigarette.
Table 31 shows the recommendations for smoking cessation strategies contained in the European guides for cardiovascular prevention.18
CHADS2-VASc scale of thrombotic risk in atrial fibrillation.
| Risk factor | Weighted value |
|---|---|
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥ 75 years | 2 |
| Diabetes | 1 |
| Ictus/TIA/previous peripheral embolism | 2 |
| Vascular disease (peripheral arterial pathology, ischemic cardiac pathology, plaque in aorta) | 1 |
| Age from 65 to 74 years | 1 |
| Female | 1 |
| Maximum score | 9 |
| Interpretation: Low risk = 0; moderate risk = 1; High risk ≥ 2 |
Every medical visit for the control of CVR should include the following items:
- -
Anamnesis on smoking:
- o
Have you ever been a smoker (regularly smoking at least 1 cigarette per month)? (No/ Yes)
- o
How many cigarettes do you smoke per day?
- o
IF you have ceased smoking, how many months have passed since you stopped?
- o
How many serious attempts to stop smoking have you made throughout your life?
- o
- -
Urge the need to cease smoking. Give information on the benefits of ceasing to smoke and the strategies that can aid this. Also inform about the potential weight gain (3−5 kg on average) and that this is less important than the benefit of cardiovascular prevention and the improved state of health in general.
- -
Evaluate the degree of addiction using Fagerström’s test132,133 (Appendix A Annex 3) as a guideline on the need to use pharmacological measures and nicotine replacement.
- -
Evaluate the patient’s attitude to smoking. Three key questions may be asked:
- o
Do you believe that smoking is bad for you?
- o
Would you like to stop smoking?
- o
Do you believe that you could stop smoking?
- o
- -
If a patient answers the above three questions affirmatively then they should be helped by means of a planned strategy, including setting a date, behavioural/motivational therapy and pharmacological support or specific visits on smoking. The patient should be informed, encouraged and urged, if this has not been done beforehand.
- -
Set a follow-up programme.
As well as the therapies based on communication during medical visits, including advice and a motivational interview, nicotine replacement therapies and different drugs can be used.134 Nicotine replacement therapies, in the form of chewing gum, patches, nasal sprays or inhalers, are effective and increase the probability of smoking cessation.4 Bupropion is an antidepressant drug which has been subjected to a broad range of clinical trials which have demonstrated its efficacy as an aid to stop smoking, as it increases the possibilities of success by more than 50%. Its main drawback is a slight risk (1/1000) of convulsions, with no increase in the risk of ACVD or neuropsychiatric disease. Varenicline is a partial nicotine-receptor agonist which also has a broad range of clinical trials that have shown that using it increases the chance of successful smoking cessation by more than twice. Its main side effect is nausea, which may be mitigated by gradually raising the dose and not taking the highest doses.135,136 However, on 6 July 2021 the Spanish Medications and Medical Products Agency (AEMPS) recommended that no new treatments with this drug should commence, as an impurity had been detected in the varenicline tablets which had made it necessary to withdraw 3 batches of the drug, and that continuity of the treatment with it could not be guaranteed for patients.
There is still insufficient information on the use of electronic cigarettes and tobacco-heating products. Use of the latter is increasing137 to above that of electronic cigarettes, and this seems to have influenced the U.S. Food and Drug Administration (FDA)to approve this as a modified risk tobacco product in 2020.138 However, many questions still have to be resolved about these products, including the most important one of their long-term effects on health.
Patients with atrial fibrillationAF is the most common arrhythmia in the population, and it affects more than 33 million people in the world.139 Due to the increase in longevity and diagnostic intensity, it is estimated that its global prevalence will stand at 15.9 million in 2050, of whom more than half will be patients ≥80 years old.140
Cardiovascular risk factors that facilitate the presence of recurrence of atrial fibrillationThe risk of AF throughout life increases with increasing CVRF. It is important to identify, prevent and treat the latter to reduce the prevalence of AF and its burden of morbidity.
