Treatment of oesophageal cancer with curative intent requires a multidisciplinary approach. Neoadjuvant therapy, the radicality of resection and extension of lymphadenectomy have been associated with increased operative morbidity and mortality. The aim of this study was to assess the results of surgical treatment of oesophageal cancer since the presence of an interdisciplinary esophagogastric tumour board.
MethodsPatients with cancer of the oesophagus and oesophagogastric junction who underwent oesophagectomy between January 2005 and March 2012 were included in this retrospective study. Data concerning type of resection, postoperative complications, mortality and survival were analysed.
ResultsOf the 392 patients with a diagnosis of oesophageal cancer over the study period, 100 underwent oesophagectomy. Seventy-four patients received neoadjuvant treatment. Eighty-two patients underwent transthoracic resection while a transhiatal was used in 10 patients. Colon interposition was required in 8 cases. An R0 resection was achieved in 98 patients. Anastomotic leaks developed in 15 patients, 9 were intrathoracic and 6 were cervical. Postoperative morbidity occurred in 42% of patients, and intra-hospital and 90-day mortality was 2%. Median length of hospital stay was 16 days. The respective actuarial survival at 1 and 5 years were 82% and 56%.
ConclusionsSurgical treatment with curative intention for oesophageal cancer is only possible in a quarter of patients diagnosed. The high morbidity rate was mainly due to intrathoracic complications.
El tratamiento del cáncer de esófago con pretensión curativa requiere un planteamiento multidisciplinar. La terapia neoadyuvante, la radicalidad de la resección y la extensión de la linfadenectomía pueden incrementar la morbimortalidad postoperatoria. El objetivo de este estudio es analizar los resultados del tratamiento quirúrgico del cáncer de esófago desde la creación del Comité de Tumores Esofagogá stricos.
MétodosEstudio retrospectivo (de enero de 2005 a marzo de 2012) de todos los pacientes con cáncer de esó fago o de la unión esofagogástrica a los que se les realizó una esofagectomía. Se analizaron el tipo de resección, las complicaciones postoperatorias, la mortalidad y la supervivencia.
ResultadosA 100 pacientes de un total de 392 diagnosticados se les realizóuna esofagec-tomía. En 74 casos se administrótratamiento neoadyuvante. Se realizaron 82 esofagecto-mías transtorá cicas en 2 o 3 campos, 10 esofagectomías transhiatales y 8 coloplastias. En 98 pacientes la resección fue R0. Se diagnosticaron 9 dehiscencias anastomóticas intratorá–cicas y 6 cervicales. La morbilidad global fue del 42% y la mortalidad hospitalaria y a los 90 días fue del 2%. La mediana de la estancia hospitalaria fue de 16 días. La supervivencia actuarial al año es del 82% y a los 5 años, del 56%.
ConclusionesEl tratamiento quirúrgico con intención curativa de la neoplasia de esófago solo es posible en una cuarta parte de los pacientes diagnosticados. La elevada morbilidad se debe, sobre todo, a complicaciones torácicas.
Esophageal cancer is a highly aggressive neoplasm whose prognosis has not significantly improved in recent years.1 In Western countries, adenocarcinoma is now more frequent than squamous-cell carcinoma,2 and gastroesophageal reflux and obesity are the main risk factors.3
Although the diagnostic and therapeutic approach to esophageal cancer should be multidisciplinary,4 esophagectomy is the best therapeutic option in tumors that infiltrate to the submucosa.5 Its combination with chemotherapy (CTx) and radiotherapy (RTx) as either adjuvant,6 neoadyuvant7,8 or radical9 treatment complete the therapeutic arsenal.
Esophageal cancer resection is technically complex, requires a large amount of human and material resources, and is associated with a high number of complications.10–12 There is a prevailing opinion that the best results are obtained both in postoperative morbi-mortality4,13,14 and in long-term survival in centers with a high volume of patients.15 The most appropriate surgical approach for performing esophageal resection (limited transhiatal [TH] or transthoracic [TT] with en bloc lymphadenectomy) is controversial,16 although it seems clear that 5-year disease-free survival was significantly better with the TT esophagectomy in patients whose number of affected lymph nodes was limited.17
Currently, the treatment of esophageal cancer is the same for the 2 histological types of the tumor, although recent papers1 seem to show a better 5-year prognosis of esophageal resection in adenocarcinoma than in squamous-cell carcinoma.
