In the last 40 years, several scientific and technological advances in microbiology of the fermentation have greatly contributed to evolution of the ethanol industry in Brazil. These contributions have increased our view and comprehension about fermentations in the first and, more recently, second-generation ethanol. Nowadays, new technologies are available to produce ethanol from sugarcane, corn and other feedstocks, reducing the off-season period. Better control of fermentation conditions can reduce the stress conditions for yeast cells and contamination by bacteria and wild yeasts. There are great research opportunities in production processes of the first-generation ethanol regarding high-value added products, cost reduction and selection of new industrial yeast strains that are more robust and customized for each distillery. New technologies have also focused on the reduction of vinasse volumes by increasing the ethanol concentrations in wine during fermentation. Moreover, conversion of sugarcane biomass into fermentable sugars for second-generation ethanol production is a promising alternative to meet future demands of biofuel production in the country. However, building a bridge between science and industry requires investments in research, development and transfer of new technologies to the industry as well as specialized personnel to deal with new technological challenges.
The automobile was one of the most impressive inventions that changed the life style of humanity. The first cars were designed to run on ethanol. However, due to the discovery of new oil fields, its abundance and low prices at the beginning of century 20, engines were being modified, giving preference to petroleum based-fuels. Then, gasoline became the primary fuel option for cars worldwide. Nevertheless, because of 1970's oil crisis, the Brazilian government launched the “Proalcool” program in 1975 with the aim to reduce the country's dependence on oil imports.1 In the first phase of the program, ethanol was added to gasoline. After the second oil crisis in 1979, the automobile industry started to produce the first car to run on ethanol only for the Brazilian market.2
In 1980, production of light vehicles that ran on ethanol reached 95% of all fleet produced in Brazil. It increased ethanol consumption and reduced significantly oil dependence. However, a combination of factors involving oil price drops, reduction of subsidies to producers and rise of sugar prices contributed to fuel shortage that led to a major downturn in the demand for ethanol-run cars.2
At the beginning of 21st century, the use of ethanol as fuel was resumed chiefly motivated by high oil prices in the international market and the development of flex-fuel technology.1 Flex-fuel cars can run either on 100% hydrous ethanol or on different blends of ethanol and gasoline. Electronic sensors detect the fuel blending and automatically adjust the engine combustion. Furthermore, the use of ethanol blends replaced toxic additives in gasoline, such as tetraethyl lead and oil-derived benzene. Leaded gasoline was banned in several countries due to serious contamination problems to the environment and humans.3 Moreover, the use of ethanol blends has improved air quality in large urban centers, reducing emissions of carbon monoxide from 50g/km driven to less than 5.8g/km driven.4 Nowadays, gasoline contains a blending of 27% of anhydrous ethanol.
The flex-fuel car technology allowed the consumer to choose for the most convenient and cheaper fuel. The production and selling of flex fuel cars and light commercial vehicles represented 85.45% of the total in 2015.5 Currently, the use of ethanol as biofuel in Brazil has been the most successful program to replace fossil fuels worldwide. Several countries have started their own programs for production and use of ethanol as fuel to reduce oil dependence and GHG (greenhouse gases) emissions.6–8
Ethanol can be produced either by chemical or microbiological processes. The chemical route is based on ethylene hydration while the microbiological process is chiefly carried out by yeast Saccharomyces cerevisiae, although other microorganisms may also produce ethanol.9–11
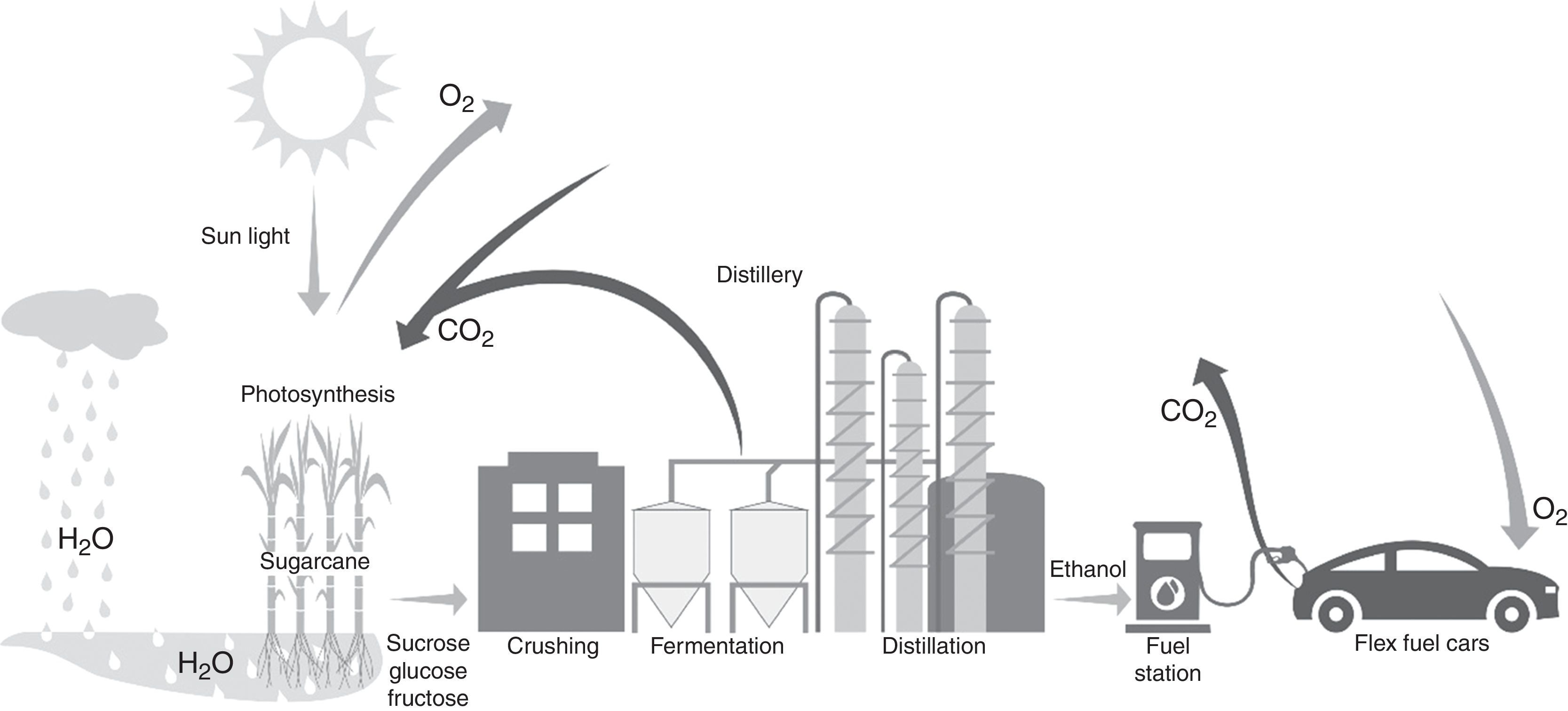
Currently, the main industrial route used for ethanol production worldwide is the microbiological process, also referred as alcoholic or ethanolic fermentation.12–15 During this process, sugars are converted into ethanol, energy, cellular biomass, CO2 and other byproducts by yeast cells. These sugars may come from different feedstocks and crop wastes.16,17 In Brazil, the main feedstock is sugarcane while the United States of America (USA) produces ethanol from corn.16,18 Sugarcane, corn and sorghum are C4 plants with high efficiency to convert atmospheric CO2 and water into sugars and polymers such as starch, cellulose and hemicellulose through photosynthesis. This process uses sun light energy to fix carbon and release oxygen into the air.19 Then, all CO2 resulting from ethanol burning is recycled through photosynthesis (Fig. 1).