Arterial hypertensionHigh and uncontrolled AP may change the structure of the myocardial wall, thereby favouring the development of AF and increasing the possibility of this becoming recurrent. On the other hand, AHT increases the risk of CVA and intracerebral bleeding events. Control of AP should therefore be an integral part of the treatment of patients with AF.141
Diabetes mellitusAlthough the influence of DM on myocardial remodelling and predisposition to the development of AF is known, intensive control of glycaemia does not reduce the rate at which new cases of AF appear. On the contrary, long-term DM leads to the predisposition for a higher risk of CVA and thromboembolic events in patients with AF. Nevertheless, treatment with metformin in diabetic patients seems to be associated with lower long-term risk of AF.142
ObesityObesity increases the probability of developing AF in parallel to the increase in the BMI.143 Reducing weight by 10−15 kg reduces the recurrence of AF.144 Likewise, improving cardiorespiratory capacity may reduce the burden of AF even more in obese patients with AF.145
Diagnosing atrial fibrillation and screening strategiesAF is diagnosed after the detection of irregularity in the RR intervals with absences of P waves during at least 30 s in an ECG. Five temporal patterns may be distinguished, depending on the presentation, duration and spontaneous resolution of AF episodes: 1) AF diagnosed for the first time. 2) Paroxysmal AF: AF that spontaneously reverts or does so after intervention within < seven days. 3) Persistent AF: AF that is maintained over > seven days. 4) Persistent long-term AF: Continuous > 1 year after applying a rhythm control strategy 5) Permanent AF: AF that is accepted by the patient and doctor, without using new medication to restore or maintain the sinusal rhythm. The terms “Isolated AF” (there is a cause of AF in each patient), “Valvular /non-valvular AF” (may create confusion) and “Chronic AF” (a contradictory definition) are no longer used in its classification.146 Depending on its clinical component, AF may be symptomatic or asymptomatic. Screening may be recommended for patients with AHT, obstructive sleep apnoea, HF or individuals older than 65 years, using pulse palpation or 12 lead ECG.146
The comprehensive management of patients with atrial fibrillationManaging a patient with AF includes informing and educating them as well as their self-care, the control of CVRF and possible underlying arrhythmogenic conditions, regulating their cardiac rhythm or frequency (CF) and preventing ictus by using anticoagulants. Nevertheless, some situations mean that a patient with AF will be referred to a specialized hospital emergency department. These include haemodynamic instability, high and uncontrolled CF, symptomatic bradycardia, severe angina, intense and irreversible dyspnoea or the presence of AIT or CVA. Transthoracic echocardiography is recommended for the initial evaluation and treatment of all patients with AF, enabling the evaluation of cardiac structure and functioning.
Control of cardiac rhythm or frequency. Antiarrhythmic drugs, catheter ablation and atrial fibrillation surgeryEarly therapy to control rhythm is associated with a lower risk of events in patients with early onset AF and those at high risk,147 while in long-term AF control of frequency is the therapy that is recommended the most.148 The selection of a therapy for rhythm control will depend on patient characteristics, their symptoms and the LVEF. Beta-blockers are the first line drug for frequency control, above all in patients with a depressed LVEF, while non-dihydropyridinic calcium antagonists are an effective alternative if the LVEF is not depressed. Digoxin should be used with care as it may be associated with increased concentration-dependent mortality.149 Amiodarone is useful in combination after failure of the previous therapy, although it should be avoided in patients with thyroid pathology.
Rhythm control treatment is recommended to improve the symptoms and quality of life of symptomatic patients with FA.146 While direct synchronized electric cardioversion is the preferred treatment for patients with AF and haemodynamic deterioration, in stable patients the use of this technique is comparable to pharmacological cardioversion. Flecainide and propafenone are indicated in patients without ischemic cardiomyopathy, hypertrophy or left ventricular systolic dysfunction. Intravenous amiodarone is used in patients with HF. Intravenous vernakalant has a faster cardioversion effect than amiodarone.146 Although dronederone may be used to control rhythm, it is associated with increased mortality in patients with recently imbalanced HF, functional class III-IV of the NYHA and permanent AF, neither concomitantly with dabigatran.150 Sotalol is contraindicated for LVH, HF and lengthening of the QT due to its risk in ventricular arrhythmia and torsades de pointes. Dronederone and sotalol should not be administered when creatinine clearing is below 30 mL/min. The use of a flecainide or propafenone “pocket pill” is safe and recommended in patients with paroxysmal AF after training.151 Surgery can also be used to control rhythm. It is based on the creation of isolation scars on the atria with the aim of preventing re-entry phenomena which trigger and perpetuate arrhythmia, permitting the redirection of the normal stimulus from the sinusal node to the atrioventricular node. Current techniques, which are based on energy transmission devices, make it possible to perform this technique thorascopically with a minimum incision. Some studies have found it to be more effect than catheter ablation, although it also has a higher probability of side effects and sometimes requires the subsequent implantation of a pacemaker152 In symptomatic persistent AF surgery may be indicated if catheter ablation has failed. This technique may also be considered in patients with AF who are going to receive cardiac surgery.