The aim of this study was to analyze the results of a consecutive series of esophagectomies due to neoplasm that were done in the Esophagogastric Surgery Unit at the Hospital Universitari de Bellvitge since the creation of the Esophagogastric Tumor Board (EGTB).
Patients and MethodsWe analyzed esophagectomy results (morbidity, hospital mortality, 90-day mortality and survival) in patients with cancer of the esophagus or the esophagogastric junction (EGJ) Siewert I from January 2005 (date of creation of the EGTB) until March 2012. EGJ tumors were classified topographically according to the Siewert classification.18
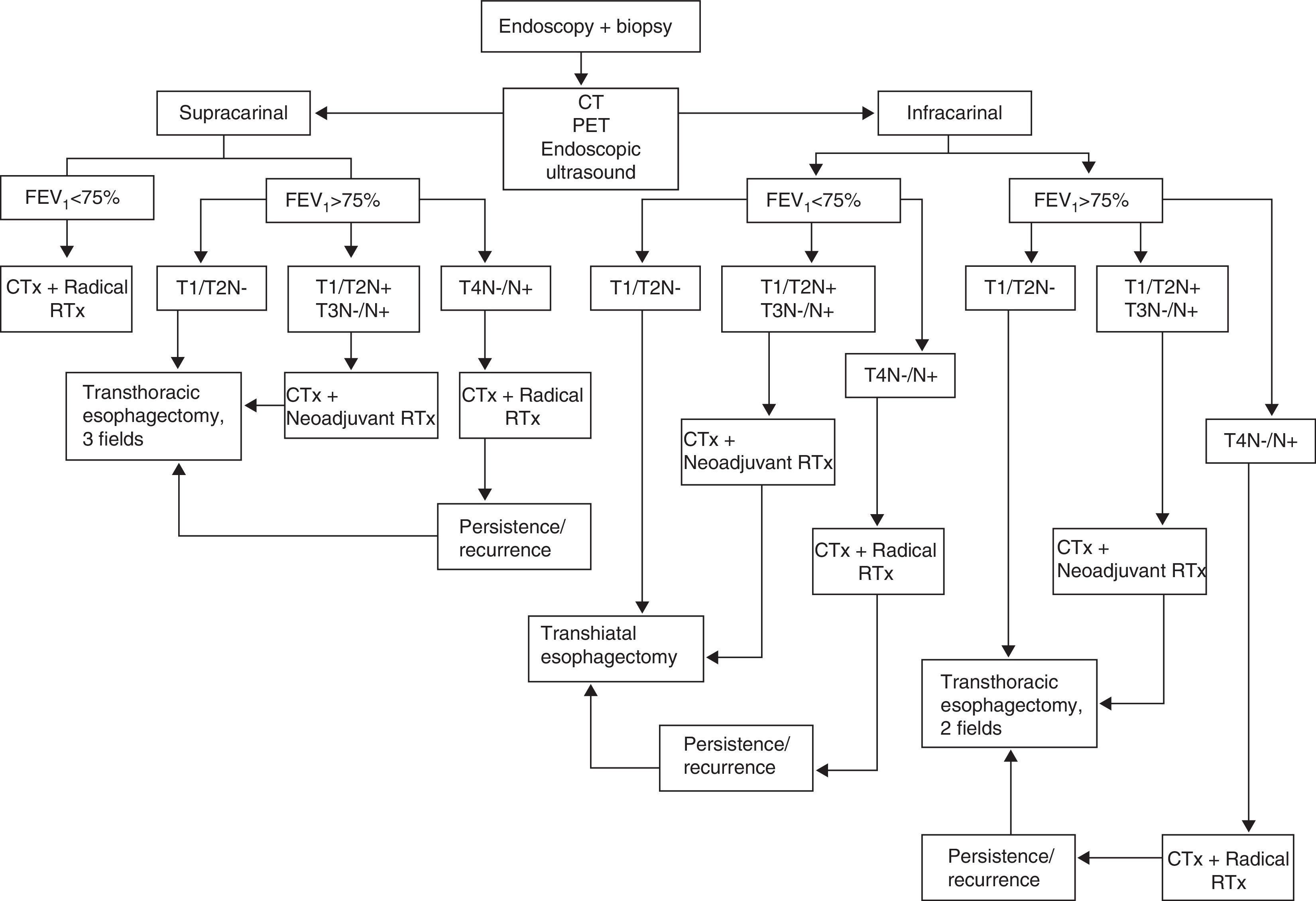
Treatment ProtocolThe EGTB protocol for our center is shown in Fig. 1. The extension study was performed with computed tomography (CT), endoscopic ultrasound and positron emission tomography (PET). The classification of the tumors was established according to the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.19
All patients underwent pulmonary function tests (PFT) to study the extension of the disease. Patients with precarinal tumors and a maximum forced expiratory volume per second (FEV1) <75% were not candidates for surgical treatment. In all other cases, our approach was as follows: T1/T2N0 underwent surgery; T1/T2N1 and T3N0/N1 received neoadjuvant treatment of CTx and RTx with intravenous cisplatin at 75mg/m2/day (day 1) and 5-fluorouracil in continuous infusion of 1000mg/m2/day (days 1–4 or 5) or carboplatin and 5-fluorouracil at the same doses in patients with a history of oto- or nephrotoxicity. We administered 2 CTx cycles in the first and fourth weeks of the RTx. Total RTx dose was 45Gy, 1.8Gy/fraction in the tumor and prophylaxis in the lymphoid areas. T4N0/N1 patients were offered radical treatment with 4 cycles of CTx (the second and third concomitant with RTx), with a total RTx dose of 66Gy, 2Gy/fraction. In cases with incomplete response to radical treatment or relapse of the neoplasia during follow-up of a complete response, possible rescue surgery was considered.
