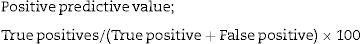Helicobacter pylori infection is usually acquired in early childhood and it can persist throughout life without antibiotic treatment. This study aimed to compare the accuracy of the noninvasive H. pylori Stool Antigen Test-applied on the stool samples with the invasive gold standart Rapid Urease Test-applied on the gastric biopy samples of patients with upper gastrointestinal complaints. After endoscopy, biopsy and stool specimens were taken in 122 patients. The infection was detected with rapid urease test which is accepted as gold standart test. Rapid, one-step H. pylori card test was applied to all patients stool specimens. In this study 106 of the 122 patients (86.8%) were positive for H. pylori infection, while 16 of the 122 patients (13.2%) were negative. H. pylori card test was negative in 13 of the 16 patients and was positive in 98 of the 106. The sensitivity, specifity, positive and negative predictive values were 92.45%, 81.25%, 97.02%, and 61.90%, respectively. H. pylori card test is rapid, easy, noninvasive and inexpensive methods for detection H. pylori infection. This test showed high sensitivity and specificity. Additionally, it may be a good alternative to invasive tests for the detection of H. pylori infections especially in children.
Helicobacter pylori (H. pylori) is classified as a gram-negative, spiral-shaped bacterium and a microaerophilic, fastidious, human pathogen. H. pylori infection is usually acquired in early childhood and it can persist throughout life without antibiotic treatment. It affects about 20% of the population in developed countries and more than 90% in the developing world.1–4 Oral–oral and fecal–oral are the most common methods of transmission.5
H. pylori specifically colonizes on the gastic mucus layer, and it has developed a variety of mechanisms to survive in the harsh acidic environment of the gastric mucosa.6H. pylori contains many virulence factors that cause the infection and contributes to gastric inflammation.7 It is a major cause of gastric and duodenal ulcer and gastritis, and the organism has been etiologically associated with Mucosal-Associated Lymphoid Tissue (MALT) lymphoma and gastric carcinoma.8,9
Invasive and non-invasive tests are used in the diagnosis of H. pylori infection. The invasive methods include culture, histology, and urease tests. Biopsy specimens obtained with upper gastrointestinal endoscopy are necessary for these tests.10–12 The noninvasive methods include stool antigen test (SAT), urea breath test and serology.13
All the tests have advantages and disadvantages. The rapid urease test (RUT) is a gold standard method for the detection of H. pylori, and it is faster and cheaper than other invasive tests.14,15 Proton pump inhibitors (PPIs), bismuth-containing compounds and antimicrobial agents may affect the performance of this test by inhibiting urease activity. In addition, other urease-producing microorganisms in the gastric mucosa can cause false positive results.12,16 SATs are non-invasive and inexpensive methods to detect active H. pylori infection. This test has two versions: enzyme immunoassay and immunochromatography. Eradication of H. pylori infection is evaluated by SATs. Therefore this test is useful before and after H. pylori therapy.2,16,17
This study aimed to compare the accuracy of the noninvasive H. pylori Stool Antigen Test (SAT) applied on the stool samples with the invasive gold standart Rapid Urease Test (RUT) applied on the gastric biopy samples of patients with upper gastrointestinal complaints.
Materials and methodsPatient selection and collection of samplesThis study was approved by the Local Ethics Committee of Ataturk University, Institute of Health Science with the number of 1466. The subjects were selected from patients with upper gastrointestinal complaints admitted to the Ataturk University, Medical Faculty and Department of Gastroenterology. Of those referred to the endoscopy unit for gastrointestinal endoscopy to evaluate dyspeptic complaints, 122 (49 male, 73 female) were included in this study. Patients taking bismuth preparations, PPIs, H2 receptor antagonists or antibiotics for the last month or taking anti-acids for the last two days were excluded from the study. The first stool samples of all patients were collected immediately after the endoscopy.