Anticoagulation is the treatment of choice for the prevention of ischemic ictus, given that the risk is similar in persistent and paroxysmal AF.147 Classically anti-vitamin K drugs (AVK) have been the choice in an international normalized ratio (INR) range of from 2 to 3, as they bring about a fall in the rate of ictus from 60% to 80%. The indication for oral anticoagulation is established by prognostic models that include patient age and comorbidities. CHA2DS2-VASc is the most widely used scale (Table 32), so that patients with a score of 0 (1 in the case of women) do not require oral anticoagulant therapy. Anticoagulation would be indicated in men with a CHA2DS2-VASc score ≥ 1 or ≥ 2 in women. This recommendation is applied to patients with non-valvular AF, on condition that there is no high risk of haemorrhagic complications as estimated by the HAS-BLED scale (Table 33).
HAS-BLED scale of risk of haemorrhage in atrial fibrillation.
| Risk factor | Weighted value |
|---|---|
| Hypertension, poorly controlled arterial pressure | 1 |
| Abnormal renal/hepatic function | 1 or 2 |
| Ictus | 1 |
| Tendency or predisposition to bleeding | 1 |
| Unstable INR | 1 |
| Advanced age (> 65 years, frail) | 1 |
| Medication (aspirin or NSAID), or excessive alcohol consumption | 1 or 2 |
| Maximum score | 9 |
INR: international normalized ratio; NSAID: non-steroid anti-inflammatory drug.
Hospital surgery resources for the overall control of the chief vascular risk factors.
| Architecture | Material resources | Computing resources |
|---|---|---|
| A surgery for each medical professional and shift. | Complete surgery furnishings: office table and examination couch. | Clinical history, digital or not, according to health department model, accessible in the reference health area. |
| Nursing space for AP measurement, health education and checking adherence to treatment. | Phonendoscope, ophthalmoscope, torch, scales, height meter and tape measure. | Online access to risk scales and questionnaires. |
| Climate control system in the hospital and surgeries. | Validated AP measurement devices170 | Electronic prescriptions, according to health department model. |
| It is advisable to have: | Possibility of online consultation by Primary Care. | |
| 1. A semi-automatic device for AP measurement so that it can be programmed to make several automatic measurements (usually three) without the presence of an observer. It is recommended that in the first visit the three automatic measurements should be made simultaneously in both arms. | ||
| 2. Access to outpatient AP measurement. | ||
| 3. Portable Doppler to determine AAI. | ||
| 4. Electrocardiograph |
AAI: ankle-arm index; AP: arterial pressure.
Adapted from Felip Benach.169
Four major randomized studies were carried out in the last decade of direct-action oral anticoagulants (DOAC): dabigatran (RELY), rivaroxaban (ROCKET-AF), apixaban (ARISTOTLE) and edoxaban (ENGAGE-AF), together with a meta-analysis. These demonstrated that they were equally or more effective than the AVK, with a lower incidence of bleeding complications, especially intracranial bleeding, so that they are now the alternative of choice in the initial treatment of AF (Fig. 11).153 “Real life” studies have also confirmed that there is less risk of bleeding with the DOAC compared to the AVK.154 Furthermore, when combined with an antiaggregant monotherapy the DOAC may be a safe and effective alternative to triple therapy in patients with AF who are subjected to percutaneous coronary intervention.155 Due to all of these factors, the latest clinical guides consider the DOAC to be the preferential option for the prevention of ictus in patients with FA.156,157
Although the European guides place the DOAC above AVK, their use is restricted in Spain. According to a therapeutic positioning report, in general these drugs are only authorized for use in patients with poor therapeutic control, a history of intracranial bleeding or the risk of this.158
In any case, it is important to involve the patient in the decision-making process regarding the different anticoagulation options, considering polymedication and the comorbidities that may favour the appearance of bleeding. It is always important to take into account age, frailty, weight and renal functioning, all of which may influence the type of anticoagulant.
