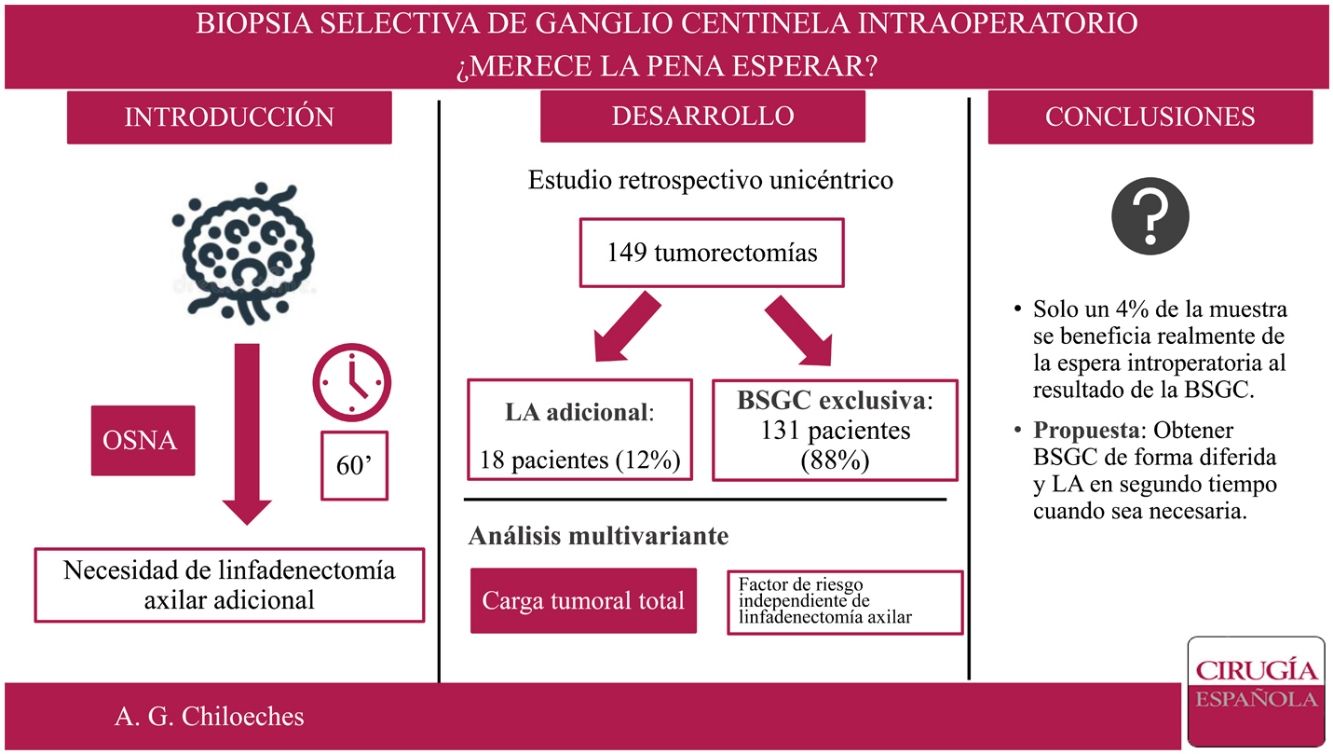
In our institution, the study of selective sentinel node biopsy (SLNB) is performed intraoperatively. The main objective of our study is to know the proportion of patients who benefits from the waiting of the results of SLNB.
MethodsA retrospective analysis of patients operated on our center between January 1st, 2018 and June 30, 2019 was carried out. We included women diagnosed with T1-T2 tumors, treated by lumpectomy and SLNB studied using OSNA method.
ResultsOur study included 149 women. There were not statistically significant differences in terms of demographic data between the group treated with axillary lymph node dissection (ALND) and exclusively SLNB group. After analysis of SLN intraoperatively, there were performed 18 axillary lymphadenectomies. Only in six of this 18 cases, three or more sentinel nodes were founded. The location of the tumor, the presence of lymphovascular permeation and the total tumor load (TTL) showed statistically significant differences between groups. Only the TTL was established as the independent factor of the need for ALND.
DiscussionObtaining a deferred result of the SLNB allowed reducing the time of anesthesia and occupation of the operating room, since in a high percentage of cases an additional procedure is not performed.
En nuestro centro el estudio de la biopsia selectiva del ganglio centinela (BSGC) se realiza de forma intraoperatoria. El objetivo principal del presente trabajo es conocer qué proporción de pacientes se beneficia de la espera intraoperatoria al resultado de la BSGC.