Through photosynthesis, sugarcane converts carbon dioxide (CO2), water (H2O) and energy from light into sugars and polysaccharides releasing oxygen (O2) into atmosphere. After harvesting and crushing, sugars are fermented by yeasts and converted into ethanol. It is separated by the distillation and used as biofuel by cars. The burning of ethanol generates CO2 that returns to the atmosphere closing the cycle.
Ethanol produced from sugarcane has reduction rates between 40 and 62% in GHG emissions compared to gasoline.20 Readily fermentable sugars such as sucrose, glucose and fructose are directly converted into ethanol during alcoholic fermentation by the yeast S. cerevisiae while starch, cellulose and hemicellulose need to be hydrolyzed to simple sugars to be fermented.21,22 In both cases, yeast cells convert a solid substrate into a liquid fuel. Moreover, to convert sugars into ethanol, yeasts charge only 7% of the energy contained in sugar molecules. The end balance shows that 93% of the energy present in sugars is conserved in ethanol.23 In addition, ethanol is a molecule that can be easily separated from water through distillation process because its boiling point (78°C) is very different from water (100°C). Moreover, ethanol production from sugarcane in Brazilian distilleries presents a highly positive energy balance. In terms of equivalence, for each unit of fossil energy consumed during its production, 9.3 units of renewable energy were produced in 2005, but with potential to reach 11.6 in 2020.6 Moreover, for second-generation ethanol processes, energy balance for ethanol production from cellulosic materials is expected to be better than that of sugarcane or corn.24 All these characteristics turned the Brazilian distilleries a renewable and environmental friendly biofuel industry.25
Several scientific studies in the microbiological field have been crucial for evolution of the ethanol industry in Brazil, namely the improvement and development of new fermentation processes, selection of robust industrial yeast strains, better control of bacterial contaminants, improvement of chemical and microbiological control at distilleries.26–29 Moreover, investments in research on second-generation ethanol have stimulated the participation of some companies to produce ethanol from sugarcane bagasse and vegetal trash. Nowadays, new scientific and technological frontiers are faced by the industry to improve the fermentation process for ethanol production in Brazil.
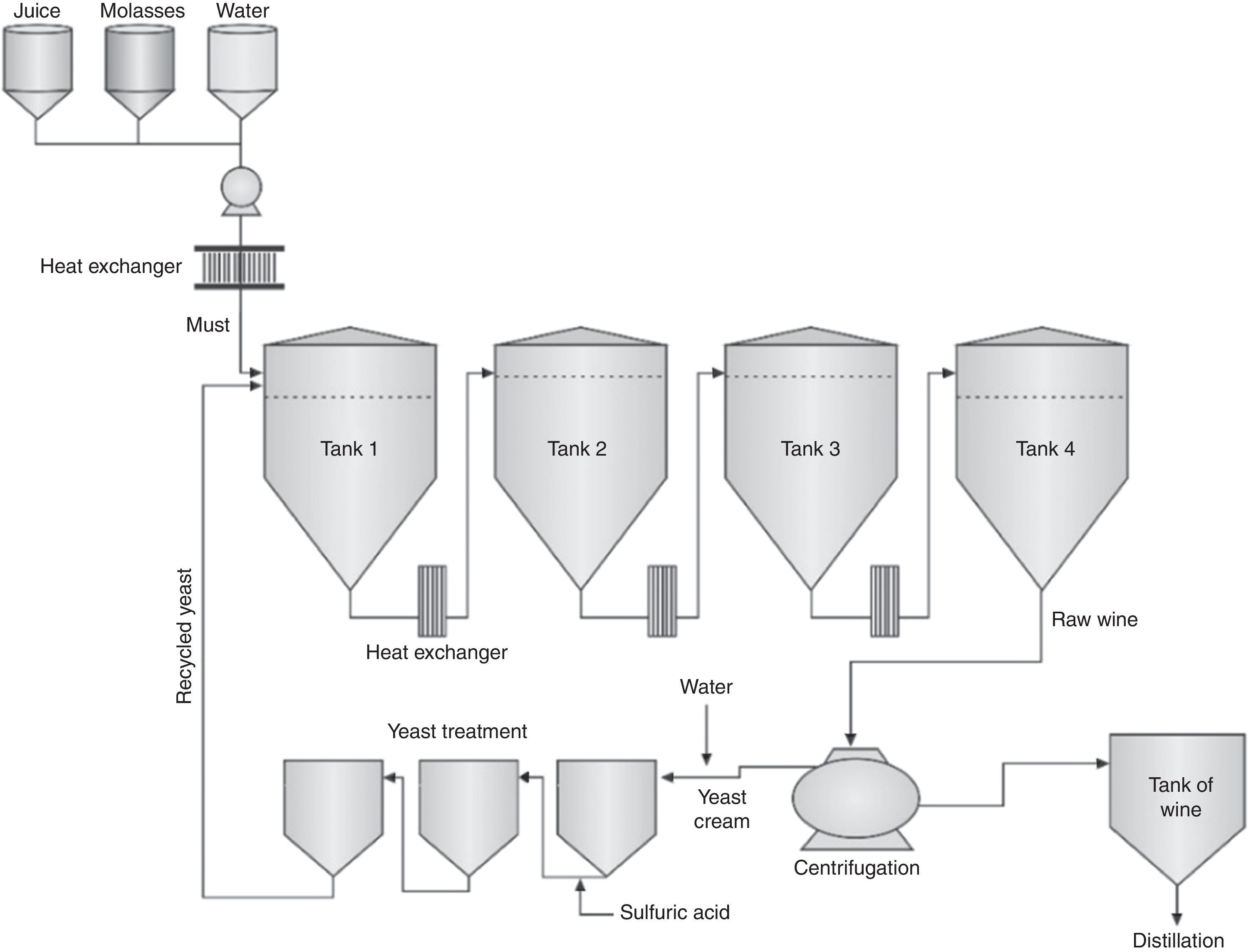
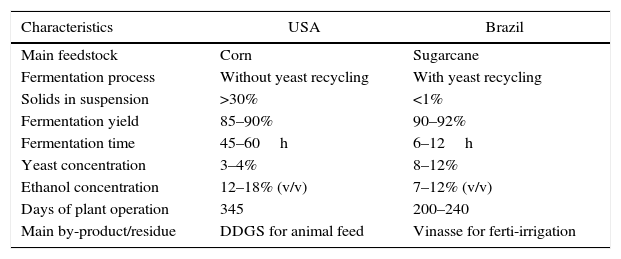
The main alcoholic fermentation processes in the worldNowadays, the USA and Brazil are the world's largest ethanol producers. Together, both countries account for more than 94 billion liters of ethanol produced per year, accounting for around 85% of worldwide production.30 However, there are huge differences in the fermentation processes (Table 1). Besides feedstock, a primary difference is the recycling of yeast cells. Brazilian distilleries use an improved fermentation process patented in 1937 by Firmino Boinot from the Melle region, France.31 After the end of each fermentation, the raw wine is centrifuged to separate the yeast cells in a concentrated cream while the wine goes to distillation. After a treatment with water-diluted sulfuric acid (pH 2.0–2.5 for 1–2h), these yeast cells return to large-volume tanks (250–3000L) for a new fermentation cycle.21,26,27 Distilleries in the USA do not recycle yeast cells due to high concentration of solids and all fermented media (including yeast cells) is distilled.32 Then, yeast cells are not recycled and more sugar is deviated for multiplication of cells rather than to ethanol production. Fermentation yield is lower in processes without recycling of cells.33
Main characteristics of ethanol production in the USA and Brazil.
