This article summarizes the clinical guidelines for the diagnosis and treatment of malignant pleural effusion (MPE) sponsored by the Spanish Society of Thoracic Surgery (SECT). Ten clinical controversies were elaborated under the methodology of PICO (Patient, Intervention, Comparison, Outcome) questions and the quality of the evidence and grading of the strength of the recommendations was based on the GRADE system. Immunocytochemical and molecular analyses of pleural fluid may avoid further invasive diagnostic procedures. Currently, the definitive control of MPE can be achieved either by pleurodesis (talc poudrage or slurry) or the insertion of a indwelling pleural catheter (IPC). It is likely that the combination of both techniques (i.e., thoracoscopy with talc poudrage and insertion of a IPC, or instillation of talc slurry through a IPC) will have a predominant role in the future therapeutic management.
Este artículo resume la guía clínica de diagnóstico y tratamiento del derrame pleural maligno (DPM) auspiciada por la Sociedad Española de Cirugía Torácica (SECT). Se elaboraron 10 controversias clínicas bajo la metodología de preguntas PICO (Patient, Intervention, Comparison, Outcome) y la calidad de la evidencia y graduación de la fuerza de las recomendaciones se basó en el sistema GRADE. El análisis inmunocitoquímico y molecular del líquido pleural puede evitar procedimientos invasivos ulteriores con finalidad diagnóstica. Actualmente, el control definitivo del DPM se puede realizar indistintamente a través de una pleurodesis (talco poudrage o slurry) o de la inserción de un catéter pleural tunelizado (CPT). Es probable que la combinación de ambas técnicas (p.ej. toracoscopia con talco poudrage e inserción de un CPT, o instilación de talco slurry a través de un CPT) ocupe un lugar predominante en el manejo terapéutico futuro.
Malignant pleural effusion (MPE) accounts for around 25% of all pleural effusions undergoing thoracentesis and is the leading cause of exudative efussions.1,2 Most MPEs are metastatic and originate from a primary lung or breast tumour. The presence of a MPE in the context of a neoplasm implies advanced or disseminated disease, with an average survival of 3 to 12 months, which varies according to the primary tumour.3
The therapeutic approach to MPE is mainly determined by the patient's prognosis. Many studies have been published in search of prognostic factors for survival in MPE. Some prognostic scoring systems for MPE have been proposed. However, only the LENT4 scoring system has been externally validated. It's calculated based on pleural fluid lactate dehydrogenase (LDH) levels, the patient's ECOG PS, blood neutrophil-to-lymphocyte ratio (NLR), and tumour type. The score obtained establish three risk groups: low (0−1 point), moderate (2−4 points), and high (5−7 points) (Table 1). The LENT score has been established as a useful, simple, and accurate tool for decision-making in MPE.
The LENT score calculation and risk categories for malignant pleural effusion.
| CALCULATION OF LENT SCORE | ||
|---|---|---|
| Variable | Score | |
| L | LDH in pleural fluid (IU/L) | |
| <1500 | 0 | |
| >1500 | 1 | |
| E | ECOG | |
| 0 | 0 | |
| 1 | 1 | |
| 2 | 2 | |
| 3−4 | 3 | |
| N | NLR | |
| <9 | 0 | |
| >9 | 1 | |
| T | Tumour type | |
| Low-risk tumours | 0 | |
| Mesothelioma | ||
| Haematological neoplasms | ||
| Moderate risk tumours | 1 | |
| Breast cancer | ||
| Gynaecological cancer | ||
| Renal cell carcinoma | ||
| High risk tumours | 2 | |
| Lung cancer | ||
| Other types of cancer | ||
| Category | Total score |
|---|---|
| Low risk | 0−1 |
| Moderate risk | 2−4 |
| High risk | 5−7 |
LDH, Lactate dehydrogenase; NLR, Neutrophil/lymphocyte ratio.