Anticoagulation in patients who require cardioversionIt is important to commence anticoagulation early in patients with planned cardioversion, given that this procedure is associated with a risk of thromboembolism. Patients who have been in AF for more than 48 h should commence anticoagulation at least 3 weeks prior to cardioversion, and after this they should continue during 4 weeks (on condition that they do not require indefinite anticoagulation). Current guidelines recommend suitable anticoagulation with an AVK or with dabigatran (both before and after the procedure). Other DOAC are under study in prospective clinical trials.159
Organization and working of cardiovascular risk consultation: professionals and devices. Quality criteriaDifferent major national and international studies in recent years have shown that there is a low level of overall control of CVRF. This is so even in secondary prevention for coronary, ictus or PAD patients.160–167 This may be due to a range of factors, including insufficient adherence to treatment, therapeutic inertia and also organizational models, which are generally found to be hardly able to improve these results.
The multidisciplinary control of CVR has clear advantages168:
- -
Make the approach to and treatment of CVRF uniform across the different levels of care, thereby ensuring the continuity of care in the prevention of vascular risk.
- -
Improve the detection rate of all CVRF in patients at high CVR, thereby facilitating the most appropriate early therapeutic intervention for each patient.
- -
Optimize medical resources, preventing the duplication of visits and complementary examinations.
- -
Define and agree criteria for referral, thereby generating a bidirectional flow that will, in the majority of cases, facilitate the return of patients to primary care after the evaluations and interventions which required referral to specialized care. This will ensure that this information reaches primary care doctors, and that patients will receive uniform messages at both levels of care.
- -
Promote teaching and research into CVR.
A Vascular Risk surgery, as an organizational unit within the context of planned care, requires:
- -
Professionals in a range of specialities (Internal Medicine, Endocrinology, Cardiology, Nephrology, Clinical Biochemistry, Family Medicine, etc.), coordinating with primary care, nutritionists and nurses, so that they are able to cover all of the main risk factors.
- -
Unified protocols based on clinical practice guides for the overall control of the main CVRF.
- -
Basic structural requisites.169 This includes the possibility of measuring AP appropriately using validated devices (Table 34).
Table 34.Indicators for the measurement of care quality in the comprehensive control of patients with high cardiovascular risk.
Indicators Structure or activity Process or quality Result –Total number of patients referred to the cardiovascular risk surgery – Number of referrals to the cardiovascular risk surgery which fulfil previous defined and agreed criteria/total number of referrals – Number of patients visited who fulfil CVRF control objectives according to their risk category/total number of patients visited – Number of cardiovascular risk team sessions – Number of patients with a first visit to the cardiovascular risk surgery with response time within set limits/total number of first visits – Fit of resource consumption (complementary examinations)/total number of patients visited – Number of consultancy sessions with primary care – Number of patients visited with diagnosis codification (according to CIE10/total number of patients visited) – Number of visits for cardiovascular risk ended with discharge to primary care with an agreed report/number of discharges from the cardiovascular risk surgery – Number of telematics visits by the members of the cardiovascular risk surgery: virtual visits, consultancy sessions, telemedicine – Number of patients in primary prevention and no diabetes whose risk has been estimated using a scale – Scientific production: communications and publications shared by several members of the group IDC-10: International Disease Classification, 10th edition; CVRF: cardiovascular risk factors.
Adapted from Armario et al.168
Certain indicators have to be established as the basic tool for measuring the quality of care (Table 35). These have to enable the detection of aspects that could be improved to optimize the overall control of risk factors, and they should be used as a self-evaluation system.