| Characteristics | USA | Brazil |
|---|---|---|
| Main feedstock | Corn | Sugarcane |
| Fermentation process | Without yeast recycling | With yeast recycling |
| Solids in suspension | >30% | <1% |
| Fermentation yield | 85–90% | 90–92% |
| Fermentation time | 45–60h | 6–12h |
| Yeast concentration | 3–4% | 8–12% |
| Ethanol concentration | 12–18% (v/v) | 7–12% (v/v) |
| Days of plant operation | 345 | 200–240 |
| Main by-product/residue | DDGS for animal feed | Vinasse for ferti-irrigation |
Once the recycling processes start with higher cell concentrations (8–12%, v/v), fermentation times of Brazilian distilleries are faster (6–12h) when compared to fermentations in USA (54–72h) without yeast cell recycling. However, corn fermentations reach higher ethanol concentrations (12–18%, v/v) in comparison to Brazilian processes (7–12%, v/v). Once corn grains can be stored for several weeks, fermentations can be carried out during 345 days a year while Brazilian distilleries run their processes for 200–240 days. Differently from corn grains, sugarcane needs to be crushed soon after harvesting to avoid loss caused by microbial contamination. In addition, Brazilian distilleries are subject to interruptions of sugarcane harvesting, crushing and fermentation due to rains.1,21
The main co-product from corn fermentations is DDGS (distillers dried grains with solubles) used for animal feed and has a high market value due to nutritional characteristics.34 On the other hand, Brazilian distilleries generate huge volumes of potassium-rich vinasse, which is used for sugarcane ferti-irrigation, reducing costs on imports of chemical fertilizers.35
Brazilian distilleries present several differences from one to another in terms of the fermentation process and sugarcane must composition. Distilleries attached to sugar factories usually ferment sugarcane musts of molasses diluted with water or a mix of juice and molasses while autonomous distilleries ferment only juice.36
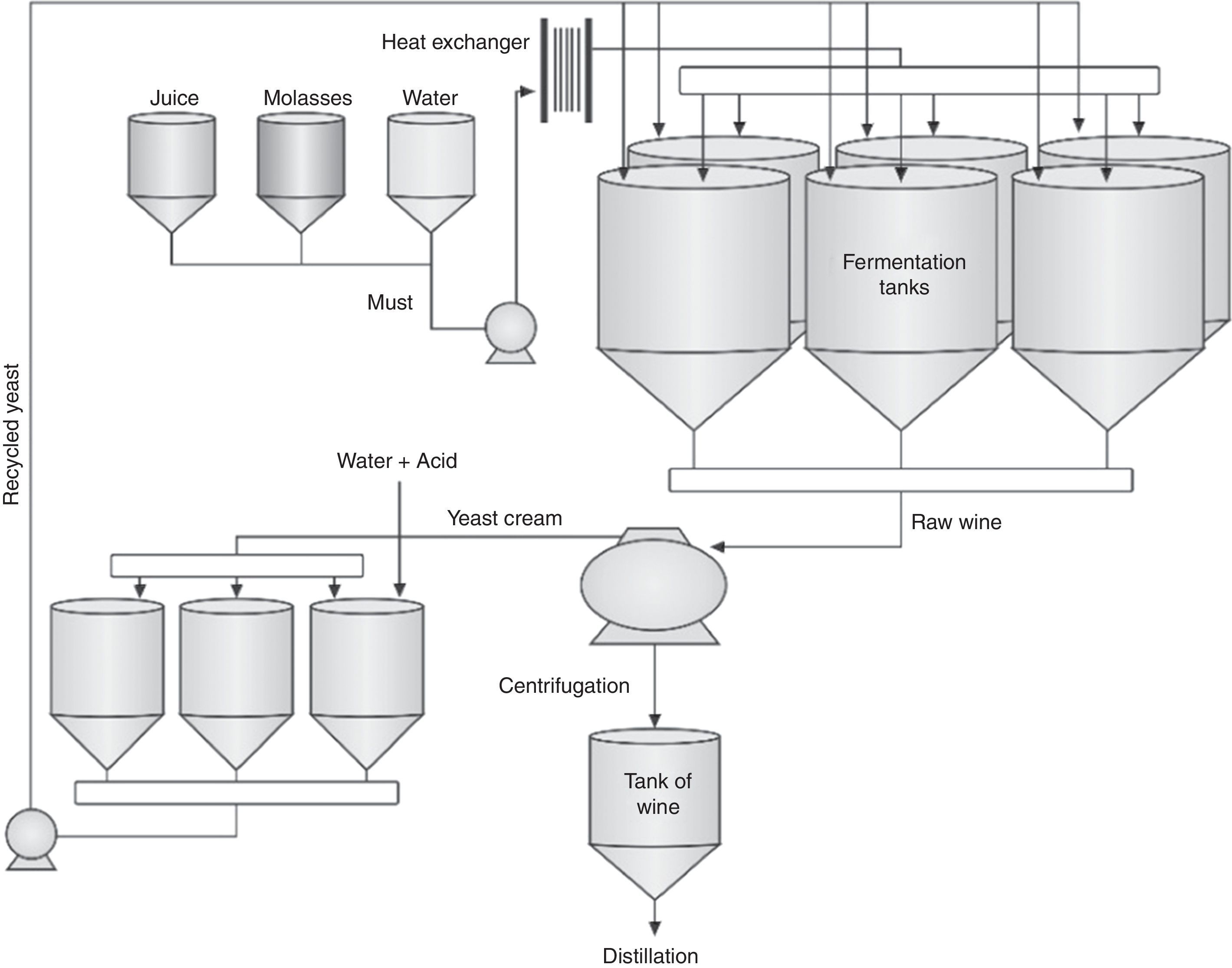
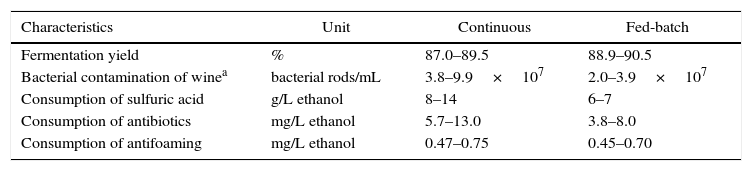
Concerning fermentation, fed-batch processes account for around 83% of the distilleries while continuous fermentations are 17%.37 Despite higher investments for installation, fed-batch processes have presented better results than continuous fermentation (Table 2). A study carried out during eight consecutive years with 62 distilleries (51 fed-batch and 11 continuous fermentation) showed that fed-batch processes reached the highest fermentation yields. In addition, it was observed a lower bacterial contamination in wine and lower consumption of chemicals such as sulfuric acid and antibiotics in comparison to continuous processes.37 However, a misconducted fed-batch process will be worse than a well-managed continuous fermentation. Sometimes, the benefits of a continuous fermentation are masked by improper engineering conception and low cost adaptations.38
