Naples yellow was widely used across different types of artwork. Technical studies identified a binary Pb–Sb type as well as modified ternary variants with either zinc or tin in the structure. Although these variants were the object of previous experimental studies, a better understanding of the impact of the glazing procedure on the chromatic, chemical and crystallographic characteristics of the pigment is still lacking. In this work, several historical Naples yellow recipes were re-worked and subsequently applied and fired on test tiles, over a white glaze. The results show that the interaction between pigment and glaze produces important modifications to the colour, chemistry and structure of the pigment. Such modifications will strongly impact the reconstruction of historical recipes, with major consequences for identifying Naples yellow variants on artwork and investigating artistic practices.
El amarillo de Nápoles se ha usado ampliamente en diferentes tipos de obras de arte. Los estudios técnicos han identificado el tipo binario Pb-Sb, así como las variantes ternarias modificadas en la estructura con cinc o estaño. Aunque estas variantes han sido objeto de estudios experimentales previos, aún falta una mayor comprensión del impacto del procedimiento de vidriado en las características cromáticas, químicas y cristalográficas del pigmento. En este trabajo, varias recetas históricas del amarillo de Nápoles han sido reelaboradas y posteriormente aplicadas y cocidas en azulejos sobre esmalte blanco. Los resultados muestran que la interacción entre el pigmento y el esmalte produce importantes modificaciones en el color, la química y la estructura del pigmento. Tales modificaciones tendrán un fuerte impacto en la reconstrucción de las recetas históricas, con importantes consecuencias para identificar las variantes del amarillo de Nápoles en obras de arte y en la investigación de las prácticas artísticas.
One of the most important pieces of Portuguese artistic heritage are the decorated tiles known as azulejos. Their chromatic palette reached a peak during the 17th century when tile panels were painted using blue, green, yellow, orange, purple and brown in various shades. The colours were obtained with natural or synthetic pigments and were imparted by several metallic oxides [1]. For yellows and oranges, Portuguese artists employed the pigment known as Naples yellow (Pb2Sb2O7), whose history of use in past technologies is long and complex. Naples yellow is among the oldest synthetic pigments, first used in the Late Bronze Age (ca. 1500 BC) in Egypt to make opaque yellow glass [2]. It remained the main yellow colourant in the glass industry until the 4th century AD, when tin replaced antimony as a glass opacifier, and the pigment disappeared from European art [3,4]. It was re-introduced sometime in the 16th century by Venetian glass makers and quickly became widely employed by Renaissance Italian painters [5] and maiolica potters [6].
Starting from the same time, lead antimonate yellow begins to feature in technical writings on art technology, where the authors provide numerous recipes to synthesise the pigment. The process involved calcining a mixture of lead and antimony compounds together with reagents that helped the reaction and influenced the final colour. Common additions were alkali-based fluxes (i.e. salt and tartar) and iron oxide, but analytical studies on European artwork discovered modified variants of the pigment, where either zinc or tin enter the otherwise binary lead-antimony structure. So far, the Pb–Zn–Sb variant of Naples yellow seems predominantly restricted to decorated glazed ceramics made in Spain [7,8] and Portugal [1,9–11], with few more examples on Italian maiolica[12,13]. Only one case of this variant in painting is known, on a miniature attributed to Valerio Mariani [14]. Some examples of lead antimonate with zinc in the molecular structure were also found in opaque yellow glasses from Late Bronze Age Egypt, though the zinc may have been introduced as an impurity from the lead or antimony source [15]. The Pb–Sn–Sb variant, on the other hand, is more common, having been found both on paintings [5,16–18] and on maiolica-style decorated pottery of various traditions [11,19–27]. The discovery of Naples yellows with ternary compositions substantiates the numerous historical recipes that call for the addition of an ingredient called tutty, as a way to improve the colour (see Section 2.1). Although traditionally associated with zinc [28], the term tutty has also been linked with tin, due to the widespread presence of the Pb–Sn–Sb ternary Naples yellow [2]. Zinc remains nonetheless the most commonly accepted translation [13].
