Robotic-assisted surgery is playing an increasingly important role in the last few years in the treatment of colorectal oncological disease. However, there are still no studies that objectively demonstrate the advantages of this type of surgery.
We present a prospective randomised study in order to compare the short-term results between colorectal robotic surgery and laparoscopic surgery.
Material and methodA total of 56 patients diagnosed with colorectal cancer between January 2008 and January 2009 were randomised and assigned to the robotic or laparoscopic group. Age, body mass index, tumour location, conversions in each group, complications during and after surgery, and histological characteristics of the specimens obtained, were all compared.
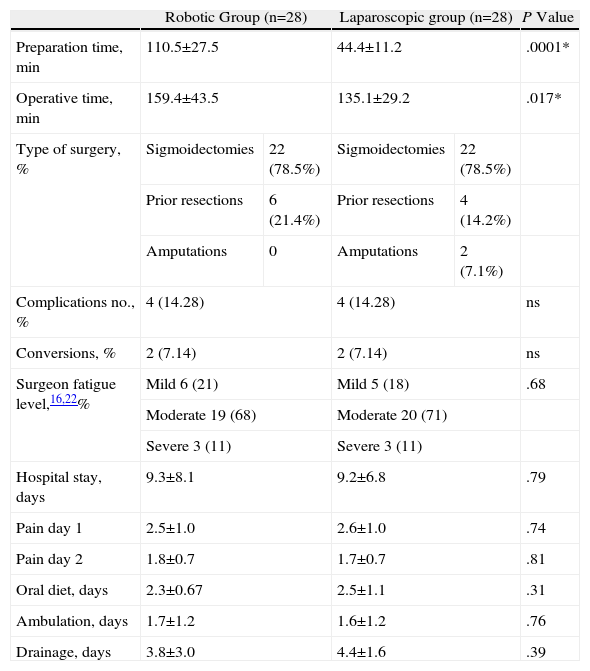
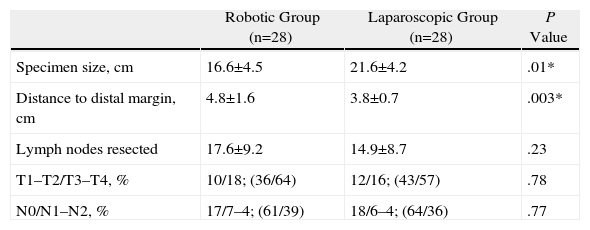
ResultsThere were no significant differences between age (P=.055), body mass index (P=.12), or tumour location (P=.91). Only one patient in the robotic group required a transfusion and none in the laparoscopic group. The percentage of conversions was the same in both groups, however, the preparation times and operating times were significantly longer in patients intervened using the robotic device (P=.0001 and P=.017, respectively). There were no differences as regards the rate of complications or in the percentage of re-interventions (14.2% and 7.1%). The mean hospital stay of the patients was 9.3 (8.1) days in the robotic group and 9.2 (6.8) days in the laparoscopic (P=.79). The distal resection margin was greater in the specimen obtained using robotic surgery (P=.003) as well as the number of lymph nodes obtained in the specimen (P=.23).
ConclusionRobotic colorectal was performed safely and effectively, and with similar clinical results.
International Trial Number for this study is: ISRCTN60866560.
La cirugía robótica está tomando protagonismo en los últimos años en el abordaje de la dolencia oncológica colorrectal. Sin embargo, no existen todavía estudios que muestren ventajas objetivas de este tipo de abordaje.
Presentamos un estudio prospectivo, aleatorizado cuyo objetivo es comparar los resultados a corto plazo entre la cirugía robótica y la cirugía laparoscópica colorrectal.
Material y métodoEntre enero de 2008 y enero de 2009, 56 pacientes diagnosticados de cáncer colorrectal fueron aleatorizados y asignados al grupo de cirugía robótica o laparoscópica. Se compararon la edad, el índice de masa corporal, la localización tumoral, las conversiones de cada grupo, las complicaciones intra- y postoperatorias y las características histológicas de las piezas obtenidas.