Comparison of continuous and fed-batch fermentations with cell recycling for ethanol production in Brazil.37
| Characteristics | Unit | Continuous | Fed-batch |
|---|---|---|---|
| Fermentation yield | % | 87.0–89.5 | 88.9–90.5 |
| Bacterial contamination of winea | bacterial rods/mL | 3.8–9.9×107 | 2.0–3.9×107 |
| Consumption of sulfuric acid | g/L ethanol | 8–14 | 6–7 |
| Consumption of antibiotics | mg/L ethanol | 5.7–13.0 | 3.8–8.0 |
| Consumption of antifoaming | mg/L ethanol | 0.47–0.75 | 0.45–0.70 |
In fed-batch fermentations, each tank is filled, managed and cleaned separately from the other, while in continuous processes all tanks ferment simultaneously in line and cannot be cleaned with the same frequency. After centrifugation, the yeast cream receives an acidic treatment and returns to fermentation tanks (Figs. 2 and 3).
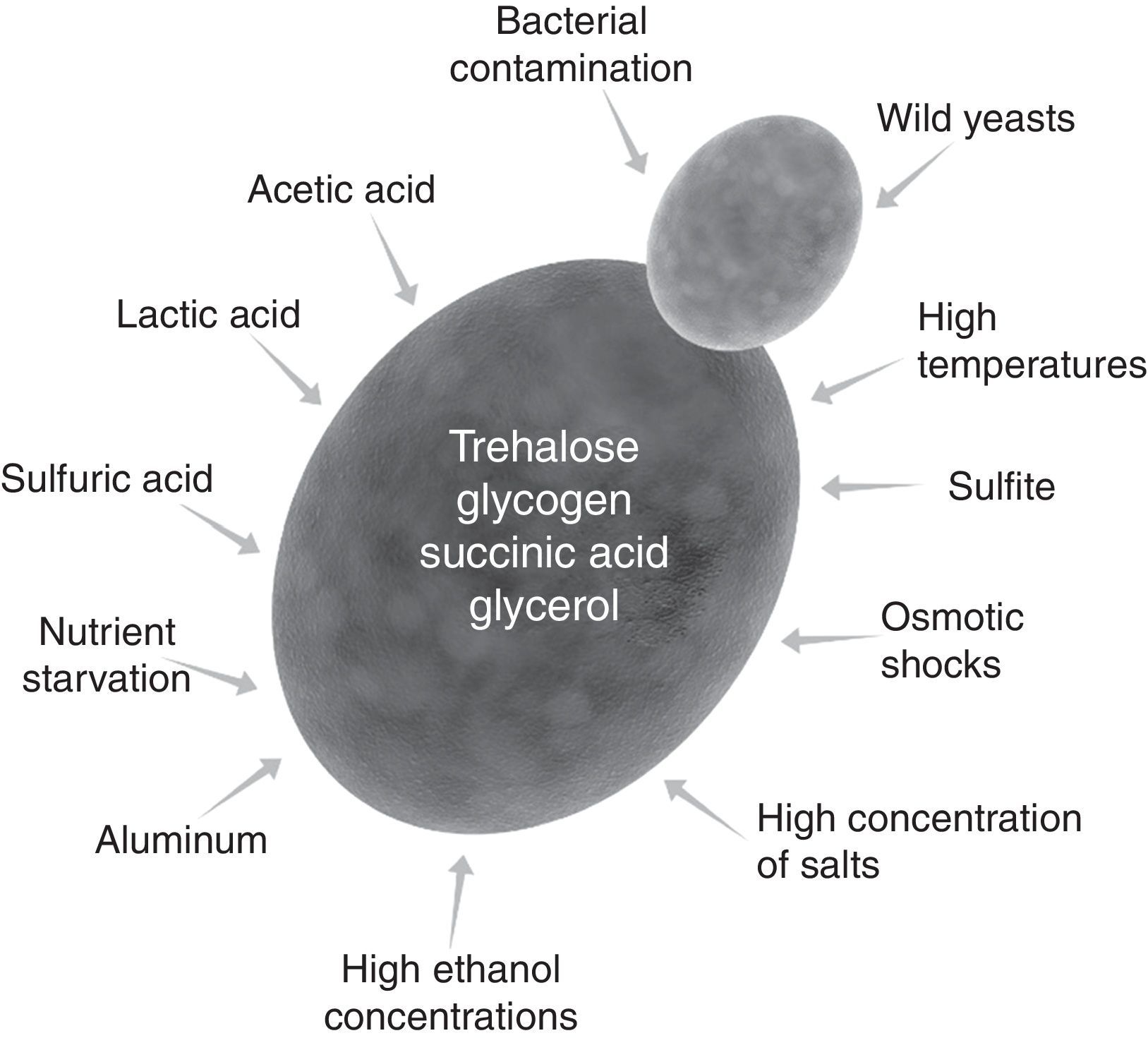
Although Eduard Buchner showed that alcoholic fermentation could be carried out with extract of dead yeast,39 the Melle-Boinot fermentation depends on living cells.31 These cells must be alive at the end of fermentation and after the acidic treatment for reuse in the next cycle. Typical Brazilian distilleries run 400 fermentation cycles during the sugarcane harvesting season. Because they are recycled several times (two cycles a day), yeast cells are subject to stressful conditions of industrial fermentations (Fig. 4). These conditions may change from one distillery to another, as well as, in the same distillery during the sugarcane harvesting season. Moreover, yeast cells are subject to effects of two or more stressing conditions.40,41
However, yeast cells have mechanisms of stress response to industrial conditions of fermentation and acid treatment.42,43 Trehalose and glycogen are reserve carbohydrates for additional energy when cells are starved for sugars.44,45 Moreover, trehalose is a protecting compound to membrane that helps cells to tolerate dehydration and high ethanol concentrations as well as other industrial stressing factors.46,47 Succinic acid in combination with ethanol has an antibacterial effect,48 while glycerol is a regulator for osmotic shocks.49,50
According to the Gay Lussac equation, theoretically, fermentation produces 511g of ethanol and 489g of CO2 from 1000g of glucose. However, in industrial processes this fermentation yield is not achieved because yeast cells drive sugars to the production of cellular biomass and secondary compounds such as glycerol, succinic, malic and acetic acids, fusel oil and other minor by-products. For this reason, the maximum fermentation yield achieved is 92–93%.28 Moreover, fermentation yield is highly dependent on the yeast strain. A fermentation carried out with cell recycles in bench scale showed that baker's yeast had a fermentation yield 3.9% lower than that of industrial strains.26 It seems little, but it means 8.5 million liters of ethanol during 200 working days for an autonomous distillery that crushes 14,000 sugarcane tons daily.