The study of the Naples yellow pigment poses different questions depending on how it was used – in oil painting, as a glass colourant or on a maiolica tile. More often than not, re-worked pigments are directly compared against unknown samples from historical artwork. This is especially the case with experimental studies focusing on painting, where the pigment is sometimes mixed with a binder and mock-up paint samples are made [29–31]. Usually, no chromatic consequences are reported as the pigment changes medium. However, the situation is different when the medium is a glass phase. As Molina and collaborators convincingly show, adding some re-worked pigment to a glass melt strongly affects the colour of the final glass [32]. As a white tin glaze provides an environment that is certainly more similar to glass than it is to oil paint, we must expect an influence too. Thus, the identification of Sn-modified Naples yellow on maiolica lead-tin glazes may raise the question of whether the tin came from the original pigment recipe or from the reaction between the glaze and the pigment during firing. Furthermore, when trying to interpret the pigment recipe, we must consider that both the pigment and the glaze have a significant amount of lead in their composition. To better understand this process, the present article focuses on the manufacturing technology of lead antimonate yellow pigments and on how it influences their chemical composition and chromatic properties when used on maiolica tiles. Our aim is to investigate how differences in the recipes and their use as maiolica pigments affected the chromatic properties of Naples yellow. This research is focused on the influence of reagents listed in the sources, such as alkali fluxes, zinc and tin, and on the formation of modified variants. Special attention was paid to the properties of the pigment before and after being applied and fired over a white glaze.
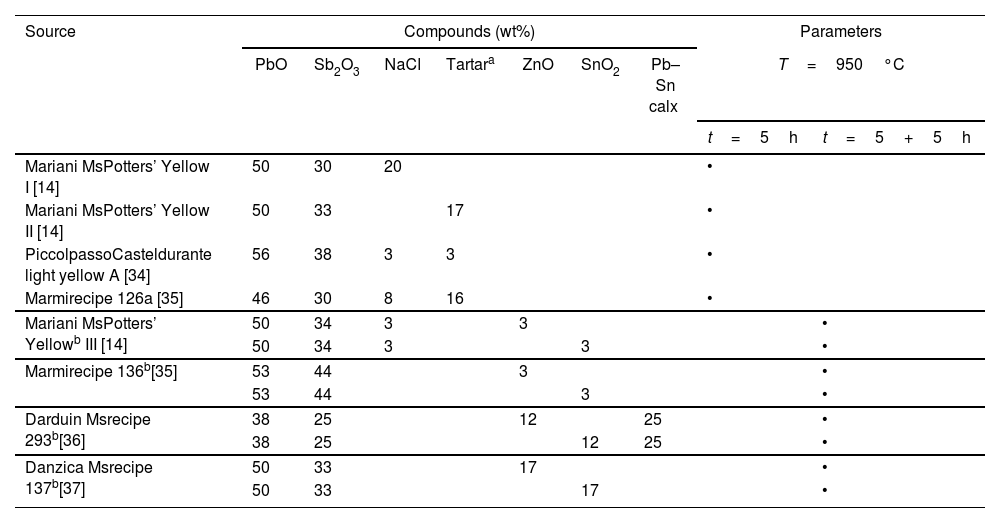
Materials and methodsReplication of historical recipesAfter a survey of available historical sources on the preparation of Naples yellow, eight recipes were chosen from five 16th and 17th-century technical texts (Table 1). The choice was based on diversification, as the recipes provide us with a variety of ingredients appearing in different quantities. Four are binary, Pb–Sb compositions, while another four are ternary pigments modified with tutty. Three recipes come from Valerio Mariani's early 17th-century treatise on miniature painting, which contains numerous pigment preparations, including different types of giallo de’ vasari (potters’ yellow) [33]; one recipe comes from The Three Books of the Potter's Art, by Cipriano Piccolpasso, a 16th-century Italian treatise on maiolica and one of the most important sources of its time, providing numerous recipes for giallolino (light yellow) to be employed for painting on pottery [34]; two recipes were taken from Segreti di Fornace (The Ceramist's Secrets), compiled by Dionigi Marmi, containing a preparation of different nature and chronology leading up to the 17th century [35]; one recipe comes from the Darduin manuscript, a recipe book composed between the 16th and 17th centuries by members of a well-known family of glass makers operating on the island of Murano [36]; and one recipe belongs to the Danzica manuscript, a collection of recipes for glass and glass pastes assembled in the town of Danzica in 1645 [37]. Since two sets of modified pigment variants were prepared from the same base recipe, one the Pb–Zn–Sb and the other the Pb–Sn–Sb variant, the total number of actual preparations re-worked in this study is twelve.