Selection criteria for type of visit.
| Preference for face-to-face visit | Preference for a telematic visit |
|---|---|
| Suspicion of potentially serious or urgent problems, having to give bad news. Clinical changes, imbalance or the patient worsening, the need to interview those accompanying them, the first visit. | Stable clinical situation |
| Difficulties communicating with the patient (language, hypoacusia, cognitive problems) | No problems with communication |
| Physical examination required | No physical examination required |
| Patient has to be trained in physical self-examination: | Patient has already been trained in physical self-examination |
| Weight | |
| Measuring arterial pressure | |
| SBPM | |
| Complementary tests required in the short term: | Complementary tests required over the medium to long term |
| Laboratory tests | (manage requests through the administration) |
| Electrocardiogram | |
| Radiology | |
| Ankle-arm index | |
| Pulse wave velocity (PWV) | |
| OBPM | |
| Patient requires more personalized health education or changes in major forms of treatment or titration of the same | They will not a priori require changes in treatment |
| Uncontrolled cardiovascular disease | No cardiovascular disease, or in a stable situation |
| Presence of multiple comorbidities | No important comorbidities |
OBPM: outpatient blood pressure monitoring; SBPM: self-blood pressure measurement.
Prepared by Gijón-Conde et al.171
Visits by patients with CVR should fulfil the requirements of the organizational system of the corresponding public or private health service. Digitalizing clinical histories, the option of electronic prescriptions (and the resulting awareness of degree of adherence which this permits) and remote medicine options (for patient-doctor as well as doctor-doctor interactions) may facilitate access to clinical information about patients and better control of CVRF.
The COVID-19 pandemic gave rise to major changes in medical care, to reduce the risk of transmission between patients and medical personnel in face-to-face visits, so that telematic consultations came to predominate. This form of consultation is now nearly ubiquitous, and we suggest that in certain specific clinical situations they could be included in the plans for care of CVR. However, this would have to be done in a previously structured way, using pre-established selection criteria regarding the situations in which face-to-face consultation would be preferable, and those in which telematics consultation could be preferred (Table 36).171
Causes of referral of patients with diabetes or AHT.
| Diabetes mellitus | Arterial hypertension |
|---|---|
| – Type 1 | – Resistant AHT |
| – Gestational | – Malign AHT and other hypertensive emergencies |
| – Diabetes not elucidated correctly | – Suspicion of secondary cause |
| – Poor glycaemic control, unstable diabetes, hypoglycaemia | – Pregnancy; a history of gestational AHT |
| – Comorbidities (such as morbid obesity) | – Infancy, adolescence |
| – Severe microvascular disease (polyneuropathy, Diabetic foot, CRF, advanced retinopatht) | – Comorbidities (heart or renal failure, cerebrovascular, coronary or peripheral arterial disease) |
| – Request 24 h OBPM when there are no resources to perform and interpret this |
AHT: arterial hypertension; CRF: chronic renal failure; OBPM: outpatient blood pressure measurement.
Patients with a high level of CVR are generally referred to surgeries for the control of certain CVRF, fundamentally AHT, DM and dyslipidaemia; this may occur in the context of primary or secondary care. It is therefore frequent for patients with established ACVD (coronary, cerebrovascular or peripheral arterial disease) to be referred to our surgeries. Sometimes these patients are young and no CVRF can be identified (for the diagnostic study of thrombophilia), or they are either unaware of the CVRF detected during their hospital assessment or do not properly control them.
It is not habitual to receive patients so that they can be helped to cease smoking, even though this is a major risk factor. Some areas of primary care or pneumology have specific centres for this. In any case, each Public Health Service usually sets its own criteria for referral to specialized units, depending on their own plans, the availability of resources and the level of care (regional hospitals as opposed to tertiary referral hospitals).
The main reasons for referral for patients with AHT and DM are shown in Table 37. Patients are referred to the AHT and Vascular Risk Unit, or to the Endocrinology Unit. The SEA has published the referral criteria for patients with dyslipidaemia to its Lipid Units172 (Table 38).
Criteria for the referral of patients with diabetes or AHT.
| Diabetes mellitus | Arterial hypertension |
|---|---|
| -–(Type 1 | -–(Resistant AHT |
| -–(Gestational | -–(Malign AHT and other hypertensive emergencies |
| -–(Diabetes not elucidated correctly | -–(Suspicion of secondary cause |
| -–(Poor glycaemia control, unstable diabetes, hypoglycaemia | -–(Pregnancy; a history of gestational AHT |
| -–(Comorbidities (such as morbid obesity) | -–(Infancy, adolescence |
| -–(Severe microvascular disease (polyneuropathy, diabetic foot, IRC, advanced retinopathy) | -–(Comorbidities (heart failure or renal failure, cerebrovascular, coronary or peripheral arterial disease) |
| -–(Request 24 h OBPM when there are no resources to perform it or interpret it |
AHT: arterial hypertension; OBPM: outpatient blood pressure measurement.