Monitoring and selection of industrial and tailored-yeast strainsBefore 1990, identification and monitoring of industrial strains belonging to S. cerevisiae were often ambiguous and uncertain when performed by cell and colony morphology, physiological and biochemical methodologies.51 Morphology of cells and colonies is quite simple to distinguish strains and different strains can frequently present the same morphology. On the other hand, the same strain can change its cell and colony morphology in response to stress conditions or nutrient starvation and overexpression of genes related to cellular dimorphism like PHD1.50,52 Several strains of Saccharomyces can alternate their multiplication pattern from single cell budding to pseudohyphae, when the buds remain attached to mother cells.53 Physiological and biochemical traits are based on the ability to assimilate and/or ferment different kinds of sugars, the use of nitrogen sources, tolerance to inhibitors, hydrolysis of complex molecules, growth at different temperatures and other conditions that may allow to distinguish different strains from others. However, all these methodologies have shown limitations to identify and monitor yeast populations in practice.
During several years, the dynamic of yeast populations in industrial processes of alcoholic fermentation in Brazil remained unknown and unexplored due to difficulties for strain identification by traditional methodologies based on morphological and physiological traits. While some distilleries claimed that the baker's yeasts, M300A and IZ1904, used as starter strains were very good fermenters, others had an opposite opinion due to low fermentation yields.54 Due to lack of proper methodologies to identify and monitor starter and wild strains, it was not possible to follow changes in population during successive recycles. For this reason, when the initial yeast population was replaced by wild Saccharomyces with good characteristics, the people believed that the strain used as starter was superior. On the other hand, when wild strains with poor fermentation abilities contaminated the process, starter yeasts were appointed as responsible for problems at the distillery. The advent of molecular techniques based on DNA analyses allowed to use differences in genome as molecular markers for strain identification, monitoring and selection.
After 1990, several laboratories have introduced molecular techniques to identify new yeast strains from industrial fermentations worldwide. One methodology was the electrophoretic karyotyping based on chromosome-length polymorphism of yeast cells.55–57 After a pulsed-field gel electrophoresis to separate large DNA molecules, yeast strains show differences in chromosome profile regarding number and size that make each karyotype unique and strain-specific.58–60 This technique had been used to identify and monitor yeast strains in grape fermentations with excellent results.56 In 1990, after a visit to INRA, Montpelier, France, Professor Luiz Carlos Basso (ESALQ-USP) introduced the electrophoretic karyotyping to identify, monitor and select industrial yeast strains based on chromosome polymorphism. Karyotyping showed that the baker's yeast, IZ1904, and laboratory strains do not survive successive fermentation cycles.56 After three or four weeks, these strains are replaced by wild Saccharomyces that contaminate the industrial process of alcoholic fermentation.26 However, several years were necessary to convince people from distilleries that baker's yeast, IZ1904 and laboratory strains do not survive in industrial fermentations with cell recycling and are quickly replaced by wild Saccharomyces.61 In addition, karyotyping has shown that PE2 and CAT1, used as starter strains, were able to replace baker's yeast even at proportions as low as 0.5kg for 12tons of pressed baker's yeast.26 Nowadays, there are only six industrial strains of major importance for ethanol production in Brazil: PE2, CAT1, FT858L and Fermel® (selected by Fermentec), BG1 and SA1 (selected by CTC). These strains have been used by Brazilian distilleries that account for 70% of all ethanol production in the country.