Material y métodosSe ha realizado un análisis retrospectivo de pacientes intervenidas en nuestro centro entre el 1 de enero de 2018 y el 30 de junio de 2019. Se incluyeron mujeres con tumores T1-T2, tratadas mediante tumorectomía y BSGC estudiado mediante método OSNA (one-step nucleic acid amplification).
ResultadosSe incluyeron 149 mujeres en el estudio. No se encontraron diferencias estadísticamente significativas en cuestión de datos demográficos entre el grupo tratado mediante linfadenectomía axilar (LA) y el grupo tratado exclusivamente con BSGC. Se realizaron 18 LA tras el análisis del GC estudiado de forma intraoperatoria. Solo en 6 de los casos se extrajeron tres o más GC. La localización por cuadrante de la lesión, permeación linfovascular y carga tumoral total muestran diferencias estadísticamente significativas entre los grupos. En el análisis multivariante, únicamente la carga tumoral total (TTL) se establece como variable independiente de necesidad de LA.
ConclusionesLa obtención del resultado de la BSGC de forma diferida permite disminuir el tiempo de anestesia de las pacientes y tiempo de ocupación de quirófano, ya que, en el momento actual, no se realiza ningún procedimiento adicional en un elevado porcentaje de casos.
Selective sentinel node biopsy (SLNB) is the optimal nodal staging method in patients with early-stage breast cancer and clinically negative axilla1–3 and can avoid the morbidity of axillary lymph node dissection (ALND)4. The first randomised clinical trial validating the technique was published in 20034.
Since then, numerous prospective randomised studies have shown that ALND is not necessary in women with disease-free sentinel lymph nodes (SLN)4–6. The results of the ACOSOG Z0011 and IBCSG 23-01 studies demonstrated that disease-free survival (DFS) and overall survival (OS) of SLNB is non-inferior to ALND in women with early-stage breast cancer and up to two metastatic sentinel nodes7–9.
These recommendations have been adapted in the latest clinical practice guidelines for the treatment of early breast cancer1–3. These guidelines state that ALND is not necessary in patients diagnosed with early breast cancer, treated with conservative surgery and SLNB, with one or two positive nodes, who are subsequently to undergo radiotherapy. It is important to note that treatment recommendations are based on histological and not molecular criteria, although with our increasing understanding of breast cancer, there is now less reliance on lymph node status to determine adjuvant therapeutic strategies.
There are two methods of studying the SLN. The cytohistological method which studies the SLN microscopically10 and the OSNA method which analyses the copy numbers of cytokeratin 19 (CK19) mRNA). Total tumour load (TTL) is defined as the sum of the copy numbers of this marker. Peg et al. established in 2013 that TTL is an independent predictor of positive non-sentinel lymph nodes in ALND. In their study, they found that a TTL above 15,000 copies translated into a 41% chance of finding positive nodes in ALND beyond the SLNs. However, below 15,000 copies this percentage reduced to 14.7%11. Studies show that OSNA has greater sensitivity to detect more cases of micrometastases12. This may lead to an increase in the number of complete axillary dissections and, consequently, an increase in patient morbidity without necessarily leading to increased DFS and OS.
ObjectiveThe primary objective of this study was to establish the proportion of patients that actually benefit from intraoperative waiting for the result of SLNB. The secondary objective was to establish a predictor model indicating which patients will require additional ALND.
Material and methodsThis article follows the STROBE (strengthening the reporting of observational studies in epidemiology) recommendations for observational studies.
Study designSingle-centre retrospective observational study.
ParticipantsAll female patients diagnosed with clinically axilla-negative breast cancer, who underwent breast-sparing surgery and SLNB (analysed by OSNA) in our service between 1 January 2018 and 30 June 2019 were included. All patients were over 18 years of age.
Patients treated by mastectomy, those who had received neoadjuvant treatment, and those with SLN study by histology or imprint cytology were excluded.
VariablesData were extracted by review of the electronic medical records.
Preoperative variables were age, personal or family history of breast cancer, hypertension, dyslipidaemia, and diabetes mellitus, smoking, symptoms at diagnosis, laterality of the affected breast, location by quadrant, BI-RADS, and size in millimetres based on ultrasound and/or MRI.
The operative data were number of SLNs localised during surgery, total intraoperative tumour load and whether or not ALND was performed.