ResultadosNo hubo diferencias significativas en la edad (p=0,055), el índice de masa corporal (p=0,12), o la localización tumoral (p=0,91). Sólo un paciente precisó ser transfundido en el grupo robótico y ninguno en el grupo laparoscópico. El porcentaje de conversiones fue idéntico en ambos grupos, sin embargo el tiempo de preparación y el tiempo operatorio sí fue significativamente mayor en los pacientes intervenidos mediante el dispositivo robótico (p=0,0001 y p=0,017 respectivamente). No existieron diferencias en cuanto al índice de complicaciones ni el porcentaje de reintervenciones (14,2% y 7,1%). La estancia media de los pacientes fue de 9,3±8,1 días en el grupo robótico y de 9,2±6,8 días en el laparoscópico (p=0,79). El margen distal de resección fue mayor en el espécimen obtenido mediante cirugía robótica (p=0,003) así como el número de ganglios obtenidos de la pieza (p=0,23).
ConclusiónLa cirugía robótica colorrectal fue llevada a cabo de manera segura y efectiva con iguales resultados clínicos.
El número de registro internacional para este estudio es: ISRCTN60866560.
Laparoscopic surgery has numerous advantages in the treatment of gastrointestinal disease, such as reduced postoperative pain and average postoperative stay, better cosmetic results and faster recovery of intestinal transit.1–3 However, some disadvantages inherent in laparoscopic surgery have been documented. These include loss of three-dimensional vision, the need to use longer instruments, thus increasing surgeon hand tremors, loss of human wrist's movement since it only allows for four degrees of freedom, and the lack of intuitive movement due to the levering effect that the trocars have on the instruments.
Theoretically, robotic systems offer a solution for overcoming some of these limitations. Currently, these surgical devices are very sophisticated and have demonstrated their advantages in numerous surgical subspecialties. Of these, the Vinci® robot (Intuitive Surgical, Sunnyvale, CA, USA) is the most widespread and the only FDA-approved robot for performing abdominal surgery.
Cadiere et al.4,5 were the first to report robotic colorectal surgeries in three patients in 2001. Since then, numerous publications have shown the multiple technical advantages of robotic colorectal surgery over conventional laparoscopic surgery. These advantages include improved vision, stability of the camera platform, freedom of movement for the instruments and precision in manual suture.6 However, experience is still limited in robotic colorectal surgery. There are few prospective randomised studies comparing robotic colorectal surgery versus conventional laparoscopic surgery, with few cases and no conclusive outcomes.