Industrial yeast strains have more complexes and heterogeneous chromosomes than laboratory strains do.62 The high polymorphism is unique for each strain. However, these chromosomes are subject to mitotic and meiotic recombination as well as segregation errors during successive mitoses under stress conditions or after sporulation and conjugation of spores.58,59,63 The introduction of a simplified methodology for the mitochondrial DNA analysis allowed to distinguish variants from starters and contaminating strains.64 The combination of karyotyping and fragment length polymorphism of mitochondrial DNA has proven to be a powerful tool to monitor yeast population diversity in industrial fermentations as well as to select new strains and variants from starter yeasts that become more adapted to conditions of each distillery in particular.61 Variability of mitochondrial DNA does not follow the Mendelian laws of segregation and independent assortment of chromosomes.65 The main differences are related to occasional mutations that are incorporated into DNA molecules of mitochondria. Thus, the combination of karyotyping and mitochondrial DNA analyses has allowed to distinguish strains derived from starter yeasts and contaminants as well as to select robust yeast strains for ethanol production.54
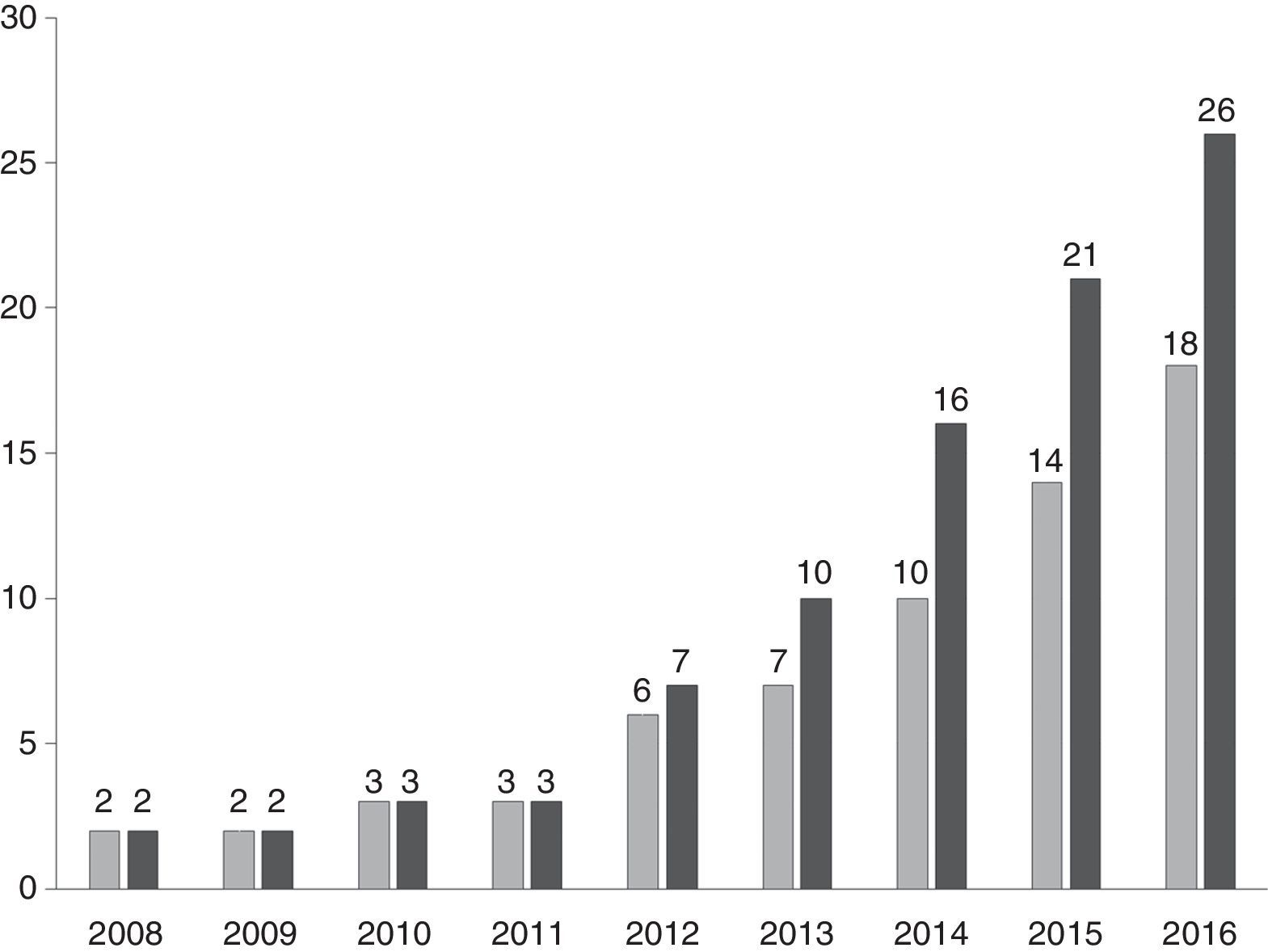
The genome complexity of industrial yeast strains represents a new opportunity to select variants with infinite combinations of genes in different fermentation processes. Two very important contributions on genome sequencing of two industrial yeast strains traditionally used by Brazilian distilleries are PE266 and CAT1.67,68 The yeast strain PE2 showed a high genome plasticity mainly in telomeric and subtelomeric regions of chromosomes. This plasticity could explain why this strain adapts to different fermentation processes.66 CAT1, selected from a continuous fermentation process presented large genomic regions of heterozygosity loss while 58% of the 6652 predicted protein-coding genes were different alleles when compared to reference genome of strain S288c.68 Both strains, PE2 and CAT1, have an increased copy number of genes for biosynthesis of thiamin (B1) and pyridoxine (B6) in comparison to the baker's yeast and laboratory strains.67 An increased copy number can confer better fitness to these strains in vitamin-free fermentation medium with high concentration of sugars. CAT1 presented a short lag phase in comparison to S288c, a laboratory strain, when both yeasts were subjected to high sugar concentration in a medium without vitamins B1 and B6.67 These results are directly related to factors that affect the yeast dominance and persistence in industrial fermentation processes of ethanol production and explain why industrial strains are more robust and tolerant than the baker's yeast and laboratory strains. This means that monitoring yeast populations in industrial fermentations regarding their dominance and persistence characteristics is the basis to select tailored and high-performance strains according to the peculiar characteristics of each distillery. Currently, 18 distilleries use Tailored Yeast Strains® in their process. Some of them have two, three or even four customized strains (Fig. 5). This approach was designed as Process-Driven Selection®.54
However, yeast selection and biodiversity of wild strains still remain a scientific challenge to be explored.22,69 Moreover, it has been proposed that biodiversity of yeasts isolated from different environments can reveal new strains with desired characteristics to industrial applications such as ethanol production.70
Contaminating yeast and bacteriaBecause of large volumes, industrial fermentations are subject to bacterial contamination, such as Saccharomyces and non-Saccharomyces wild species.26,71,72 Several wild yeasts compete with starter strains causing serious operational difficulties to industrial processes of ethanol production.26,54,73
A research carried out during five years demonstrated that all 73 wild yeasts isolated from two distilleries were classified as S. cerevisiae.74 However, contaminant strains can present different characteristics in relation to starter strains. After to evaluate 340 contaminating yeasts isolated from fermentation tanks of 50 Brazilian distilleries it was shown that 80% had undesirable characteristics for an optimum fermentation such as foaming, flocculation and high concentrations of residual sugars in the wine.26 Moreover, some strains presented a combination of these traits and most of them belonged to genus Saccharomyces. The main contaminant yeasts, classified as non-Saccharomyces, were Dekkera, Schizosaccharomyces and Candida. Dekkera is a common genus of contaminant yeasts causing significant reduction of fermentation yield in distilleries that does not warm the sugarcane juice and works at very low ethanol concentrations in the wine.75,76
Contaminating wild Saccharomyces show a high frequency of undesirable traits such as flocculation, pseudohyphae development and foam excess. Although flocculation and chain formation of cells are characteristics well documented, foam production by different strains is not well known. However, it has been associated to hydrophobicity of the yeast cell wall in ethanol and sake production.77,78
Although contaminants can enter the process through different ways, sugarcane is the major source and new strategies based on non-cultural techniques have been applied to access microbial communities that can affect industrial processes of bioethanol production.79,80 Besides wild yeasts, bacteria compete for sugars and nutrients with starter yeast strains causing many problems, such as inhibition of fermentation, production of organic acids, reduction of industrial yields and flocculation.81–83
A common procedure adopted by Brazilian distilleries that recycle yeast cells is the use of diluted sulfuric acid (pH 2.0–2.5 for 1–2h) to kill bacteria.26 Without acidic treatment, industrial fermentations are subject to sugar losses due to bacterial contamination. Microbiological analyses carried out in a distillery demonstrated that sulfuric acid treatment of the yeast cream reduced bacterial population by 44.55%.84 Control of bacterial contamination in industrial fermentations for ethanol production has been one of the most important factors that contributed to increase the fermentation yield in Brazilian distilleries.37
The development of a methodology to count living bacteria by microscopy in 15min, evolution of fast tests to identify the best antibiotics in only 6h and the use of antimicrobial products reduced significantly bacterial contamination in the industry.29,71 From 2007 onward, new antimicrobials were introduced to control bacterial contamination.85 This change was necessary due to pressure to reduce the use of antibiotics, mainly by distilleries that produce inactive dry yeast for animal feed. Chlorine dioxide and hop acids derivatives (alpha and beta fraction) are among the new antimicrobials used. These products are alternatives to control bacterial contaminants without use of antibiotics. Microscopic countings as low as 105 bacterial rods per mL of wine have been obtained in several distilleries. This means a thousand and ten thousand times less bacteria in fermentation than 40 years ago.37 Moreover, several compounds can affect not only bacteria but also the yeast cells and should be evaluated before to be used by the industry.86