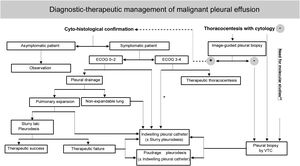
The European5 and American6 guidelines on the management of MPE were published in 2018. In recent years, growing clinical evidence based on randomised clinical trials (RCTs) has resulted in a change in the diagnostic and therapeutic approach to this entity. The Spanish Society of Thoracic Surgery (SECT) has considered necessary the development of a clinical guideline that reflects the numerous advances and controversies still existing in the management of PMD (Table 2)(Fig. 1).7
Summary of recommendations from the SECT guidelines for Malignant Pleural Effusion.
| Summary of recommendations |
|---|
| CLINICAL CONTROVERSY 1How should pleural fluid be processed to maximise the diagnostic yield of cytological studies? |
Recommendations
|
| CLINICAL CONTROVERSY 2Which type of pleural biopsy is most cost-effective in patients with MPE? |
Recommendations
|
| CLINICAL CONTROVERSY 3Is drainage of the pleural effusion necessary in patients with MPE, or is observation an option? |
Recommendations
|
| CLINICAL CONTROVERSY 4In patients with MPE undergoing therapeutic thoracentesis, is pleural manometry useful in predicting a non-expandable lung or complications of the procedure? |
Recommendations
|
| CLINICAL CONTROVERSY 5What should be the approach to a non-expandable lung diagnosed after placement of a pleural drain? |
Recommendations
|
| CLINICAL CONTROVERSY 6Is pleurodesis more effective with talc slurry or talc poudrage? |
Recommendations:
|
| CLINICAL CONTROVERSY 7What should be the approach to pleurodesis failure? |
Recommendations:
|
| CLINICAL CONTROVERSY 8Can placement of a IPC provide similar therapeutic success to pleurodesis in patients with a diagnosis of MPE? |
Recommendations
|
| CLINICAL CONTROVERSY 9What should be the frequency of IPC drainage in patients with MPE? |
Recommendation
|
| CLINICAL CONTROVERSY 10In patients with an expected response to systemic antitumour treatment and symptomatic MPE, should definitive procedures (pleurodesis or IPC) be performed initially, or should a therapeutic thoracentesis be performed and wait for the effect of systemic treatment? |
Recommendations
|
IPC,indwelling pleural catheter; MPE, malignant pleural effusion; VTC, videothoracoscopy.
This document was drawn up with a view to standardising the care of patients with metastatic MPE, i.e., those with a primary tumour extending into the pleura and developing a secondary uni- or bilateral pleural effusion (PE). We excluded pleural mesothelioma, because of its particular characteristics and its low prevalence in our setting.
Based on an initial script, "Clinical Controversies" regarding diagnostic or therapeutic aspects of MPE were agreed, using the methodological approach of a PICO (Patient, Intervention, Comparison, Outcome) question. A scoping search was performed, selecting potentially relevant studies. It was conducted in May 2019 in the electronic databases PubMed, Trip Database, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials (CCRCT). In a second stage between August and October 2019, specific searches were conducted for controversies where insufficient studies were identified during the first stage. Embase, Pubmed and CCRCT were consulted, with no language or time limits, with updates through 2020. Further details on the search strategy are described in Supplementary Material 1.
Each author conducted a review and critical reading of the literature in response to these clinical controversies. The GRADE (Grading of Recommendations, Assessment, Development and Evaluations)8 criteria were used to quantify the strength of the recommendations in each of the controversies raised (Tables 3 and 4). After three review processes, the final document was approved by the working group and then reviewed and approved by the Scientific Committee and the SECT Board of Directors.