The following variables were collected on the pathological anatomy of the lesion: type of carcinoma, immunohistochemical profile, grade, presence of perineural invasion, or lymphovascular permeation. With regard to ALND the following variables were analysed: number of non-sentinel lymph nodes, number of total lymph nodes at lymph node dissection, and number of positive nodes at lymph node dissection.
Statistical analysisCategorical variables were reported as absolute value and percentage. Quantitative variables were described as mean and standard distribution (SD) in case of normal distribution, and as median and interquartile range (IQR) if they followed a non-normal distribution. Quantitative variables were tested for normality using the Shapiro-Wilks test. For hypothesis testing, the χ2 test was used for categorical variables, Student's t-test for normally distributed quantitative variables, and the Mann Whitney U test for non-normally distributed quantitative variables.
A multivariate logistic regression model was used to assess risk factors for ALND, initially including the variables that had shown a significant relationship with the variable of interest.
Statistical significance was set at <.05. SPSS Statistics 23 (IMB Corp., Armonk NY, USA) was used).
Lymph node dissection criteriaIn our centre, the operating room protocol consisted of removal of the SLN (or SLNs) and subsequent intraoperative analysis by OSNA. In the case of a TTL of more than 15,000 copies (sum of the copy number of all the SLNs), surgery was completed with ALND. In the case of a TTL below 15,000 copies, ALND was not performed.
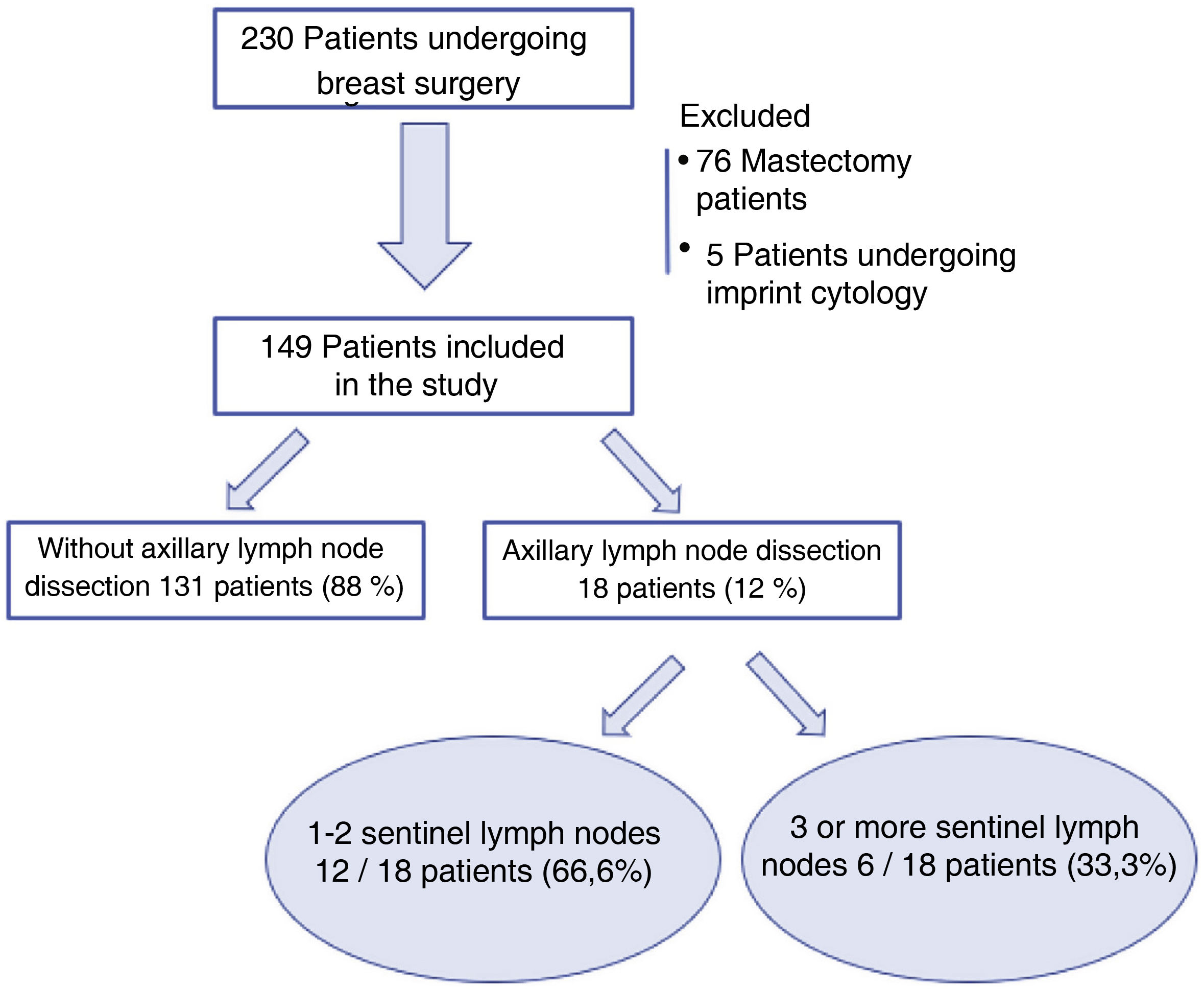
ResultsFrom 1 January 2018 to 30 June 2019, 230 patients underwent breast surgery at our centre without prior neoadjuvant treatment. Seventy-six patients undergoing mastectomy and five cases of SLN study by imprint cytology were excluded. Finally, 149 patients were included in the analysis. Of these, in 18 cases (18/149; 12%) an ALND was performed after intra-operative SLN analysis, and three or more sentinel nodes were removed in only six cases (6/149; 4%) (Fig. 1).