Surgical TreatmentIn all those cases with planned cervical esophagogastrostomy (TH or TT esophagectomy, 3 fields), gastric conditioning was carried out using percutaneous arterial embolization of the left gastric, right gastric and splenic arteries for 2 or 3 weeks prior to surgery.20,21
For the induction of anesthesia, we administered 2g of ceftriaxone and 1g of intravenous metronidazole as antibiotic prophylaxis. Peridural catheters were inserted in all patients. Surgical treatment depended on the location of the tumor: in patients with subcarinal involvement and FEV1 >75%, we opted for TT in 2 fields in accordance with the Ivor Lewis technique; with FEV1 <75%, we opted for TH. In those patients where the tumor was located proximal to the carina and had an FEV1 >75%, TT esophagectomy was performed in 3 fields in accordance with the McKeown technique. Regardless of the technique used, we made a gastroplasty measuring about 3cm wide and as long as possible.
At the abdominal level, we performed a D2 lymphadenectomy with the exclusion of group 10, which was only included when affected. In these cases, splenectomy was also done.
Mediastinal lymphadenectomy included: the lower mediastinal group and paraesophageal, subcarinal, bronchial, paratracheal and thoracic duct nodes. Since 2008, in all precarinal tumors we have performed left cervical functional lymphadenectomy. In tumors of the esophagogastric junction with involvement of the distal esophagus and gastric fundus, we performed esophagogastrectomy and reconstruction with coloplasty (preferably right).
In all cases, jejunostomy was done for early enteral nutrition and we left a drain for suction at the esophageal hiatus. In transthoracic approaches, we drained the pleural cavity with 2 Argyll 28 CH chest tubes.
Postoperative Follow-upFollowing surgery, patients were transferred to an Intensive Care Unit for at least 72h. On the third day, the peridural catheter was removed. Early enteral nutrition (EN) was initiated 24h after surgery and an imaging test was done with water-soluble contrast 7 days after surgery, prior to commencement of oral diet. The anterior chest drain was withdrawn after 72h, as was the posterior drain after ruling out anastomotic dehiscence.