Detection of H. pylori in biopsy samplesThe RUT (Salubris Helicheck, Boston, USA) was used for the detection of H. pylori on biopsy samples in this study. H. pylori produce an abundance of urease. The urease enzyme hydrolyses urea to release CO2 and NH3. The release of ammonia increases the pH of the medium. The urease activity causes a change in the pH indicator color for positive H. pylori results. All the biopsy specimens were taken from the patients during endoscopy and the RUTs were performed by the clinicians according to the manufacturer's protocol.2,18
Investigation of H. pylori antigens in stool samplesThe rapid, one-step H. pylori card test (H+R H. pylori CARD, Madrid, Spain) was used to investigate the presence of H. pylori antigens in the stool samples. This test is a qualitative immunochromatographic assay for the determination of H. pylori in stool samples. The membrane is precoated with monoclonal antibodies, on the test band region, against H. pylori antigens. During testing, the sample is allowed to react with the colored conjugate (anti-H. pylori monoclonal antibodies-red polystyrene microspheres) predried on the test strip. The mixture then moves up the membrane by capillary action. As the sample flows through the test membrane, the coloured particles migrate. For a positive result, the specific antibodies present on the membrane will capture the colored conjugate. The mixture continues to move across the membrane to the immobilized antibody placed in the control band region, where a red band always appears. The presence of this red band serves as; an internal control for the reagents and verification that sufficient volume was added and proper flow was obtained.
The stool samples were evaluated by the card test according to the manufacturer's protocol. A single red band appearing across the central window in the site marked with the control line was considered negative. A red band appearing in the site marked with the result line and in the site marked with the control line was considered positive. A total absence of the control band, regardless of the appearance of the result site was considered invalid.
Statistical analysisThe statistical analysis was performed using SPSS for Windows Version 17.0 (Statistical Package for Social Sciences version 17.0). Positive predictive value, negative predictive value, sensitivity and specificity were evaluated with the following formulas.
ResultsThe mean age of the 122 patients’ was 45.02±15.134 years. The male patients’ ranged from 18 to 84 years old with a mean age of 44.41±16.075 years. The female patients’ ranged from 18 to 80 years old with a mean age of 45.42±14.568 years.
The RUT detected H. pylori in 63 of the 73 female patients (59.4%) and 43 of the 49 male patients (40.6%) with upper gastrointestinal complaints. The RUT-positive patients with upper gastrointestinal complaints had a mean age of 45.21±15.641 years and the RUT-negative patients with upper gastrointestinal complaints had a mean age of 43.75±11.538 years. The relationship between patients with upper gastrointestinal complaints and their ages are shown in Fig. 1. The upper gastrointestinal complaints are most seen in 28–37 and 48–57 age ranges. It means that middle-age patients are under risk of upper gastrointestinal diseases.
Of the subjects, 106 (86.8%) were diagnosed with gastritis and 16 (13.2%) were diagnosed with ulcer by endoscopic biopsy. For the 106 RUT-positive patients, 91 (85.8%) were diagnosed with gastritis and 15 (14.2%) were diagnosed with ulcer. For the gastritis patients diagnosed by endoscopy, 91 (85.8%) were H. pylori positive according to the RUT. The RUT also tested positive for 15 (93.75%) of the patients with ulcers diagnosed by endoscopy (Table 1).
H. pylori infection was positive in 106 patients according to the RUT. Among these patients, 98 (93.75%) were positive for H. pylori according to the immunochromatographic assay (rapid one-step H. pylori card test). In addition, 3 (18.75%) of the RUT-negative patients were positive according to this method (false positive). In regards to the endoscopy diagnosis, 85 of the 91 (93.4%) H. pylori-positive gastritis patients and 13 of the 15 (86.6%) H. pylori-positive ulcer patients were positive according to the immunochromatographic assay.
In addition, 12 of the 15 (80%) H. pylori-negative gastritis patients and the single H. pylori-negative ulcer patients were negative according to the card test (Table 2).
The H. pylori card test and RUT results were compared and positive predictive value, negative predictive value, sensitivity and specificity were evaluated for the card test (Table 3).