We therefore present a prospective randomised study whose primary objective is to show the safety of robotic colorectal surgery. The secondary objective is to demonstrate the feasibility of treating rectal and sigmoid cancers with this technique, compared to conventional laparoscopic colorectal surgery.
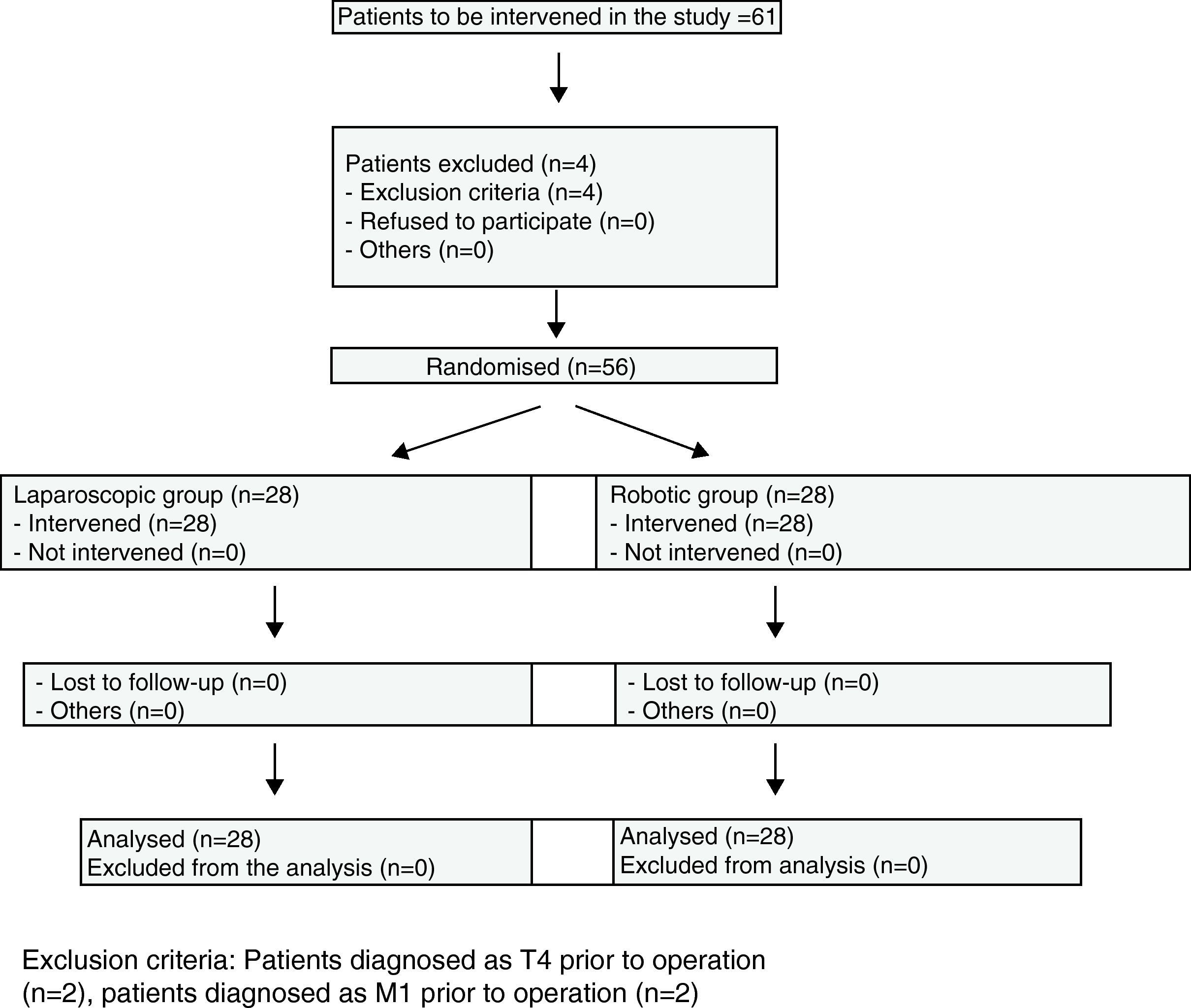
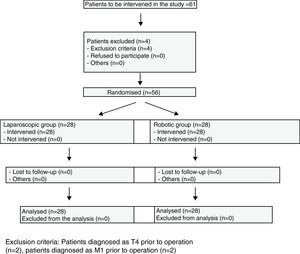
Materials and MethodsBetween January 2008, when the robotic surgery programme started, and January 2009, 298 colorectal cancer patients were operated on at the Hospital Universitario Virgen del Rocio. Of these, 56 cases of colorectal cancer located in the sigmoid colon and rectum were randomly distributed, by means of a sequence created by a computer program, into the robotic colorectal surgery and laparoscopy groups (Fig. 1). Three surgeons with experience in laparoscopic and robotic surgeries (J.D.P., E.P.S, H.C.D.) operated on all the patients.
All patients underwent a complete preoperative analysis, including haemograms, biochemistry and hepatic function tests, chest X-rays and electrocardiograms. All patients underwent a colonoscopy with biopsy for the histological diagnosis of the lesion, along with a thoracoabdominal CT, MRI, and ultrasound tests for those patients diagnosed with rectal cancer.
All patients diagnosed with stage T4 or M1 were excluded, as well as those under 18 years of age and those who did not sign the informed consent.
The following patient data were collected prospectively and stored in the database: demographic characteristics (age, weight, height), classification according to the American Society of Anesthesiologists (ASA), body mass index, surgical procedure, clinical results (preparation time of surgical system; operative time; need for transfusion; hospital stay; pain at day 1, day 2 and at discharge, according to the visual analogue scale, with the same analgesic treatment for all patients; days to intake and ambulation; days to withdrawal of drainage), complications and conversions, results of the histological analysis of the specimen and postoperative complications.
Criteria for hospital discharge were recovery of intestinal transit, oral diet tolerance, and ambulation, as well as a lack of complications (fever and uncontrolled pain).
The results of the histological analysis included distance to the distal margin, total number of affected lymph nodes resected and total length of the specimen.
This study was approved by the ethics committee of the Hospital Virgen del Rocio of Seville and the Agencia de Evaluación de Tecnologías Sanitarias de la Junta de Andalucía (healthcare technology evaluation agency of the Government of Andalusia). Informed consent was obtained from all patients.