GRADE system: meaning of the 4 levels of evidence.
| Quality levels | Current definition | Former concept |
|---|---|---|
| High | High confidence in the match between the actual and estimated effect. | Confidence in the estimate of effect will not change in subsequent studies. |
| Moderate | Moderate confidence in the estimate of effect. | Further studies may have a major impact on our confidence in the estimate of effect. |
| Low | Limited confidence in the estimate of effect. The actual effect could be far from the estimated. | Further studies are very likely to change our confidence in the estimate of the effect. |
| Very low | Low confidence in the estimated effect. The true effect is very likely to be different from the estimated effect. | Any estimate is very uncertain. |
Classification of the level of evidence according to the GRADE system.
| Type of study | A priori level of quality | Decreases if | Rises if | Ex post level of quality |
|---|---|---|---|---|
| Randomised studies | High | Risk of bias-1 significant-2 very significantInconsistency-1 significant-2 very significant | Effect+1 large+2 very largeDose-response+1 evident gradient | HighModerate |
| Observational studies | Low | No direct evidence-1 significant-2 very significantImprecision-1 significant-2 very significantPublication bias-1 likely-2 very likely | All confounders+1 would reduce the observed effect+1 would suggest a spurious effect if there is no observed effect | LowVery low |
Cytological analysis of pleural fluid is diagnostic in about 55% of MPE, although this percentage is influenced by a number of factors such as the type of primary tumour, the experience of the cytologist, the number of specimens submitted for analysis, the manner in which they are examined and the volume of pleural fluid processed.9 The most widely accepted recommendations would be: (1) send at least 20−40 mL of pleural fluid for cytological analysis; (2) perfome a Papanicolaou- or May-Grunwald Giemsa-stained smear and a formalin-fixed, paraffin-embedded, haematoxylin-eosin-stained cell block, as they provide complementary information. In a study of 414 MPEs, 11% of smear-negative pleural fluids showed a positive block for malignancy, while the opposite occurred in 15% of cases10; (3) request a second cytological study by repeat thoracentesis if the first was negative and there is a well-founded suspicion of malignancy. In a study of 214 patients with MPE whose first cytological study was negative, examination of a new sample was diagnostic in 55 (26%) cases.2 Further cytological studies are not recommended; (4) use immunocytochemical stains on the cell block to define the origin of pleural metastases, as well as molecular biology techniques on the supernatant, smear or cell block of pleural fluid for therapeutic decisions.9
Pleural biopsy is recommended when the pleural fluid cytology study is negative, malignancy is suspected, and the diagnosis needs to be confirmed because the patient would be a candidate for active oncological treatment. There are three types of pleural biopsy: closed or "blind" biopsy, image-guided biopsy (ultrasound or computed tomography (CT)) and video thoracoscopy (VTC). Closed biopsy is less cost-effective than fluid cytology because tumour invasion of the pleura is mostly patchy or focal. However, closed biopsy can diagnose up to 20% of MPEs with false-negative pleural fluid cytology.11
Imaging-guided pleural biopsy is much more cost-effective, provided there is sufficient pleural thickening (>1 cm) or focal nodular lesions. In two randomised studies, CT-guided Tru-cut needle pleural biopsy had a diagnostic sensitivity of 87% versus 40%–47% for closed Abrams needle pleural biopsy.12,13 A randomised study found that ultrasound-assisted (non-real-time) Tru-cut needle pleural biopsy was less sensitive than CT-guided Abrams needle pleural biopsy (61% vs 77%).14 It is plausible that the use of real-time ultrasound would have increased this sensitivity. In addition to pleural thickening, a larger biopsy needle size is significantly associated with a higher diagnostic yield.15
VTC has a sensitivity of 93%–95% in confirming the malignant nature of a PE.11 It can be performed under intravenous sedation and local anaesthesia or under general anaesthesia, the former is preferred due to lower costs and shorter hospital stay.16 Its only contraindications are obliteration of the pleural space by extensive adhesions and baseline hypercapnia. In addition to taking pleural biopsies under direct vision, VTC allows talc pleurodesis (poudrage) and placement of a indwelling pleural catheter (IPC). In a randomised study of 90 patients, the diagnostic yield of rigid VTC was superior to that of the semi-rigid option (97.8% vs 73.3%).17 This difference was attributed to the smaller size of pleural biopsies obtained with the semi-rigid thoracoscope, which could theoretically be overcome using cryobiopsy. However, a recent meta-analysis comparing 311 pleural cryobiopsies versus 275 obtained by forceps found no differences in diagnostic yield (96.5% vs 93.1%).18 Another study randomising 73 patients found no statistically significant differences between the sensitivity of pleural biopsy obtained by mini-thoracoscopy or semi-rigid VTC (69.4% vs 81.1%).19 Finally, the comparison between medical VTC and CT-guided pleural biopsy (Abrams needle) in a single randomised study showed the former procedure to be numerically, but not statistically significantly, superior in diagnosing malignancy (95.2% vs 86.8%).20