Evaluation of H. pylori card test with rapid urease test.
| H. pylori Card Test | Rapid urease test | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 98 | 3 | 101 |
| Negative | 8 | 13 | 21 |
| Total | 106 | 16 | 122 |
Sensitivity; 98/(98+8)×100=92.45%
Specificity; 13/(3+13)×100=81.25%
Positive predictive value; 98/(98+3)×100=97.02%
Negative predictive value; 13/(8+13)×100=61.90%
False positive rate; 1−0.812 = 0.188=18.8%
False negative rate; 1−0.924 = 0.076=7%.
Approximately half of the world's population is infected with H. pylori. H. pylori infection is especially a public-health problem in developing countries. It is the leading cause of various upper gastroduodenal tract diseases such as gastric and duodenal ulcer, gastritis, MALT and gastric carcinoma.9,13,15H. pylori infection can be detected by invasive and noninvasive methods. Endoscopy is necessary for the invasive tests. All these methods have advantages and disadvantages.14,16
The RUT is an invasive method and considered a gold standard. This test is inexpensive and allows for the rapid detection of H. pylori. The test may result in false negative if the patient recently used antimicrobial agents, PPIs or bismuth-containing compounds or a heterogeneous distribution of bacteria is present in the gastric mucus layer. In addition, contamination of the biopsy with saliva or other urease producing microorganisms in the gastric mucosa can cause false positive results. This test's sensitivity, specificity, and positive predictive values are more than >98%, 99%, and 99%, respectively.12,15,19 The RUT was used as the gold standard method and compared with the immunochromatographic assay (H. pylori card test) in this study.
The study examined 122 patients with upper gastrointestinal complaints especially ulcer and gastritis. The patients’ biopsy specimens and stool samples were examined with the RUT and one-step H. pylori card test, respectively. Of these patients, 106 (86.8%) were positive for H. pylori infection; while 16 (13.2%) were negative. The sensitivity, specificity, positive predictive value and negative predictive value of the H. pylori card test were 92.45%, 81.25%, 97.02%, and 61.90%, respectively. In current literature, the sensitivity and specifity of the card test were 87.8%, 93.8% and 75–100%, respectively.11,20–25 These results are similar to our study and showed that performance of the card test was excellent for the noninvasive detection of H. pylori. In addition, previous studies have shown that the H. pylori card test exhibits good performance before and after eradication therapy.22,26
In our study, 3 of the 16 (18.75%) RUT-negative patients were found positive according to the H. pylori card test (false positive). A cross-reaction with an antigen from Helicobacter species or other microorganism of the intestinal flora may cause false positive results.27,28 On the other hand, the irregular distribution of bacteria in the gastric mucosa can cause suspect false negative results with the RUT.19
Among the 106 RUT-positive patients, 8 (7.25%) were considered as negative (false negative) according to the immunochromatographic assay of the stool samples. Card test characteristics or the application of the test may have caused false negative results. Additionally, contamination of the biopsy with saliva or other urease producing microorganisms in the gastric mucus layer can cause false positive results with the RUT.12,19
Infections caused by H. pylori usually affect adults. In many previous studies, patient's age ranged from 23 to 89 years.20,29,30 In the current study, patient's age ranged from 18 to 84 years. No children were included; however, previous studies have shown that the rapid one-step immunochromatographic card test performs well for the detection of H. pylori infection in children's stool samples.26,30
ConclusionsIn conclusion, the H. pylori card test is rapid, easy, noninvasive and inexpensive method for the detection of H. pylori infection. This test showed high sensitivity and specificity. No significant difference was found in sensitivity or specificity between the RUT (the gold standard method) and the one-step H. pylori card test. On the other hand, H. pylori in the stool is not a diagnosis of ulcer and/or gastritis. So, invasive examination should also be done to confirm the diagnosis. However, this test can be used in some insufficient conditions such as if endoscopy is not available. Additionally, the H. pylori card test has exhibited good performance before and after eradication therapy and it is a good alternative to invasive tests for the detection of H. pylori infection in children.
Conflicts of interestThe authors declare no conflicts of interest.