In the absence of gastroparesis, the nasogastric tube was removed 3 days after surgery.
Postoperative Complications and MortalityPostoperative complications were classified according to the scale proposed by Dindo-Clavien22 and both hospital and 90-day mortality rates were recorded.
Statistical AnalysisFor the description of the population with qualitative variables, frequency and percentage tables were created. Quantitative variables were expressed as mean and standard deviation (SD) or as median and interquartile range (25th–75th percentile). We evaluated survival with population characteristics and sample size using the actuarial method. The SPSS version 13 (SSPS, Inc., Chicago, IL, USA) statistical package was used.
ResultsFrom January 2005 to March 2012, 392 patients were treated in the EGTB who had been diagnosed with esophageal or EGJ cancer, 100 of which (90 males) had a mean age of 58 (SD 6) and were treated surgically.
Fifty-one patients presented symptoms of gastroesophageal reflux disease (GERD) and 17 were histologically diagnosed with Barrett's esophagus; 3 patients had previously undergone an antireflux technique.
The most common tumor was adenocarcinoma (63) and the most predominant location was subcarinal (79) (Table 1). After the extension study, 62 patients received neoadjuvant treatment and 26 were operated on directly. During this period, 88 patients were treated with radical RTx+CTx, 12 of which underwent rescue surgery due to persistence or recurrence of the disease.
We performed 58 transthoracic esophagectomies in 2 fields (Ivor Lewis), 24 transthoracic esophagectomies in 3 fields (McKeown), 10 transhiatal esophagectomies and 8 esophagogastrectomies and reconstruction with right coloplasty (Table 2). We performed a minimally invasive abdominal approach in 10 of the 27 patients without neoadjuvant treatment, completing the technique in 8 of them (8/27, 29.6%).
Overall morbidity was 42%, with a predominance of thoracic complications (Table 3). According to the Dindo-Clavien classification,22 23 patients had a complication grade II, 13 grade III and 6 grade IV.
Complications of the Esophagectomy According to the Reconstruction Technique.
| Transthoracic, 2 fields | Transthoracic, 3 fields | Transhiatal | Coloplasty | Overall rate | |
| (n=58) | (n=24) | (n=10) | (n=8) | (n=100) | |
| Atelectasis | 11 (19) | 8 (33.3) | 3 (30) | 2 (25) | (24) |
| Dysphonia | 2 (3.4) | 10 (41.6) | 3 (30) | 1 (12.5) | (16) |
| Pneumonia | 7 (12) | 2 (8.3) | 0 | 0 | (9) |
| Hemothorax | 2 (3.5) | 0 | 0 | 1 (12.5) | (3) |
| Chylothorax | 4 (6.9) | 0 | 0 | 0 | (4) |
| Empyema | 8 (13.8) | 2 (8.3) | 1 (10) | 3 (37.5) | (14) |
| Hemoperitoneum | 2 (3.5) | 0 | 0 | 0 | (2) |
| Reoperation | 6 (10.3) | 0 | 0 | 1 (12.5) | (7) |
| Cervical anastomotic fistula | 3 (12.5) | 2 (20) | 1 (12.5) | (14.3) | |
| Intrathoracic anastomotic fistula | 9 (15.5) | (15.5) | |||
| Mortality | 1 (1.7) | 0 | 0 | 1 (12.5) | (2) |
The results are expressed as n (%).
Fifteen cases were diagnosed with diagnostic dehiscence. The intervention types included: 9 (15.5%) in intrathoracic anastomoses and 6 (14.3%) in cervical anastomoses. Out of the 9 patients with intrathoracic fistula, 4 were reoperated and 5 were treated conservatively with broad-spectrum antibiotic therapy, no oral intake and enteral nutrition. In 3 of them, we used a partially covered endoscopic stent (Wallflex® Esophageal Stent, 18×153, Galway, Ireland) to cover the defect.
Seven patients were reoperated: 6 Ivor Lewis (4 dehiscences, 1 hemothorax, 1 hemoperitoneum) and 1 coloplasty (biliary peritonitis).