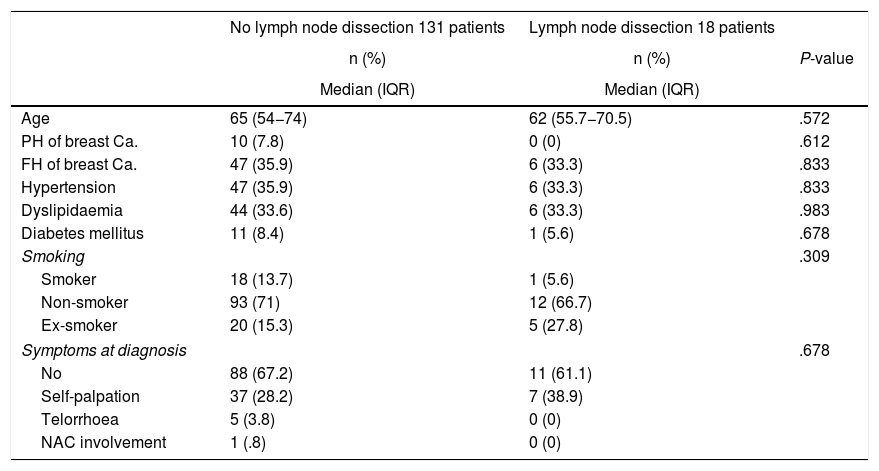
Preoperative dataNo differences were found between the ALND and the non-ALND groups in terms of age, family or personal history of breast cancer, smoking, common cardiovascular risk factors, or symptoms at diagnosis (Table 1).
Baseline characteristics of the patients.
| No lymph node dissection 131 patients | Lymph node dissection 18 patients | ||
|---|---|---|---|
| n (%) | n (%) | P-value | |
| Median (IQR) | Median (IQR) | ||
| Age | 65 (54−74) | 62 (55.7−70.5) | .572 |
| PH of breast Ca. | 10 (7.8) | 0 (0) | .612 |
| FH of breast Ca. | 47 (35.9) | 6 (33.3) | .833 |
| Hypertension | 47 (35.9) | 6 (33.3) | .833 |
| Dyslipidaemia | 44 (33.6) | 6 (33.3) | .983 |
| Diabetes mellitus | 11 (8.4) | 1 (5.6) | .678 |
| Smoking | .309 | ||
| Smoker | 18 (13.7) | 1 (5.6) | |
| Non-smoker | 93 (71) | 12 (66.7) | |
| Ex-smoker | 20 (15.3) | 5 (27.8) | |
| Symptoms at diagnosis | .678 | ||
| No | 88 (67.2) | 11 (61.1) | |
| Self-palpation | 37 (28.2) | 7 (38.9) | |
| Telorrhoea | 5 (3.8) | 0 (0) | |
| NAC involvement | 1 (.8) | 0 (0) | |
Ca.: Cancer; FH: Family history; IQR: Interquartile Range; NAC: Nipple-areola complex; PH: Personal history.