The prevalence of asymptomatic MPE ranges from 14% to 41%, although it has not been established how many of these patients will eventually develop symptoms.21 In a multicentre retrospective study, 46 (41%) of 113 patients with asymptomatic MPE associated with lung cancer developed symptoms requiring pleural space intervention over one year of follow-up, despite appropriate cancer treatment.22 In an international multicentre study of 540 patients with MPE, 53.6% required definitive treatment with pleurodesis or IPC.23 In another retrospective study of 556 lung cancer patients, 40% developed PE at some point during the course of the disease. In half of these cases the PE was too small for diagnostic thoracentesis. Such minimal PE did not progress to symptomatic PE, but was associated with worse survival.24 Of the MPEs demonstrated by thoracentesis, 84% required some palliative intervention on the pleural space. The general consensus is not to act on PE when it is asymptomatic.6,10,25–27
Predicting a trapped lung is crucial when pleurodesis is planned, as it would contraindicate pleurodesis and leave IPC as the only therapeutic option. The lung does not expand in approximately 30% of patients with MPE.6,28,29 A rare complication of therapeutic thoracentesis (<0.1%) is re-expansion pulmonary edema (RPE).30 To avoid this, it is recommended to stop drainage when the patient presents with symptoms such as chest discomfort, pain, persistent cough or dyspnea.
Pleural manometry allows elastance (change in pleural space pressure in cm H2O/l of fluid removed) to be calculated to predict whether the lung will expand.31,32 An elastance >14.5 cm H2O/l suggests a non-expandable lung and values >19 cm H2O/l are related to pleurodesis failure.33,34
In a randomised study of 124 patients, the use of pleural manometry during therapeutic thoracentesis did not reduce the risk of complications compared to stopping the procedure when the patient presents symptoms or all fluid has been drained.35 When manometry is used, thoracentesis is discontinued if a pleural pressure of −20 cm H2O is reached.33,36,37
A "trapped or non-expandable lung" is one that is unable to expand and occupy >50% of the chest cavity after PE drainage.38 There are no randomised studies specifically investigating the therapeutic management of trapped lung in MPE. A systematic review concluded that the best therapeutic option for trapped lung in the context of MPE is IPC.39 IPC improves symptoms,40 reduces hospital stay and has few adverse effects in patients with MPE and non-expandable lung.41 Several observational studies conclude the same, with symptomatic improvements in more than 94% of cases.42–44
Loculated MPE presents several liquid collections due to compartmentalisation of the pleural space. In these cases, PE drainage does not achieve complete lung re-expansion, and therefore pleurodesis would be contraindicated, and IPC would also fail to completely evacuate the pleural space, thus limiting improvements in the patient’s symptoms.45 Several randomised studies have investigated the role of fibrinolytics in the treatment of loculated MPE, showing radiological improvements and subsequent pleurodesis with success rates of between 80% and 95%. The most recent study, TIME3, analysed dyspnea and pleurodesis outcome in 71 patients with multiseptal MPE who received intrapleural urokinase versus placebo. No differences were found in the degree of improvement of dyspnoea or in the pleurodesis failure rate. However, urokinase was associated with a decrease in radiological PE volume and a reduction in hospital stay.46
More than half of MPEs recur within a few weeks of an initial therapeutic thoracentesis, and definitive pleural procedures are recommended in these cases. Talc is the most effective sclerosing agent for pleurodesis, once complete drainage of the MPE and lung re-expansion have been achieved.1,28,47
The administration of talc slurry is as effective as the administration of poudrage.28,47 Success rates for talc slurry range from 81% to 100%48–50; similar rates to those described for talc poudrage. In cases of recurrence of MPE after a first attempt at pleurodesis, a second procedure using talc poudrage via VTC or insertion of a IPC are the most common procedures.