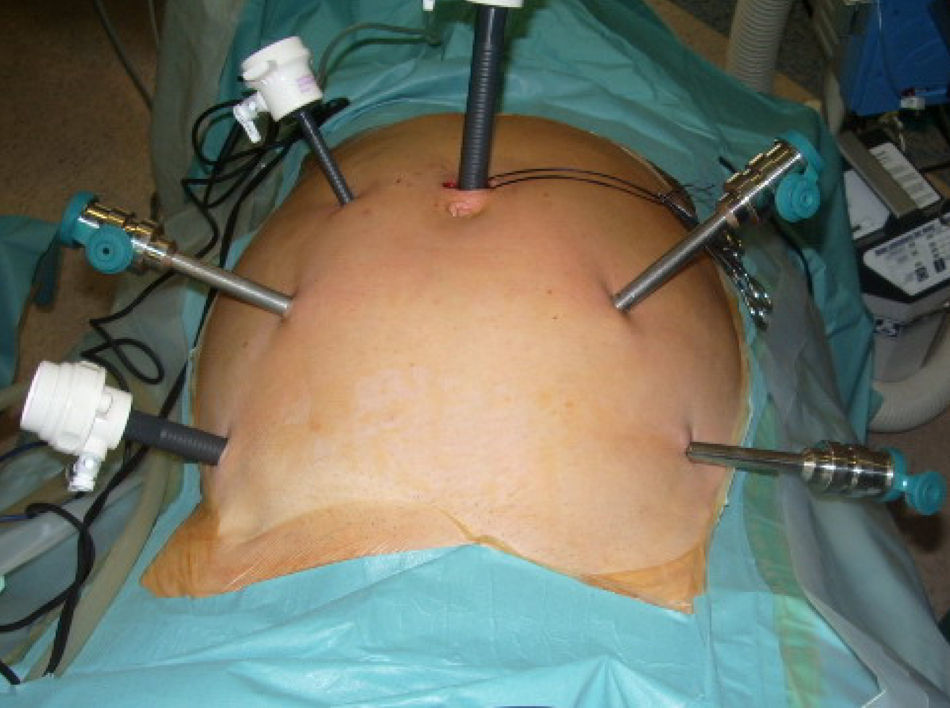
Surgical TechniqueRobotic ProcedureThis was performed under general anaesthesia and orotracheal intubation, with the patient in the lithotomy position (Lloyd-Davies) and after obtaining pneumoperitoneum with Veress needle in the left hypochondrium, maintaining intra-abdominal pressures of 12mmHg to 14mmHg. The first trocar (Covidien, Norwalk, CT, USA) was placed in the navel (12mm) for the optics. Next, a general review of the entire cavity was performed to rule out disseminated disease, thus enabling continuation of the procedure according to the study design. Three 8mm robotic trocars and one 5mm trocar (Covidien, Norwalk, CT, USA) were placed for the assistant (Fig. 2).
First, the lower mesenteric vein was located and ligated at the angle of Treitz using a 30mm endoGIA stapler (Covidien, Norwalk, CT, USA). Then, a medial to lateral dissection was performed on the splenic flexure of the colon. The opening to lesser sac was opened to fully take the colon down. After locating the lower mesenteric artery, the root was ligated using Hem-O-Lock silicone clips (Weck Closure Systems, Triangle Park, North Carolina, USA) after locating the ureter. The medial to lateral dissection was completed by opening the left parietocolic fold. In cases with rectal cancer, the approach to the upper rectum began with the opening of the peritoneal reflection, continuing with total or subtotal mesorectal excision. An endoGIA stapler (Covidien, Norwalk, CT, USA) is inserted to section the specimen in order to complete the cut. The specimen was extracted through a protected Pfannenstiel-type assistant incision through which the anvil was placed so that once it is secured with purse-string sutures using non-absorbable monofilament 2/0 it could be reintroduced for anastomosis. This anastomosis was performed using a 29mm or 31mm CEEA circular stapler (Covidien, Norwalk, CT, USA) under direct laparoscopic vision. In all cases, the lack of leaks was confirmed using the gas emboli test with water seal. Juxta-anastomotic drain was systematically installed.