Recipes and firing parameters.
| Source | Compounds (wt%) | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PbO | Sb2O3 | NaCl | Tartara | ZnO | SnO2 | Pb–Sn calx | T=950°C | ||
| t=5h | t=5+5h | ||||||||
| Mariani MsPotters’ Yellow I [14] | 50 | 30 | 20 | • | |||||
| Mariani MsPotters’ Yellow II [14] | 50 | 33 | 17 | • | |||||
| PiccolpassoCasteldurante light yellow A [34] | 56 | 38 | 3 | 3 | • | ||||
| Marmirecipe 126a [35] | 46 | 30 | 8 | 16 | • | ||||
| Mariani MsPotters’ Yellowb III [14] | 50 | 34 | 3 | 3 | • | ||||
| 50 | 34 | 3 | 3 | • | |||||
| Marmirecipe 136b[35] | 53 | 44 | 3 | • | |||||
| 53 | 44 | 3 | • | ||||||
| Darduin Msrecipe 293b[36] | 38 | 25 | 12 | 25 | • | ||||
| 38 | 25 | 12 | 25 | • | |||||
| Danzica Msrecipe 137b[37] | 50 | 33 | 17 | • | |||||
| 50 | 33 | 17 | • | ||||||
Historical terminology may pose problems of interpretation, as substances were often referred to with more than one name, while the same term sometimes came to define different substances over time [38]. The nature of components such as lead, salt and tartar is relatively straightforward, and we employed lead (II) oxide (PbO), sodium chloride (NaCl) and potassium sodium tartrate 4-hydrate (NaK(COO)2(CHOH)2·4H2O). However, sources on Naples yellow do not specify in which form antimony entered the mix – whether metallic, as a sulfide or an oxide. Previous experimental studies have shown that the oxide would have afforded the best results in terms of pigment purity [2], therefore we used antimony (III) oxide (Sb2O3). As already mentioned, tutty is possibly the most relevant terminological issue at play here and, due to our specific interest in modified Naples yellows, we decided to test the relative recipes using both zinc oxide (ZnO) and tin IV oxide (SnO2). Finally, the recipe from the Darduin manuscript mentions calcina, lead and tin mixed and calcined together. For this ingredient, a recipe from L’Arte Vetraria was followed, the 1612 treatise on glass making written by Antonio Neri [39,40].
Original recipes report quantities in pounds (libbra) and ounces (oncia), whose exact weight also tended to shift from place to place. Based on previous experiments in the literature [41], a pound-to-ounce ratio of 1:12 was maintained. Quantities were normalised to 100% and a total of 10–20g was prepared for each recipe. The ingredients were thoroughly mixed and spread on unglazed ceramic tiles. The tiles were placed in an electric kiln and fired to 950°C for five hours, with a 5°C per minute ramp rate. All ternary recipes but one mention repeated firings to improve the quality of the colour. Therefore, those recipes were re-fired to the same temperature for an additional five hours. All final mixtures were ground to a fine powder in an agate mortar to homogenise the colour and texture, and to facilitate the successive painting on test tiles.