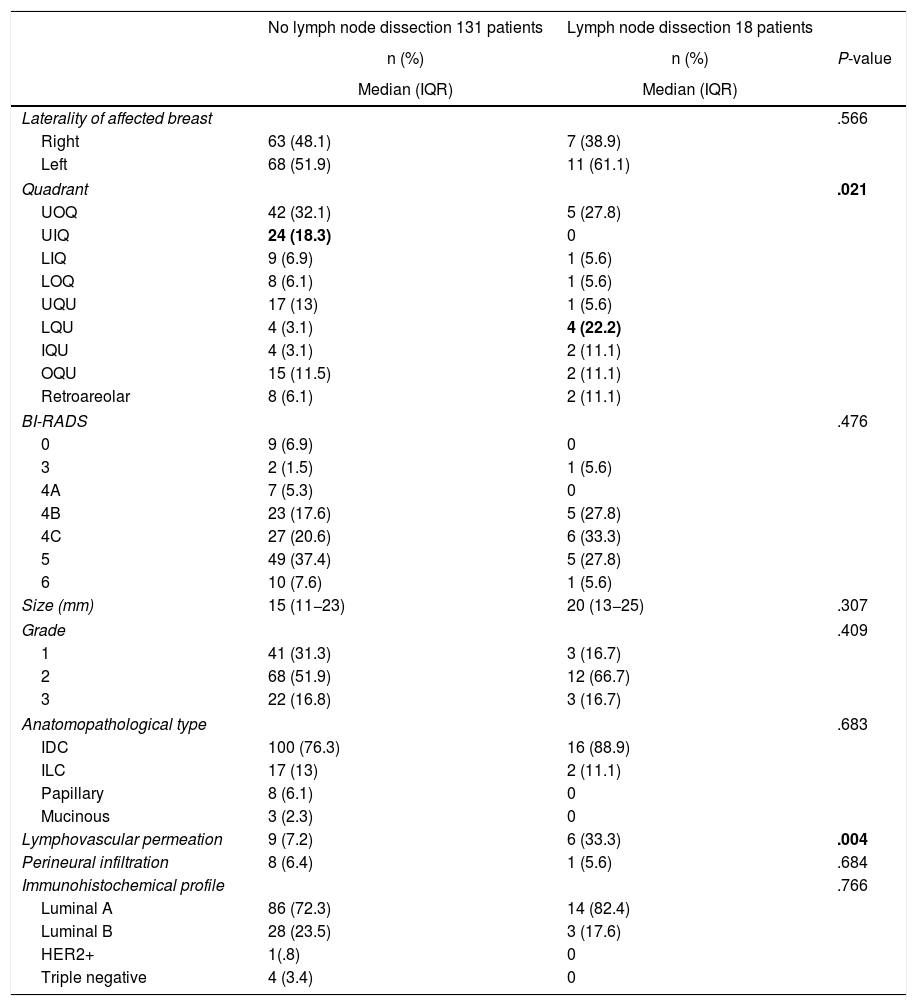
Regarding baseline breast lesion characteristics, no differences were found in BI-RADS or lesion size. Regarding the quadrant location of the lesion, there was a higher number of lymph node dissections in the patients with a lesion located in the lower quadrants union (3% vs. 22%; P = .021), with statistically significant differences. In relation to the anatomopathological characteristics of the preoperative biopsy, no statistically significant differences were found in terms of carcinoma type or immunohistochemical characteristics, although the presence of lymphovascular permeation was statistically significant in patients who underwent lymph node dissection (7% vs. 33%; P = .004) (Table 2).
Baseline characteristics of the breast lesion.