Two patients died: one after reintervention due to hemothorax and another due to bronchoaspiration secondary to early intestinal obstruction. 90-day mortality was also 2%.
According to the NCCN Guidelines from 2/2011 of the AJCC,19 the operated patients were classified as: 8 stage 0, 13 stage IA, 8 stage IB, 7 stage IIA, 30 stage IIB, 17 stage IIIA, 4 stage IIIB and 13 stage IIIC. All stage 0 patients (T0N0M0) had received neoadjuvant or radical therapy.
The mean number of resected lymphadenopathies was 23.1,5,17–29 In 13 patients with precarinal neoplasm, a cervical functional lymphadenopathy was added. In these cases, the average number of cervical lymphadenopathies was 10,9–12 and 3 patients presented lymph node affectation.
In 98 cases, the resection was R0 and in 2 it was R1 due to affectation of the surgical margin.
No significant differences were found in the morbidity and mortality of patients who were given radical RTx+CTx compared to those who received neoadjuvant RTx+CTx. In the 5-year survival analysis, mortality was higher in the group with radical RTx + CTx (54.5% vs 34%), although this was not statistically significant.
Out of the 8 patients with complete pathological response (stage 0), 2 died at 27 and 7 months after surgery. The rest are alive at 56, 47 (relapse), 30, 20, 13 and 7 months.
The mean stay in the Intensive Care Unit was 43–6 days and mean hospital stay was 161,5,12–17,19–21,23,24–27 days. Overall actuarial survival rate was 85% at 1 year, 59% at 3 years and 56% at 5 years, with a mean follow-up of 19 months (9–37).
DiscussionIn the last 2 decades, adenocarcinoma has surpassed squamous-cell carcinoma as the most common esophageal neoplasm in Western countries, and most neoplasms of this type are found in the infracarinal region.2 Our series confirms these data: 63% of the esophageal tumors resected were adenocarcinomas and 79% were subcarinal. These epidemiological changes are mainly attributed to gastroesophageal reflux and obesity.3
The aggressive nature of these neoplasms entails poor mid- and long-term survival.1 Surgical resection is still the main therapeutic option,5 but several studies have demonstrated improved survival with the combination of surgery, CTx and RTx.6–9 This complex treatment means that a multidisciplinary approach is essential.4 In our hospital, since January 2005 all patients with gastroesophageal neoplasms are treated by a specific unit compiled of specialists from 8 different departments.
Out of the 100 patients operated on in this period, 62 received neoadjuvant therapy and 12 had radical therapy prior to surgery. There is controversy about whether the TH or TT esophagectomy surgical approach should be used. TH esophagectomy has a lower morbidity than TT, without affecting mortality,16 but Omloo et al.17 demonstrated a better 5-year survival in patients with a limited number of affected lymph nodes who underwent TT esophagectomy. Our group is in favor of TT esophagectomy with lymphadenectomy in 2 or 3 fields as radical surgical treatment of esophageal cancer versus TH esophagectomy (82 patients TT and 10 patients TH). The increased postoperative morbidity has not been associated with increased mortality (1.2% TT and 0% TH). We only indicate TH esophagectomy in patients with subcarinal neoplasms who present an FEV1 <75% (since the baseline respiratory condition contraindicates thoracotomy), or with clinical stage T1aN0M0. Tumors in stage T1bN0M0 may present with lymph node metastases in 20%–25% of adenocarcinomas and in up to 40% of squamous-cell carcinomas,5 so we believe it is necessary to carry out standard mediastinal lymphadenectomy.
Rizk et al.23 propose that an optimal lymphadenectomy should contain a minimum of 18 lymphadenopathies, while the AJCC recommends the removal of at least 10 lymphadenopathies for T1, 20 for T2 and 30 for T3/T4.19 In our series, the mean number of excised lymph nodes was 23,1,5,17–29 whereas in patients with precarinal neoplasms who underwent left cervical lymphadenectomy, the mean number of lymph nodes removed was 109–12; in 3 of these 13 cases (23%), lymph node involvement was found. This datum forces us to consider whether the lack of cervical lymphadenectomy in precarinal tumors reduces the effectiveness of oncologic treatment.