Referral criteria to lipid units of the Spanish Arteriosclerosis Society.
| Dyslipidaemia | Thresholds | Clinical context | Diagnosis | Treatment |
|---|---|---|---|---|
| Hypercholesterolaemia | TCh > 300 mg/dL | Tendinous xanthomas | Screened in FH cascade | Triple therapy |
| LDL-c > 200 mg/dL | Corneal arch < 45 years | Genetic tests | New treatments (anti-PCSK9) | |
| Lp(a) > 50 mg/dL | Family history +++ | Imaging techniques to detect SVD | Pharmacological intolerance | |
| CI or premature PAD | Refractory to the treatment | |||
| Recurring CI | Apheresis | |||
| Vascular disease without evident CVRF | ||||
| Suspicion of FH | ||||
| Steatosis and/or cirrhosis | ||||
| Hypertriglyceridaemia | Fasting TGS > 1000 mg/dL | Xanthomas | Analysis of secondary causes | Exclude secondary cause |
| TGS > 500 mg/dL in spite of treatment | Hepatomegaly | Biochemical tests. | Ineffective treatment | |
| Hypertriglyceridaemia and TCh > 350 mg/dL | Splenomegaly | Molecular diagnosis | Special diets | |
| Lipemia retinalis | ||||
| Debut in infancy | ||||
| Pancreatitis | ||||
| HDL-c | HDL-c < 20 mg/dL | Hepatomegaly | Exclude secondary causes | Control secondary causes |
| HDL-c > 100 mg/dL | Splenomegaly | Biochemical tests | ||
| Tonsillar hypertrophy | Molecular diagnosis | |||
| Corneal opacity | ||||
| Renal failure | ||||
| Hypocholesterolaemia | LDL-c < 50 mg/dL without treatment | Malabsorption | Exclude secondary causes | |
| Steatosis | Biochemical tests | |||
| Molecular diagnosis |
HDL-c: HDL cholesterol; IC: ischemic cardiomyopathy; LDL-c: LDL cholesterol; TCh: total cholesterol; PAD: peripheral arterial disease; FH: familial hypercholesterolaemia; Lp(a): Lipoprotein (a); TGS: triglycerides. +++ Positive.
Adapted from Sánchez-Chaparro et al.172
Within the context of CVR, type 2 is the main type of DM to be considered. This is associated with dyslipidaemia, obesity, AHT and MS; the most usual cause of referral will therefore be a lack of appropriate control of glycaemia, even though in recent years the therapeutic arsenal in the field of DM2 is enormous.173
In the case of AHT, in our country the most common reason for referring patients to an AHT unit is to rule out the cause of secondary AHT, followed by poor therapeutic control and resistant AHT.174 The latter is defined as inability to bring AP under control (<140/90 mmHg) in spite of using three drugs, one of them a diuretic.175
The most common causes for referring patients in the case of dyslipidaemia are poor control of cholesterol levels and triglycerides in serum, the diagnosis and treatment of FH, adverse effects of medications, especially intolerance of statins (fundamentally because of muscular toxicity) or the need for combined treatments, especially with iPCSK9.172
Discharge criteria for patients with cardiovascular riskIt is obvious that one a definitive diagnosis has been made and the risk factor which led to the visit has been brought under control, patients should be referred to primary care by the specialized unit in question for follow-up. Nevertheless, it is not uncommon for patients to be examined in specialized surgeries for a range of reasons, including failure to achieve therapeutic goals, the need for unconventional procedures (bariatric surgery, LDL-c apheresis or perfusion in day hospitals for replacement lysosomal therapy), the need to prescribe and dispense certain drugs in hospital pharmacies (as is the case for iPCSK9 drugs), polymedication with a high risk of pharmacological interactions (e.g., in subjects with HIV infection or those who have received transplants) and those who suffer adverse effects due to their medication, such as hypoglycaemia. Some patients with secondary AHT remain hypertensive in spite of aetiological treatment of their disease, suggesting the long-term effect of AHT on the vascular tree (remodelling) or that some patients may also have essential AHT.176
FinancingLaboratorios Ferrer provided support for publication together with administrative support in connection with the updating of the document “SEA 2022 standards for the control of cardiovascular risk”. The authors of the document are responsible for the contents of the same. Laboratorios Ferrer did not intervene in writing it or its content.