Painting of test tilesIn order to address the influence of the glazing process on the colour, chemistry and structure of different Naples yellows, the re-worked pigments were applied on test tiles. First, a white glaze was prepared by mixing 100g of a commercial lead-silicate frit (TR29 ®Ferro: 65wt% PbO and 35wt% SiO2) with laboratory-grade oxides and carbonates (60g SiO2, 15g SnO2, 13.23g K2CO3, 3.6g CaCO3, 3.4g Na2CO3, 1.5g MgCO3), according to the recipe used in [42] for historical tiles. The mixture was ground in an electric mortar for fifteen minutes and water was added until a moderately runny paste was obtained. The glaze was applied to the tiles by quickly dipping them in the water suspension, after which they were left to air dry for an hour. Then, small amounts of pigment were mixed with water and painted directly on the white glaze using a brush. Once the paint layer dried, the test tiles were fired at 1000°C with a 120°C per hour ramp rate and 1h dwell.
Analytical methodologyAll pigments and test tiles were analysed using a range of techniques for chemical and chromatic characterisation.
μ-Raman spectroscopyRaman microscopy was carried out using a Labram 300 Jobin Yvon spectrometer, equipped with a He–Ne laser of 17mW power operating at 632.8nm. Spectra were recorded as an extended scan. The system was calibrated using a silicon standard. The laser beam was focused either with a 50× or a 100× Olympus objective lens and discrete pigment crystals were targeted. The laser power at the surface of the samples was controlled with neutral density filters (optical densities 0.3 and 0.6). Raman data analysis was performed using LabSpec 5 software. All spectra are presented as acquired without any baseline correction or other treatment.
Energy dispersive X-ray fluorescence spectrometryEDXRF analyses were carried out using a Bruker Tornado M4 spectrometer. The instrument is equipped with a side window X-ray tube (Rh, 50kV, 600μA) and an energy dispersive silicon drift detector with a 30mm2 sensitive area and an energy resolution<145eV at 5.9keV. The X-ray tube uses a polycapillary lens accounting for a spot size down 25μm at the sample. Considering the inhomogeneity of the samples, it was decided to proceed with 2D area scans of 2mm×2mm, with a step size of 20μm and a time per step of 10ms. The X-ray tube operated at 50kV and 300μA for 90s. Spectra acquisition of the test tiles was run under 20mbar pressure. Quantitative results were obtained through the analysis of 3 areas per sample and the data was treated using the Bruker M-Quant software considering the oxides present in the test tiles.
ColorimetryThe AvaSpec-2048 Fiber Optic Spectrometer is based on the AvaBench-75 symmetrical Czerny-Turner design with a 2048 pixel CCD Detector Array. The spectrometer has a fibre optic entrance connector, collimating and focusing mirror and diffraction grating. A choice of 15 different gratings with different dispersion and blaze angles enables application in the 200–1100nm range. The light source was AvaLight-HAL Tungsten Halogen. To perform the colorimetry analysis, an integrating sphere was used.
ResultsThe results of our multi-analytical investigation are structured according to the twofold aims of this study: (i) understand how variations in the recipe affect pigments’ colour, chemical composition, and molecular structure; (ii) assess the influence of the interaction between pigment and glaze, and if and how we can reconstruct aspects of the original pigment recipe from the analysis of yellow decorations.