Our group proposes the laparoscopic approach in localized esophageal tumors that do not require neoadjuvant treatment, as this (especially RTx) increases the difficulty of dissection and may influence the radical nature of the lymphadenectomy. We believe that systematization of the technique should involve complete minimally invasive approaches (laparoscopy and thoracoscopy) in this type of patients.30
Overall morbidity was 42% (Table 3), and thoracic complications were the most frequent.9,12 Among these, there was a notable prevalence of pleural empyema (14%). Coloplasty was the reconstruction technique which resulted in a greater number of this type of complication.
Dysphonia affected 16 patients and was much more frequent in TT esophagectomy with 3-field lymph node dissection (41.6%). Although 13 cases recovered phonation, 2 patients had permanent damage to the left recurrent laryngeal nerve. Cervical lymphadenectomy could probably favor this complication. Paresis or paralysis of a vocal cord makes the efficacy of respiratory effort more difficult and may lead to more pulmonary complications in the immediate postoperative period.5
Fifteen anastomotic dehiscences were diagnosed: 9/58 (15.5%) intrathoracic anastomoses, and 6/42 (14.3%) cervical anastomoses (Table 3). Out of the 9 patients with intrathoracic dehiscence, 4 (6.9%) had severe clinical impact that required reoperation and removal of the plasty; the diagnoses of the remaining 5 were radiological and treatment was conservative. The approach of our group to an intrathoracic dehiscence is always conditioned by the clinical situation of the patient. When faced with the suspicion of mediastinitis secondary to a fistula, we opt for emergency surgery with excision of the plasty and terminal cervical esophagostomy. In cases with no clinical impact and late, well-drained dehiscence, we attempted endoscopic treatment. Endoscopic stent placement is an effective, less-aggressive solution, as long as the patient's clinical status allows for it.26
The incidence of cervical anastomotic dehiscence was 14.3%. Since 2002, our group has been systematically performing prior conditioning of the gastroplasty using the technique described by Akiyama20,21 in all patients candidates for gastroplasty with cervical anastomosis. We believe that the increased vascularization through the right gastroepiploic artery that occurs after the completion of this technique is one of the factors that reduces the risk of ischemia of the gastroplasty and favors a lower incidence of dehiscence. Based on these results, our objective is to implement the gastric conditioning in all patients who are candidates for esophagectomy and reconstruction with gastroplasty.
Two patients died in the postoperative period (Fig. 2). This mortality rate is similar to published reports by different authors17,24 and corresponds with international recommendations regarding esophageal cancer surgery.19 Neoadjuvant treatment and technically complex surgery in a patient with a significantly affected nutritional status require precise patient selection, which can only be done by a multidisciplinary unit. This selection, surgical expertise and the number of cases treated are the main factors involved in reducing mortality.4
In 1986, Matthews et al.25 demonstrated the inverse correlation between the number of resected tumors and hospital mortality. Numerous subsequent studies have confirmed this association4,13–15 and it now seems clear that the surgical treatment of these patients should be performed in a few highly-specialized units that treat significant number of cases and have joint protocols with hospitals in their area of influence. A first step in this direction was made apparent in the Regulations published this year by the Department of Health of the Generalitat de Catalunya, which limited the number of hospitals allowed to perform esophageal oncologic surgery to just seven.27
Actuarial survival was 85% the first year, 59% at 3 years and 56% at 5 years (Fig. 2), which was equivalent to other published series.28,29 Over 40% of the operated patients died during the first 2 years, while survival became relatively stable from the third year on. In 21 of the 26 patients who underwent surgery without neoadjuvant therapy, the final stages were IA in 13 and IB in 8, which probably influenced survival.
In conclusion, only 25% of patients diagnosed with esophageal cancer can be operated on with curative intent. Surgical morbidity is high and especially important, including thoracic complications and anastomotic dehiscence.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Teixidor LF, Talaverón JL, Guzmán MG, Danso HA, Miró Martín M, Larrañaga CB, et al. Resultados de la esofagectomía por cáncer tras la creación de un Comité de Tumores Esofagogástricos. Cir Esp. 2013;91:517–523.