Regarding bacterial biodiversity in industrial fermentations, 334 strains were isolated from Brazilian distilleries and classified using a matrix of similarity.84 The main bacterial communities were represented by Gram-positive (98.52%), rods (87.76%) and not-sporulated (73.95%). Among the main bacterial groups, the largest were Lactobacillus (59.75%) and Bacillus (26.58%) while other representatives were at minor proportion (14.67%). For the Lactobacillus group, the main species observed was L. fermentum (15.04%).84 Despite bacterial contamination is caused chiefly by Gram-positive bacteria, it was reported a case with drastic reduction of fermentation yield and ethanol production due to Gram-negative bacteria identified as Acetobacter pasteurianus by the sequencing of 16S rDNA gene.83
Lactic acid bacteria are the main contaminants of alcoholic fermentations. These bacteria are classified into two biochemical groups, according to metabolism of glucose: homo and heterofermentative. The first group is characterized by the production of lactate from glucose, while the second drives the metabolism for the production of lactate, acetate, ethanol and CO2.87
Since lactic acid bacteria are the main contaminants of alcoholic fermentations, lactic acid is an important indicator to monitor bacterial contamination. However, some bacterial species can produce different isomers of lactic acid or a mix of both. A research carried out with 27 strains of Lactobacillus isolated from industrial fermentations of ethanol production demonstrated that 70% of them produced dl-lactate while the other 30% produced only one isomer (d or l)-lactate.88 Furthermore, heterofermentative bacteria also produce mannitol which has been used as an indicator of sugarcane deterioration by microorganisms as well as of contamination of alcoholic fermentation processes by heterofermentative bacteria.89,90 Homo and heterofermentative lactic acid bacteria can affect the fermentation of different ways.91 Because of their importance, some distilleries have monitored mannitol, lactate, acetate and residual sugars in wine through high performance liquid chromatography improving their process control.29
Another very important issue is the effect of bacterial contamination on the yeast. A very common effect is flocculation of yeast cells. This kind of flocculation depends on physical contact between bacteria and yeast cells. Among lactic acid bacteria, L. fermentum, L. fructosus, L. buchneri and L. plantarum can cause yeast flocculation.92 When the ratio bacteria:yeast reaches 4.8:1, flocculation occurs.93 Furthermore, a fermentation assay carried out with three strains (baker's yeast, PE-2 and M-26), contaminated by L. fermentum, demonstrated that baker's yeast was the most sensible strain to bacterial contamination. Fermentations with baker's yeast presented the lowest rates of cell viability and the highest rates of bacterial multiplication. The results showed that dead cells release nutrients such as amino acids, vitamins and minerals that stimulate bacterial metabolism. In addition, PE-2 and M-26 showed the highest cell viability rates and lowest bacterial contamination by Lactobacillus.94 Later, the high tolerance of industrial and tailored yeast strains to bacterial contamination by L. fermentum while the baker's yeast was more sensible.54
One of the most important bacterial contamination sources of sugarcane juice is the soil. There is one billion of bacterial cells for each 1g of soil.95 Another important source of bacterial contamination is sugarcane infestation by borer. In addition, sugarcane stalks perforated by borer accumulate organic acids and phenolic compounds that can inhibit the fermentation.96
Bacterial contamination can affect the ethanol production differently as well as the indirect determination of fermentation yield based on by-products such as glycerol, yeast biomass, residual sugars and acidity. Some years ago, a distillery presented a fermentation process with contamination above 107bacterial rods/mL and a wine acidity that was lower than acidity of sugarcane must. It was not expected for an industrial fermentation. After isolation and biochemical tests were identified bacterial species belonging to malolactic group.97 These bacterial species have been isolated from grape wine fermentations but not from ethanol production. Malolactic bacteria are able to convert malic acid into lactic acid for ATP production.98 Malic acid is a dicarboxylic acid present in sugarcane juice, but it can also be produced by yeasts during the fermentation.49,99 Once lactic acid is monocarboxylic, these bacteria reduce the overall acidity of fermented medium. Because the acidity balance at the end of fermentation was negative, it overestimated the fermentation yield by indirect methodology based on by-products. In 2013, another case observed was the contamination by Acetobacter indonesiensis. It is a Gram-negative bacteria associated to soil and roots of sugarcane. This contamination was associated to a drastic glycerol reduction in wine, overestimating the real fermentation yield based on by-products.
All of these cases show the diversity and complexity of yeast and bacterial communities that contaminate industrial process of ethanol production and the different ways that these microorganisms can affect alcoholic fermentation and compromise the current ethanol production. The biodiversity of contaminants may affect not only ethanol production of first generation but also new technologies.
New technologies for production of first and second-generation ethanolDespite reaching alcoholic fermentation yields as high as 92%, there are several scientific and technological challenges to be overcome for first and second-generation ethanol.22,100 In comparison to corn fermentations in the USA, Brazilian distilleries run their processes for eight months (or less) in a year because sugarcane cannot be harvested during the rainy season and cannot be stored as corn can. However, new Brazilian distilleries were built to ferment cornstarch, reducing the off-season without ethanol production.101 It has opened a new opportunity to increase the production of first-generation ethanol, reducing industrial fixed costs, employing specialized labor force and improving competitiveness of distilleries. Moreover, these processes have an enormous advantage because they do not generate vinasses. The main by-product is distillers dried grains with solubles (DDGS). It has a commercial value as high as that of ethanol, it is richer in protein than corn grain and can be used directly for animal feeding.102
More recently, a new Brazilian process of corn fermentation has been developed based on the reuse of surplus yeast cells from sugarcane fermentation that would be discarded by the plant. This Brazilian process integrates the fermentation of sugarcane juice (or molasses) and starchy feedstocks, providing a number of advantages over conventional distilleries.103 This process has a faster fermentation (34–36h) in comparison with the traditional process (45–60h) adopted by distilleries in the USA and less sugar is deviated for yeast multiplication and production of cellular biomass. In addition, this technology allows to use the same distillation system used for sugarcane and extend the period of ethanol production to 345 days a year, reducing initial investments and fixed costs. Each corn ton allows to produce 415L of ethanol and 250kg of DDGS.103 Moreover, integration of ethanol production from sugarcane and corn has the potential to offer significant economic advantages in comparison to stand-alone units, since important operations, yeast, feedstock, laboratories and technical personnel may be shared between both processes.