| No lymph node dissection 131 patients | Lymph node dissection 18 patients | ||
|---|---|---|---|
| n (%) | n (%) | P-value | |
| Median (IQR) | Median (IQR) | ||
| Laterality of affected breast | .566 | ||
| Right | 63 (48.1) | 7 (38.9) | |
| Left | 68 (51.9) | 11 (61.1) | |
| Quadrant | .021 | ||
| UOQ | 42 (32.1) | 5 (27.8) | |
| UIQ | 24 (18.3) | 0 | |
| LIQ | 9 (6.9) | 1 (5.6) | |
| LOQ | 8 (6.1) | 1 (5.6) | |
| UQU | 17 (13) | 1 (5.6) | |
| LQU | 4 (3.1) | 4 (22.2) | |
| IQU | 4 (3.1) | 2 (11.1) | |
| OQU | 15 (11.5) | 2 (11.1) | |
| Retroareolar | 8 (6.1) | 2 (11.1) | |
| BI-RADS | .476 | ||
| 0 | 9 (6.9) | 0 | |
| 3 | 2 (1.5) | 1 (5.6) | |
| 4A | 7 (5.3) | 0 | |
| 4B | 23 (17.6) | 5 (27.8) | |
| 4C | 27 (20.6) | 6 (33.3) | |
| 5 | 49 (37.4) | 5 (27.8) | |
| 6 | 10 (7.6) | 1 (5.6) | |
| Size (mm) | 15 (11−23) | 20 (13−25) | .307 |
| Grade | .409 | ||
| 1 | 41 (31.3) | 3 (16.7) | |
| 2 | 68 (51.9) | 12 (66.7) | |
| 3 | 22 (16.8) | 3 (16.7) | |
| Anatomopathological type | .683 | ||
| IDC | 100 (76.3) | 16 (88.9) | |
| ILC | 17 (13) | 2 (11.1) | |
| Papillary | 8 (6.1) | 0 | |
| Mucinous | 3 (2.3) | 0 | |
| Lymphovascular permeation | 9 (7.2) | 6 (33.3) | .004 |
| Perineural infiltration | 8 (6.4) | 1 (5.6) | .684 |
| Immunohistochemical profile | .766 | ||
| Luminal A | 86 (72.3) | 14 (82.4) | |
| Luminal B | 28 (23.5) | 3 (17.6) | |
| HER2+ | 1(.8) | 0 | |
| Triple negative | 4 (3.4) | 0 | |
IDC: Invasive ductal carcinoma; ILC: Invasive lobular carcinoma; IQU: Inner quadrants union; LIQ: Lower inner quadrant; LOQ: Lower outer quadrant; LQU: Lower quadrants union; OQU: Outer quadrants union; UIQ: Upper inner quadrant; UOQ: Upper outer quadrant; UQU: Upper quadrants union.
In bold: statistically significant values.
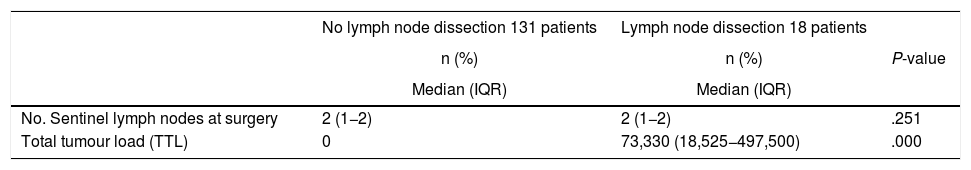
The surgical data are shown in Table 3. No statistically significant differences were found between groups in the number of sentinel lymph nodes located intraoperatively during the surgery (median: 2 [IQR: 1−2]). Sentinel node analysis was performed intraoperatively by OSNA in all operated patients, with a higher TTL in the ALND group with 73,330 copies (IQR: 18,525–497,500) versus a median of 0 in the group that did not require lymph node dissection (P = .000).
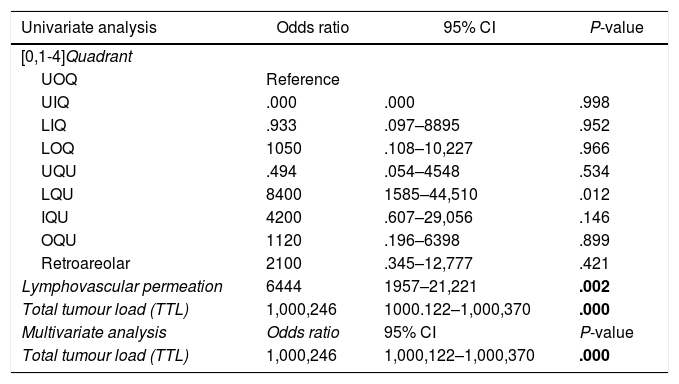
A univariate analysis was performed with the variables that showed significant differences between the groups: quadrant location of the lesion, lymphovascular permeation, and total tumour load (Table 4). Once these three variables were entered into the multivariate analysis, only total tumour load proved to be a predictor of the need for lymph node dissection (odds ratio: 1000246; 95% confidence interval [CI]: 1,000,122–1,000,370; P = .000).
Univariate analysis and multivariate analysis.
| Univariate analysis | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| [0,1-4]Quadrant | |||
| UOQ | Reference | ||
| UIQ | .000 | .000 | .998 |
| LIQ | .933 | .097–8895 | .952 |
| LOQ | 1050 | .108–10,227 | .966 |
| UQU | .494 | .054–4548 | .534 |
| LQU | 8400 | 1585–44,510 | .012 |
| IQU | 4200 | .607–29,056 | .146 |
| OQU | 1120 | .196–6398 | .899 |
| Retroareolar | 2100 | .345–12,777 | .421 |
| Lymphovascular permeation | 6444 | 1957–21,221 | .002 |
| Total tumour load (TTL) | 1,000,246 | 1000.122–1,000,370 | .000 |
| Multivariate analysis | Odds ratio | 95% CI | P-value |
| Total tumour load (TTL) | 1,000,246 | 1,000,122–1,000,370 | .000 |
IQU: Inner quadrants union; LIQ: Lower inner quadrant; LOQ: Lower outer quadrant; LQU: Lower quadrants union; OQU: Outer quadrants union; UIQ: Upper inner quadrant; UOQ: Upper outer quadrant; UQU: Upper quadrants union.