Laparoscopic ProcedureUnder identical conditions, five trocars (Covidien, Norwalk, CT, USA) were placed in the patient: a 12mm, two 10mm, and two 5mm trocars. The surgical steps were similar to those performed using robotic surgery with ligation of the lower mesenteric vein at the angle of Treitz, ligation of the lower mesenteric artery at the root and medial to lateral dissection of the mesocolon. Resection of the left parietocolic fold and total or subtotal mesorectal excision was performed (according to tumour location) after opening the peritoneal reflection in cases where tumours under 15cm were located. The rectum was sectioned with a 45mm endoGIA stapler (Covidien, Norwalk, CT, USA) and extracted through the Pfannenstiel incision. The anvil was placed in the distal end of the colon after sectioning the specimen. Anastomosis was then performed under direct laparoscopic vision with sealing test, using the same system used in robotic surgery. Finally, juxta-anastomotic drainage was installed.
Statistical AnalysisWe used the software program Statistical Product and Service Solutions (SPSS) 11.5 for Windows (SPSS Inc. Chicago, IL, USA). Sample size was calculated based on the formula for the kind of sampling employed, with a 95% confidence level, which was at least 28 patients per study branch.
The quantitative variables in the descriptive study that followed a normal distribution were defined by mean and standard deviation. Qualitative variables were defined by number of cases and percentages. The quantitative variables in the analytical study of both samples were analysed with the Student's t-test for independent variables. A value of P<.05 was considered statistically significant.
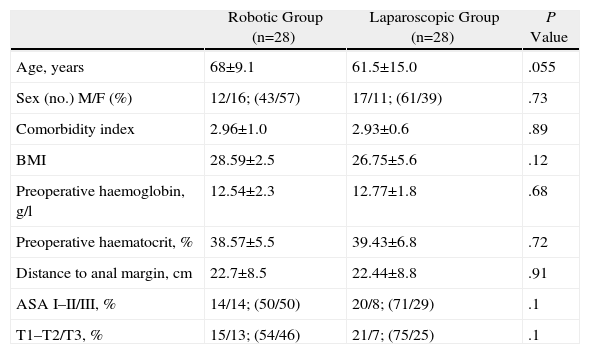
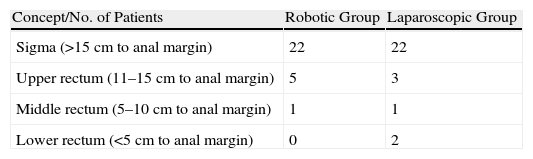
ResultsPatient CharacteristicsWe analysed patient characteristics, comparing the robotic group with the laparoscopic group. A total of 28 patients were included in each group (Tables 1 and 2). There were no differences according to gender (P=.73) or by distribution according to the patients’ preoperative stage (P=.1). There were significant differences between the groups in terms of mean age (P=.055), body mass index (P=.12), ASA classification (P=.1), and location of neoplasm in cm from anal margin (P=.91) (Tables 3 and 4).
Characteristics of Population Subjected to Study: Results Expressed as Mean±Standard Deviation and Percentages.
| Robotic Group (n=28) | Laparoscopic Group (n=28) | P Value | |
| Age, years | 68±9.1 | 61.5±15.0 | .055 |
| Sex (no.) M/F (%) | 12/16; (43/57) | 17/11; (61/39) | .73 |
| Comorbidity index | 2.96±1.0 | 2.93±0.6 | .89 |
| BMI | 28.59±2.5 | 26.75±5.6 | .12 |
| Preoperative haemoglobin, g/l | 12.54±2.3 | 12.77±1.8 | .68 |
| Preoperative haematocrit, % | 38.57±5.5 | 39.43±6.8 | .72 |
| Distance to anal margin, cm | 22.7±8.5 | 22.44±8.8 | .91 |
| ASA I–II/III, % | 14/14; (50/50) | 20/8; (71/29) | .1 |
| T1–T2/T3, % | 15/13; (54/46) | 21/7; (75/25) | .1 |
Results expressed as mean±standard deviation.
F: female; BMI: body mass index; M: male.