Flex fuel plants of corn and sugarcane have an energy balance and reduction of GHG emissions that do not compromise the fermentation performance. They are economically viable in regions with corn supply at low prices and high demand of DDGS for animal feed. It is an opportunity for Brazilian distilleries in corn producing regions that are far from ports.104
Besides ethanol production from starchy feedstocks, the last frontier of biofuels has been the use of lignocellulosic biomass-derived sugars.105 Cellulose and hemicellulose account for more than 60% of dry weight of sugarcane bagasse and can be converted into fermentable sugars either by acid or enzymatic hydrolysis.106 To achieve this goal, several research groups and companies worldwide have expended efforts and resources to produce not only ethanol, but also other biofuel molecules such as butanol and isobutanol.107–109
Research on synthetic biology and metabolic engineering has improved productivity and expectations of high yield for different biofuels. Moreover, selection of microorganisms to be used as biotechnological platform for metabolic engineering approaches has been considered a success key factor.110 These strains need to be improved regarding velocity of sugar assimilation, co-fermentation of pentoses and hexoses, tolerance to inhibitors produced from hydrolysates, tolerance to ethanol concentrations and recycling processes.22,110 Selection of industrial yeast strains has been a viable approach for a new generation of strains that can be used as platform to produce second-generation ethanol while other initiatives have focused on bacteria.111,112
Nowadays, in Brazil, there are two industrial plants in operation for the production of second-generation ethanol and one that use a semi-industrial process. These processes were originated from investment programs of Brazilian government in second-generation ethanol launched in 2011. Nevertheless, production costs of second-generation ethanol are still high, mainly concerning equipment handling at bagasse pre-treatment and the use of enzymes.113 Several initiatives have focused on selection of microbial strains and optimization of conditions for production of enzymes required for hydrolysis and fermentation in industrial processes.114–118
Lignocellulosic pre-treatments, such as steam explosion, alkaline hydrogen peroxide, diluted acid, ammonia, use of solvents, among others, are carried out prior to enzymatic hydrolysis in order to make cellulose more accessible to attack by enzymes.119 However, the pre-treatment of sugarcane bagasse still has technological challenges to be overcame in industrial scale. New technologies to disassembly the sugarcane fiber as well as the use of enzymes will improve the efficiency of bagasse hydrolysis as well as the production of ethanol 2G.120,121
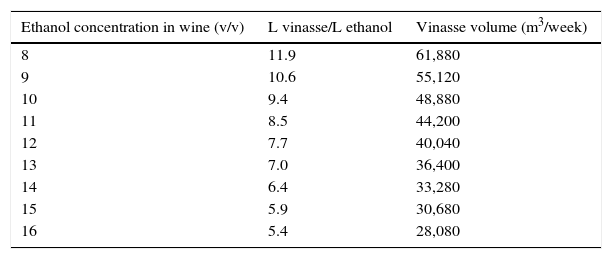
Reduction of vinasse volumesVinasse is the resulting residue after distillation of wine. For each liter of ethanol produced in Brazil, on average, are generated other 12L of vinasse.122 Considering the ethanol production in 2015 of 30 billion liters, it means a production of 360 billion liters of vinasse per year. Vinasse is potassium-rich and it has been used in ferti-irrigation of sugarcane fields, reducing costs with chemical fertilizers.35 In addition, vinasse increases the soil pH some days after its application due to microbial activity stimulated by organic compounds and other nutrients.123
However, vinasse transport and application in the field are costly and require careful procedures to avoid contamination of table waters and soil saturation with cations.124 A reduction of vinasse volume increases the economic distance of transport and application in sugarcane crops.125–127 More concentrated, vinasse can be transported to distant areas from distillery reducing costs of application in the field.
The vinasse volume is directly related to ethanol concentration in wine (Table 3). Despite the overall yield of fermentation process increased from 75–80% to 90–92% in the last 30 years, ethanol concentrations in wine (8.0–8.5) has remained almost the same with exception of some distilleries that have run their processes with alcoholic contents above 10% (v/v).22,128,129
Increase of ethanol concentration in wine and reduction of vinasse volumes for a distillery with an ethanol production of 5200m3/week.
| Ethanol concentration in wine (v/v) | L vinasse/L ethanol | Vinasse volume (m3/week) |
|---|---|---|
| 8 | 11.9 | 61,880 |
| 9 | 10.6 | 55,120 |
| 10 | 9.4 | 48,880 |
| 11 | 8.5 | 44,200 |
| 12 | 7.7 | 40,040 |
| 13 | 7.0 | 36,400 |
| 14 | 6.4 | 33,280 |
| 15 | 5.9 | 30,680 |
| 16 | 5.4 | 28,080 |
Source: Fermentec.
A key factor to increase the ethanol concentrations in wine and reduce vinasse volumes has been the selection and use of robust yeast strains. Tests carried out at a pilot scale revealed that some industrial strains are more robust and viable for fermentations with high ethanol concentrations in wine than other traditional yeasts.130 These yeast strains open a new perspective to Brazilian distilleries increase the concentration of ethanol in wine for reduction of vinasse volumes.
Final remarksNowadays, several Brazilian distilleries are looking for technological innovations to improve their performance and ensure competitiveness of ethanol production. Evolution of first and second-generation ethanol depends of new feedstocks and a continuous improvement of the microbiological processes.22,131,132
Although production of first-generation ethanol has been considered a mature technology, there are huge opportunities of research, development and innovation for Brazilian distilleries.133 It includes reduction of sugars losses, new strategies to control bacterial contaminants, selection of new yeast strains, technologies for reduction of vinasse volumes and alternative uses by the industry, energy saving, better use of water, development of new fermentation processes for alternative feedstocks and biorrefineries for high-value added products.22
Moreover, it is necessary to develop strategies for transfer of new technologies to the industry.134 This movement of technologies is fundamental to increase efficiency and reduce costs. A study conducted in 2016 showed that for every R$ 1.00 invested in research and development, there is potential to return R$ 17.11 only in terms of reduction of production costs in Brazilian distilleries.135 Finally, investments in scientific and technological development, formation of researchers and specialized professionals will build solid bridges between science and industry for sustainable future of ethanol production in Brazil.136
To Marta Maria Moraes Leitão for drawings and illustrations.