Bold signifies statistically significant value.
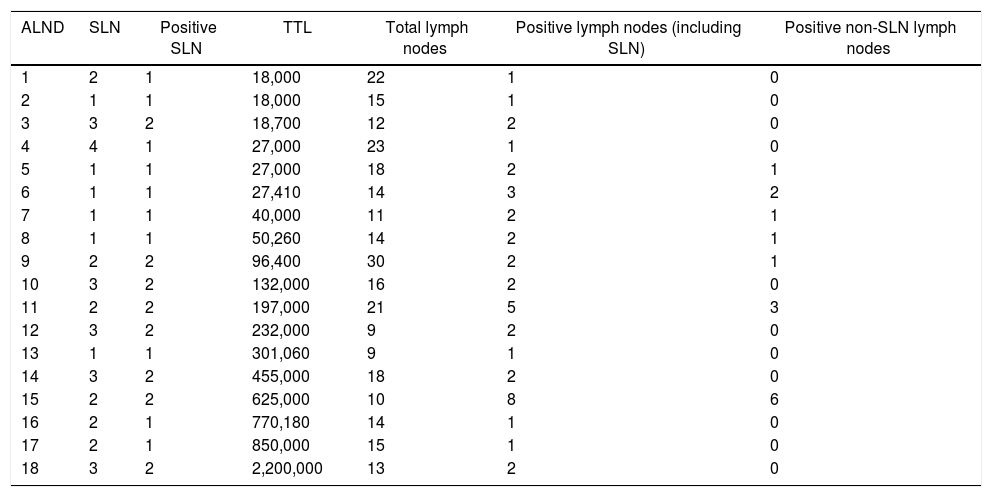
Of the 18 ALNDs performed, the median number of lymph nodes removed was 1511–18. The mean number of positive lymph nodes was 2 (1−2), while the median number of positive non-sentinel lymph nodes was 0 (0–1). In 12 of the 18 lymph node dissections (67%), no positive nodes other than SLN were found. Neither TTL nor the number of copies per positive SLN appeared to correlate with the total number of positive lymph nodes at lymph node dissection (Table 5).
Analysis of lymph node dissections performed.
| ALND | SLN | Positive SLN | TTL | Total lymph nodes | Positive lymph nodes (including SLN) | Positive non-SLN lymph nodes |
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 18,000 | 22 | 1 | 0 |
| 2 | 1 | 1 | 18,000 | 15 | 1 | 0 |
| 3 | 3 | 2 | 18,700 | 12 | 2 | 0 |
| 4 | 4 | 1 | 27,000 | 23 | 1 | 0 |
| 5 | 1 | 1 | 27,000 | 18 | 2 | 1 |
| 6 | 1 | 1 | 27,410 | 14 | 3 | 2 |
| 7 | 1 | 1 | 40,000 | 11 | 2 | 1 |
| 8 | 1 | 1 | 50,260 | 14 | 2 | 1 |
| 9 | 2 | 2 | 96,400 | 30 | 2 | 1 |
| 10 | 3 | 2 | 132,000 | 16 | 2 | 0 |
| 11 | 2 | 2 | 197,000 | 21 | 5 | 3 |
| 12 | 3 | 2 | 232,000 | 9 | 2 | 0 |
| 13 | 1 | 1 | 301,060 | 9 | 1 | 0 |
| 14 | 3 | 2 | 455,000 | 18 | 2 | 0 |
| 15 | 2 | 2 | 625,000 | 10 | 8 | 6 |
| 16 | 2 | 1 | 770,180 | 14 | 1 | 0 |
| 17 | 2 | 1 | 850,000 | 15 | 1 | 0 |
| 18 | 3 | 2 | 2,200,000 | 13 | 2 | 0 |
ALND: Axillary lymph node dissection; SLN: Sentinel lymph node; TTL: Total tumour load.