The authors have no other relevant links or financial participation in any organization or entity with an interest in or financial conflict with the subject or materials discussed in the manuscript, apart from those which are declared.
Conflict of interestsLaboratorios Ferrer has provided support for publication together with administrative support in connection with the updating of the document “SEA 2022 standards for the control of cardiovascular risk”. Laboratorios Ferrer did not intervene in writing it or its content.
Some authors have received fees from different pharmaceutical companies including Laboratorios Ferrer for taking part in congresses and as advisors, and these are described in the corresponding section. The authors have not received any remuneration for preparing this report and nor do they have any other direct conflicts of interest to declare according to this work.
The authors’ declarations of conflict of interestsMasana L has received consultancy fees from Amgen; Sanofi; Amarin; Daiichi; Novartis, for congresses, presentations, manuscripts or educational events and meeting from Amgen; Sanofi; Mylan; Sevier; Amarin; Amryt; Daiichi; Novartis.
Valdivielso P has received consultancy fees, for expert witness, for congresses, Advisory Boards, presentations, manuscripts or educational events, support for meetings from Amarin; Amgen; Novartis; Ferrer; MSD; Akcea; Ionis; Daiichi-Sankyo; Sanofi Mylan and grants or contracts from Ferrer; Akcea.
Arrobas-Velilla T has received fees for congresses, presentations, manuscripts or educational events from Sanofi; Amgen; Novartis.
Cebollada J has received fees for congresses from Daiichi-Sankyo; Servier; Chiesi.
Díaz-Díaz JL has received fees for congresses, presentations, manuscripts or educational events from Sanofi; MSD; Amgen; Viatris, support for meetings from Viatris; Sanofi; Amgen; MSD and participation in Advisory Boards from Amgen; Sanofi.
Fernández Pardo J took part in the preparation of the Textbook of Emergency Medicine and Manual of medical diagnosis and therapy for Menarini; MSD, fees for presentations from Ferrer Internacional and support for congresses from Menarini.
Guijarro C has received administrative support and for meetings to prepare this manuscript from Laboratorios Ferrer, consultancy fees from Sanofi; Amgen and Daiichi Sankyo, fees for congresses, presentations, manuscripts or educational events from Rubio, Sanofi; Amgen; Daiichi Sankyo, participation in Advisory Boards from Sanofi; Amgen; Daiichi Sankyo.
Jericó C has received fees for congresses, presentations, manuscripts or educational events from Amgen; Boehringer; Mylan; Novo Nordisk; Rovi; Sanofi, and support for meetings from Sanofi.
López-Miranda J has received fees for congresses and educational activities from Amgen; Sanofi; MSD; Ferrer and Laboratorios Esteve, consultancy fees or Advisory Boards and support for meetings from Amgen and Sanofi.
Pedro-Botet J has received fees for Advisory Boards from Amgen; Daiichi-Sankyo; Ferrer; Mylan (Viatris); Sanofi and congresses from Amarin Pharma; Amgen; Daiichi-Sankyo; Esteve; Ferrer; Merck Sharp & Dohme; Mylan (Viatris); Rovi; Sanofi; Servier.
Puzo J has received consultancy fees from Beckman-coulter, for congresses, presentations, manuscripts or educational events from Sanofi and Amgen, and leadership in boards, society, committee or defence groups from SEQC.
We would like to thank our colleagues who gave their time to answer the survey, together with the invaluable collaboration by Burgos PH Management, which made this work possible for everyone.
Please cite this article as: Mostaza JM, Pintó X, Armario P, Masana L, Real JT, Valdivielso P, et al. Estándares SEA 2022 para el control global del riesgo cardiovascular. Clin Investig Arterioscl. 2022;34:130–179.