Perioperative Clinical Characteristics: Expressed as Mean±Standard Deviation and Percentages.
| Robotic Group (n=28) | Laparoscopic group (n=28) | P Value | |||
| Preparation time, min | 110.5±27.5 | 44.4±11.2 | .0001* | ||
| Operative time, min | 159.4±43.5 | 135.1±29.2 | .017* | ||
| Type of surgery, % | Sigmoidectomies | 22 (78.5%) | Sigmoidectomies | 22 (78.5%) | |
| Prior resections | 6 (21.4%) | Prior resections | 4 (14.2%) | ||
| Amputations | 0 | Amputations | 2 (7.1%) | ||
| Complications no., % | 4 (14.28) | 4 (14.28) | ns | ||
| Conversions, % | 2 (7.14) | 2 (7.14) | ns | ||
| Surgeon fatigue level,16,22% | Mild 6 (21) | Mild 5 (18) | .68 | ||
| Moderate 19 (68) | Moderate 20 (71) | ||||
| Severe 3 (11) | Severe 3 (11) | ||||
| Hospital stay, days | 9.3±8.1 | 9.2±6.8 | .79 | ||
| Pain day 1 | 2.5±1.0 | 2.6±1.0 | .74 | ||
| Pain day 2 | 1.8±0.7 | 1.7±0.7 | .81 | ||
| Oral diet, days | 2.3±0.67 | 2.5±1.1 | .31 | ||
| Ambulation, days | 1.7±1.2 | 1.6±1.2 | .76 | ||
| Drainage, days | 3.8±3.0 | 4.4±1.6 | .39 | ||
Results expressed as mean±standard deviation.
Histological Results: Expressed as Mean±Standard Deviation and Percentages.
| Robotic Group (n=28) | Laparoscopic Group (n=28) | P Value | |
| Specimen size, cm | 16.6±4.5 | 21.6±4.2 | .01* |
| Distance to distal margin, cm | 4.8±1.6 | 3.8±0.7 | .003* |
| Lymph nodes resected | 17.6±9.2 | 14.9±8.7 | .23 |
| T1–T2/T3–T4, % | 10/18; (36/64) | 12/16; (43/57) | .78 |
| N0/N1–N2, % | 17/7–4; (61/39) | 18/6–4; (64/36) | .77 |
Results expressed as mean±standard deviation.
Mean operative time was 159.4±43.5min for the robotic group and 135.1±29.2min for the laparoscopic group. Only one patient required transfusion in the robotic group while none required it in the laparoscopic group.
Both the time that patients took to start ambulation and maintain an oral food intake and the postoperative pain was similar for both groups. There were no differences in conversions due to tumour infiltration and technical difficulties in the laparoscopic group, or due to obesity and having to deal with a large tumour in the robotic group.
Postoperative stay, which was 9.38±8.1 days in the robotic group and 9.2±6.8 days in the laparoscopic group, did not show significant differences either.
The rate of postoperative complications was 14.28% for both groups. The reported complications for the laparoscopic group were rectal bleeding (n=1), deep vein thrombosis (n=1), intestinal obstruction by internal hernia (n=1) requiring reoperation, and dehiscence (n=1), also requiring surgery. The reported complications for the robotic group were anastomotic bleeding (n=1) requiring urgent surgery, AHT crisis (n=1) from a head injury due to an accidental fall requiring prolonged hospitalisation, anastomotic leakage (n=1) that was treated conservatively and strangulated inguinal hernia (n=1) requiring urgent surgery. One patient in the laparoscopic group had an infection of the surgical wound. The mortality rate was 0% for both groups.
Histological Results (Table 4)The mean number of lymph nodes obtained for each group was 17.6±9.2 in the robotic group and 14.9±8.7 in the laparoscopic group (P=.23). The mean distance of the resection from the distal margin was 4.8±1.6cm in the robotic group and 3.8±0.7cm in the laparoscopic group, with significant differences between the two groups. There were no significant differences in the remaining parameters.
DiscussionEver since Ballantyne et al.7 performed the first robotic cholecystectomy in 2001, the popularity of this technique has been increasing. Experience with robots in colorectal surgery has demonstrated several advantages over traditional and laparoscopic approaches: three-dimensional view of the operative field, a stable platform for the camera and freedom of movement thanks to articulated instruments that have bending abilities similar to the human hand.6
It has been postulated that these characteristics may facilitate certain steps in colorectal surgery and thus reduce conversion rates, steps such as mobilisation of the splenic flexure,8–10 dissection of lower mesenteric vessels,11–13 preservation of nerve plexus,8,11,14 dissection and mobilisation of the rectum in narrow pelvises8,10–14 and suturing.8 For these reasons, robotic surgery has attracted special attention in rectal cancer surgery13 as a solution to some of the problems with complete laparoscopic mesorectal excision, especially those related with narrow pelvis and reduced manual dexterity of laparoscopic instruments.