None of the four published RCTs have found significant differences in terms of pleurodesis success when comparing the two techniques51–54 (Table 5). In the largest randomised study, no differences were observed in pleurodesis rate with talc poudrage or slurry at 30 days in patients with initial lung re-expansion >90% (78% vs 71%).51 However, talc poudrage was superior in the subgroup of patients with breast or lung cancer (82% vs 67%). Recently, Bhatnagar et al. found no significant differences when comparing the two techniques in the failure rate of pleurodesis at 30 (10% vs 14%), 90 (22% vs 24%), or 180 days (29% vs 28%).52 The study, however, was not statistically powered to find differences of less than 15% between treatment groups, which some might consider clinically relevant.
Randomised studies comparing pleurodesis with talc poudrage and talc slurry in MPE.
| Author | Year | N | Results |
|---|---|---|---|
| Yim and cols.53 | 1996 | 57 (28 poudrage; 29 slurry) | No significant differences in recurrence (3.5% poudrage vs. 10.3% slurry; p = NS).Similar rate of complications (10.7% poudrage vs. 6.9% slurry; p = NS). |
| Dresler and cols.51 | 2005 | 482 (240 poudrage; 242 slurry) | Similar rate of pleurodesis at 30 days (78% vs. 71%; p = .169).Greater rate of pleurodesis with the use of poudrage in breast/lung cancer (82% vs. 67%; p = .022). |
| Terra and cols.54 | 2009 | 60 (30 poudrage; 30 slurry) | With no significant differences in recurrence (16.6% poudrage vs. 13.3% slurry; p = .999).Similar rate of complications (23.3% poudrage vs. 30% slurry; p = .559). |
| Bhatnagar and cols.52 | 2020 | 330 (166 poudrage; 164 slurry) | Similar pleurodesis failure rate at 30 days (10% vs. 14%; p = .29).Similar pleurodesis failure rate at 90 days (22% vs. 24%; p = .74).Similar pleurodesis failure rate at 180 days (29% vs. 28%; p = .86). |
NS, not significant.
Pleurodesis usually fails in about 25%–30% of cases,55 particularly in patients with lung cancer and mesothelioma.56,57 In the event of pleurodesis failure, a IPC can be placed or a new pleurodesis attempted, either by VTC or through a thoracic drain. The published experience with re-pleurodesis is anecdotal and from the description of isolated cases.56,58,59 Therefore, the general recommendation is to use IPC if technically possible.60
Five RCTs have been published comparing chemical pleurodesis with IPC,61–65 the most relevant being TIME262 and AMPLE.64 TIME2 studied whether IPC is more effective than insertion of a drainage tube followed by talc slurry pleurodesis in relieving dyspnea in patients with MPE. A total of 106 patients were randomised; 52 to the IPC group and 54 to the talc pleurodesis group.62 Baseline dyspnea improved equally in both groups. However, at 6 months, the results favoured the IPC group. IPC was associated with fewer days of hospital stay over 1 year (1 vs. 4.5 days) and less need for subsequent pleural procedures (6% vs. 22%), but a higher risk of side effects, mainly infections. The AMPLE study randomised 144 patients with symptomatic MPE to treatment with either IPC or talc slurry pleurodesis, to assess the total number of days the patient required in hospital from the procedure until death or 12 months follow-up, excluding time spent on day hospital chemotherapy.64 This number of days was significantly lower in the IPC group (10 vs 12 days), although the magnitude of this difference is of uncertain clinical significance. The study also showed that both groups improved dyspnea and quality of life equally and that fewer subsequent pleural procedures were required in the IPC group (4% vs 22%), although there were greater overall non-serious adverse effects (30% vs 18%).