Breast cancer can now be diagnosed early thanks to widespread mammography screening, which has reduced the incidence of extensive axillary disease13. Since the beginning of SLNB, the percentage of SLN-positive patients has decreased from the 35.5% reported by Veronesi et al. in 20034 to 23.3% in Gupta's study in 202014.
However, the SLN is the only node involved in 40%–60% of patients with clinically negative axilla15. Again, Giuliano et al. were the first to establish that SLNB in SLN-positive patients was non-inferior to ALND. The ACOSOG Z0011 study showed that there was no difference in terms of locoregional recurrence, DFS, and OS between patients with ALND and patients with removal of up to three axillary lymph nodes7,8. Galimberti et al. corroborated these results with a new randomised, multicentre, non-inferiority study, which established that SLNB was an alternative to ALND in patients with early-diagnosed breast cancer, low tumour load, and involved SLN9. Radiotherapy also plays a role in the control of axillary disease thanks to the results of the AMAROS study16. This study showed that in patients with small tumours (T1-T2), clinically negative axilla, and positive SLNB, axillary radiotherapy after SLNB had disease control comparable to ALND, with a lower incidence of lymphoedema. These advances form the basis of current clinical practice guidelines1–3 and have led to a decrease in ALND and its associated morbidity17.
Thus, ALND has increasingly fewer indications. In our study, ALND was only performed in 12% of the sample (18 of 149 patients). If we were to apply the criteria established by Giuliano7, this percentage would be even lower, as in only 6 of the 18 cases were three or more SLNs found during surgery. It would be this 4% of the sample that would really benefit from intraoperative waiting for the SLN result, which corresponds to a number needed to treat (NNT) of 25. It is important to bear in mind that with technical advances and current knowledge, the lymph node status is increasingly less of a determinant for adjuvant systemic therapies. Biological information such as oestrogen, progesterone, and HER2 receptors as well as the Ki67 proliferation index is increasingly influencing decision making. Neither must we forget the significant morbidity and emotional impact of complete axillary dissection in breast cancer patients18.
In our institution, obtaining the intraoperative SLN result involves a wait of approximately 40 min, during which time the patient is kept under general anaesthesia. The time required to review the lumpectomy specimen by radiological and anatomopathological study is usually much shorter, about 15 min, and therefore the wait usually depends on the SLN result. In our study of 149 patients who underwent surgery, 131 intraoperative SLN studies by OSNA were performed, which did not lead to a subsequent ALND. Optimal use of a surgical day is considered to be 80%, corresponding to five and a half hours in a typical seven-hour day. The performance of 131 intraoperative OSNA SLN studies corresponds to approximately 87 h of operating theatre occupancy while awaiting results, which corresponds to approximately 15 operating days.
Therefore, we consider it more useful to obtain the result on a deferred basis. Eliminating the time spent waiting for intraoperative SN results reduces the time that patients are under general anaesthesia after which, in most cases, no additional procedure is performed. In addition, it allows the use of this time to schedule a larger number of patients per working day. Moreover, it allows the case to be discussed in a multidisciplinary committee to make a joint decision on the individual need to perform an ALND at a later stage, reducing the number of unnecessary ALNDs and the overall morbidity of the patients.
In relation to the secondary endpoint of the study, only the total tumour load was shown to be an independent predictor of the need for ALND after SLNB. This is consistent with the results published by Peg et al. in 201311. This team established that TTL by OSNA was a useful and standardised tool for predicting axillary node status, regardless of the number of SLNs present. It also established a cut-off point of 15,000 copies, above which the number of involved axillary lymph nodes increased by a significant percentage, and it was therefore advisable to perform an ALND.
One of the strengths of this study is that no patients were lost to follow-up. Because the SLN was assessed intraoperatively and the decision to perform ALND was made at the same time, a possible attrition bias was avoided.
Among the limitations of the study are that the results are not applicable to patients who have received neoadjuvant treatment, as in these cases any TTL is an indication for ALND. In addition, no other variables were found that could serve as a predictive model for ALND beyond TTL. Another limitation found in our study is that it analyses a short period of time and not the medium and long-term course of the patients. This project could be the starting point for new studies that analyse our results in the medium and long term, and thus a project for continuous improvement in the quality of our service.
ConclusionThe indication for ALND in early-diagnosis breast cancer is decreasing every day. Given the small number of patients requiring ALND after SLNB, we consider it more useful to defer SLN analysis, which reduces the patient’s time under anaesthesia and optimises operating theatre occupation time. In our study, TTL was the only predictor of the need for ALND after SLNB.
FundingNo funding was received for this work.
Conflict of interestsThe authors have no conflict of interests to declare.