Another added advantage lies in the reduction of fatigue. Some studies15,16 have reported the physical damage that laparoscopy incurs in the surgeon, who has to adopt anti-ergonomic postures that may cause fatigue and musculoskeletal damage. Robotic surgery reduces physical stress and allows surgeons to adopt more ergonomic postures, allowing them to remain comfortably seated throughout the procedure.
We can see why robot-assisted colorectal surgery is being implemented in a consistent and progressive manner,17 although there are few studies in the literature currently available, and these studies include few cases and present low levels of evidence.
As for the length of robotic procedures, three studies reported longer surgery times when compared to laparoscopic assisted surgery,11,18,19 similar to the results in our study. Of note is the minimal increase in surgical time documented in more complex pelvic processes. Most of these publications attribute this time increase to the need for changing the position of the device in order to perform complete mesorectal excision. However, we performed all procedures with the robotic system, so it is not necessary to change its site according to the patient situation. Despite this, operating times were still longer using robotic surgery. Nevertheless, it has also been observed that surgical times and robotic system preparation decrease after the initial cases.
In published data referring to mean postoperative stay,14 some authors suggest that it is lower in the robotic group, which may be due to less damage during pelvic dissection. In our study, the mean postoperative stay was similar for both groups, probably due to the tumours having a higher location, which meant that pelvic dissection was not necessary in all cases.
As in the current literature,14 we did not find significant differences in our study in terms of blood loss.
The complications found were not due to the robotic system. Only two patients in each group required surgery (intestinal obstruction and dehiscence in the laparoscopic group, and strangulated hernia and anastomotic bleeding in the robotic group), with no mortality in either group. If we review all published complications occurring during robotic colorectal surgery, we find that only two cases may be directly attributable to the robot.6,8,11,18,19 Both cases were due to damage caused by the lack of tactile sensation and loss of control over the robotic clamps’ pulling force. However, the lack of tactile sensation may be overcome with small visual tricks during surgery, as proposed by Baik et al.20
The main disadvantages of laparoscopy, such as camera platform instability, which can cause the loss of the surgical field due to minor unexpected movements, and the restricted movement of instruments, make surgical dissection complex, especially in the case of the pelvis in rectal cancer. These disadvantages, along with the large size of some lesions, are the main cause of conversion. We should also consider the difficulty in transection of the rectum. Given the advantages provided by the da Vinci robot, we should expect a reduction in conversion rates, although in our case they were similar in both groups. In the laparoscopic group, conversions were due to technical difficulties, for reasons already mentioned and tumour infiltration. Meanwhile, conversions in the robotic group were due to the high body mass index of one of the patients and the large tumour size in another patient.
If we focus on the oncology results, several authors have reported similar length for the surgical specimen,8 similar number of lymph nodes11–15 and similar distances from the tumour to the distal margin of the specimen.11,14,15 Our study shares both the specimen size and the number of lymph nodes resected. However, in our experience, robotic surgery helped us obtain more extensive distal margins with a significant difference, which was probably due to the greater ease in distal dissection in cramped surgical fields. We should also take into consideration that there were no differences between both groups in terms of gender distribution.
One of the major disadvantages of robotic surgery is its cost. Perhaps because of this, Pigazzi and García-Aguilar21 suggest limiting its indications to those conditions where the robot will provide objective benefits over laparoscopy. Another disadvantage is the large size of the robotic system, which in emergencies may be difficult to handle and may become a drawback during surgery. However, work is proceeding on new systems that are lighter and easier to handle, which will be available in the very near future.
ConclusionThis study shows that colorectal surgery performed with robotic assistance is feasible, safer and provides results similar to those achieved with conventional laparoscopic assistance, since postoperative complication and conversion rates were similar in both groups.
Nevertheless, longer-term follow-up studies are needed to determine if these data correspond to greater disease-free survival and whether they reduce local recurrence rates.
Conflicts of InterestThe authors have no conflicts of interest to declare.
Please cite this article: Jiménez Rodríguez RM, et al. Estudio prospectivo, aleatorizado: cirugía laparoscópica con asistencia robótica versus cirugía laparoscópica convencional en la resección del cáncer colorrectal. Cir Esp. 2011;89:432–8.
This study was presented at the Second Worldwide Conference of Robotic Surgery held in Chicago on October 1 and 2, 2010.