A recent network meta-analysis concluded that IPC is as valid a first-line treatment for MPE as pleurodesis.66 Compared to talc slurry, IPC achieves pleurodesis less frequently, but similarly controls dyspnea and is associated with less need for subsequent pleural procedures. The disadvantages are the need to fit a IPC and the risk of infection. Approximately 50% of IPCs can be removed, due to spontaneous pleurodesis, after a median of 50 days after insertion.67 Infections, either of the skin at the entry site of the IPC or of the pleural fluid, occur in 5%–8% of cases.67,68 It should be noted that the percentage of infections is similar in patients receiving chemotherapy or who are neutropenic, therefore these circumstances should not contraindicate the insertion of a IPC.69 Infection of the pleural space does not mean the IPC has to be removed. Other minor complications include obstruction of the IPC (5%), which may resolve with saline instillations or fibrinolytics, or the development of multiple septa (<15%), which may require intrapleural fibrinolytics.67
Several non-randomised studies with a limited number of patients have shown that placement of a IPC during VTC with talcage reduces hospital stay, achieves pleural symphyses after a median of one week and, in the case of non-expandable lung, avoids further procedures since the IPC has already been placed beforehand.70–72
For many patients with a IPC, the therapeutic goal is not only improvement of dyspnea and quality of life, but also removal of the IPC in the shortest possible time. The shorter the time with a IPC, the lower the associated costs and potential complications.
The ideal IPC drainage schedule is not known. In practice, there are two options: (1) symptom-based drainage, which usually involves 2 or 3 weekly drains; and (2) intensive or daily drainage. The ASAP73 and AMPLE274 trials demonstrated that intensive drainage achieves a higher degree of self-pleurodesis and, consequently, IPC removal (47% vs 24% at 12 weeks in ASAP, and 37.2% vs 11.4% at two months in AMPLE2). Another approach that reduces pleurodesis time is instillation of talc slurry (4 g) through the IPC (43% self-pleurodesis vs 23% in the placebo group at 5 weeks in the IPC-plus study).75
A frequently encountered scenario is whether initial definitive pleural treatment (pleurodesis, IPC) is necessary in patients with symptomatic MPE secondary to malignancy who are expected to respond to systemic anti-tumour treatment. The European guidelines state that, due to the lack of evidence, no strong conclusions can be drawn on the value of systemic treatment to control MPE.4 Therefore, since definitive treatment on MPE is safe and effective, it is recommended to use it in these situations as well.
In general, early initiation of chemotherapy is recommended in haematological malignancies,76,77 although some investigators advocate definitive pleural procedures from the outset in symptomatic MPE.78
In breast cancer, a retrospective series of 108 patients with MPE divided patients into two groups according to whether they received pleurodesis from baseline in addition to the corresponding systemic therapy. The median pleural progression-free survival was longer in those who underwent pleurodesis initially (8.5 vs. 4.1 months).79
In patients with small cell lung carcinoma (SCLC) an initial approach could be to wait for the effect of systemic therapy, but there are no solid studies to support this. A retrospective study of 373 patients with SCLC showed a 55% resolution rate of MPE after the first line of treatment.80 On the other hand, draining PE prior to chemotherapy could avoid toxicity potentially resulting from its accumulation in a third space.
In non-small cell lung carcinoma (NSCLC), waiting for the effect of systemic treatment does not seem to have any benefit on the control of MPE. In the few studies available, the use of new therapies (target therapies, immunotherapy, etc.) does not improve MPE control in NSCLC.38,81 In a cohort study of 233 patients with MPE secondary to lung cancer (mostly NSCLC), early control of MPE with pleurodesis or IPC, in addition to target therapies, significantly reduced the need for pleural space reinterventions versus those who received only target therapies (23.5% vs. 53.8%).81
Conflict of interestThe authors have no conflict of interest to declare.
Juan Ignacio Martín Sánchez
Head of the Area of Evidence-Based Medicine. Knowledge Transfer Area.
Instituto Aragonés de Ciencias de la Salud (IACS), Centro de Investigación Biomédica de Aragón (CIBA), Zaragoza.