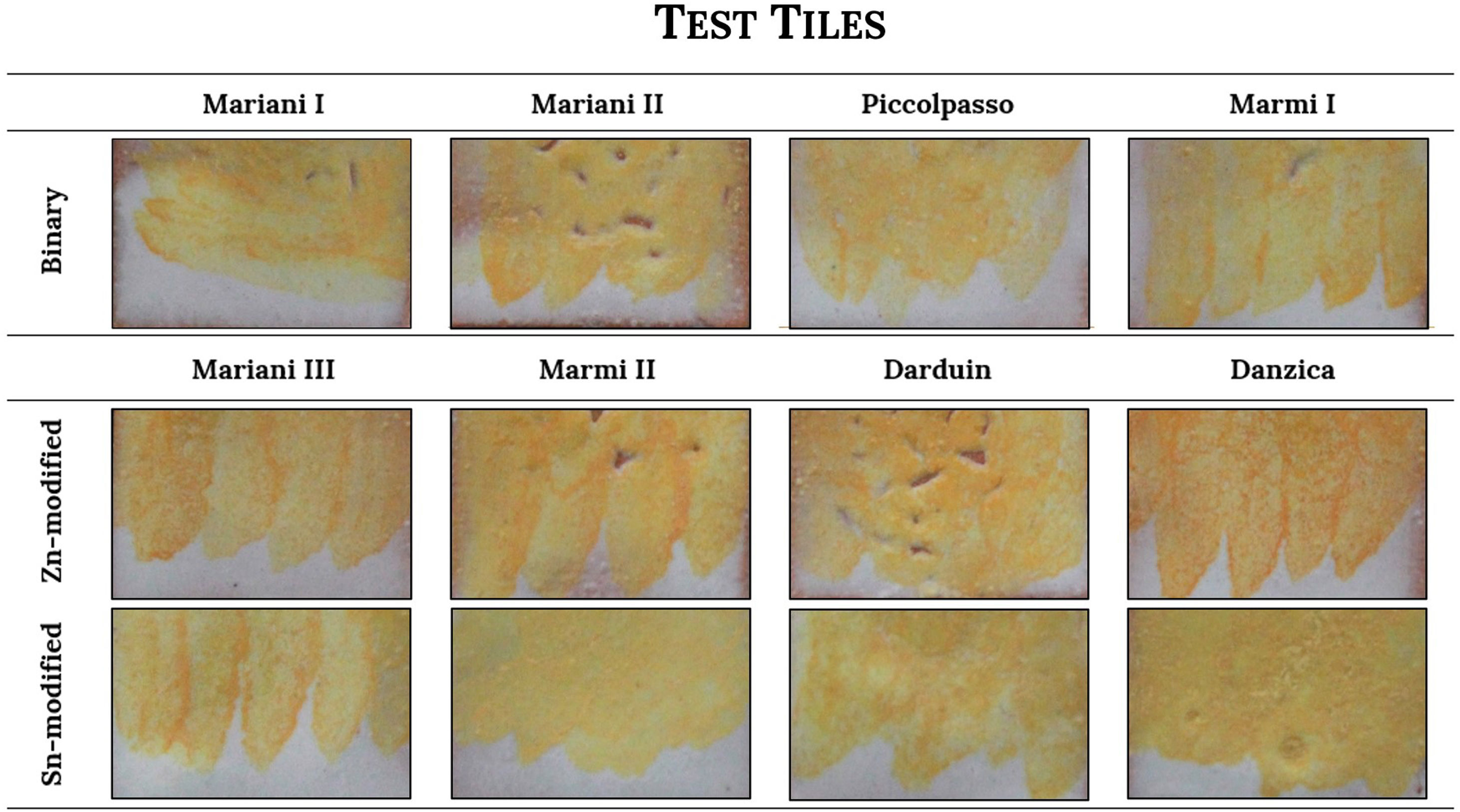
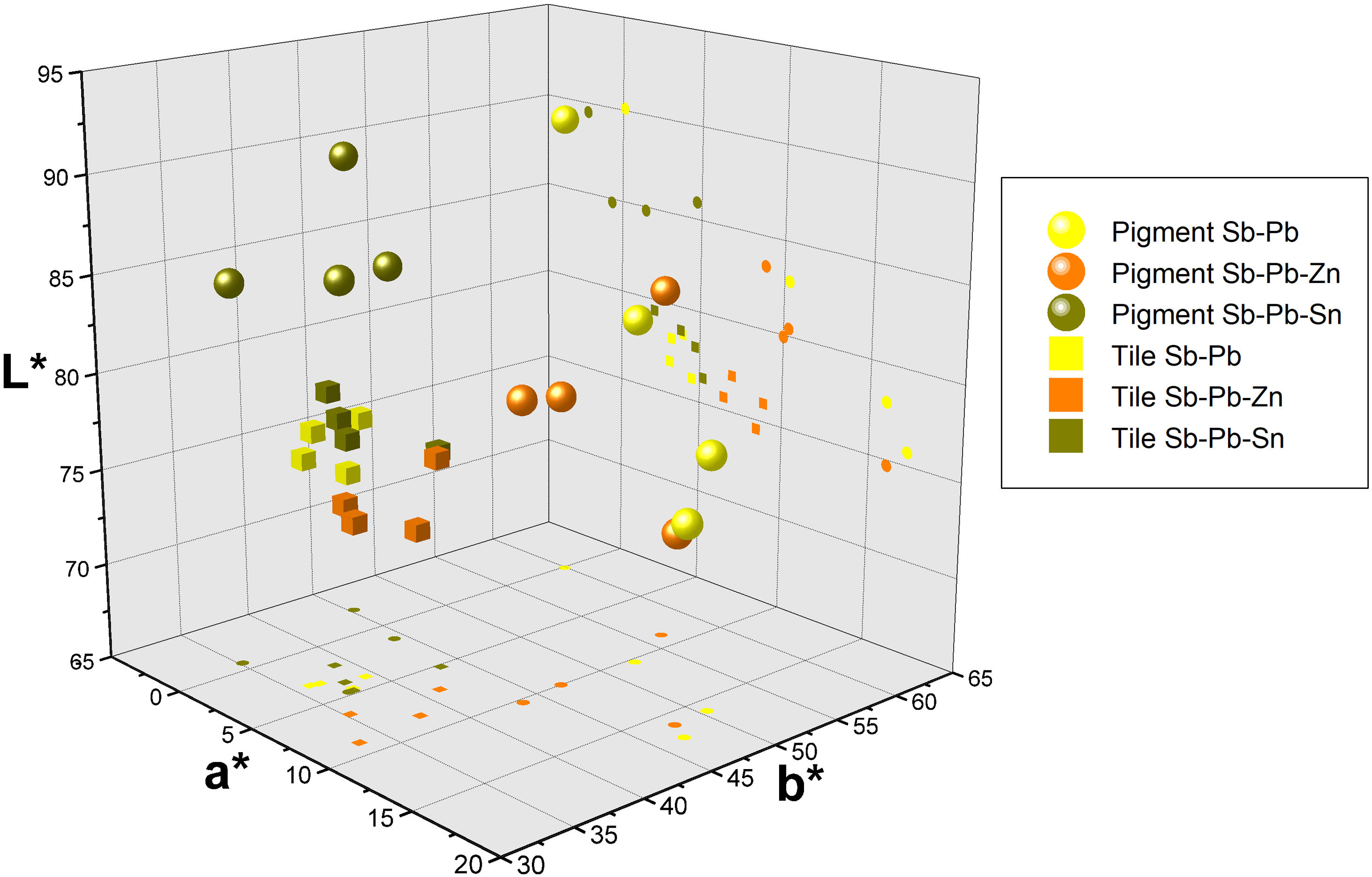
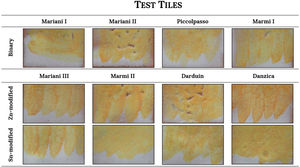
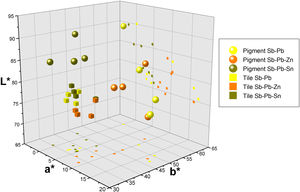
ColourFigs. 1 and 2 illustrate the yellow hues obtained from the selected recipes both as pigments (Fig. 1) and fired on the white lead-tin glaze (Fig. 2). Colorimetric data is available as Supplementary Material (Table S1) and presented as a plot in Fig. 3.
After firing the mixtures, the pigments resulted in a wide spectrum of yellow hues, ranging from pale to bright yellow and through to deep orange (Fig. 1). Since the lead-to-antimony ratio is rather stable at around 1.5:1 (Table 1), the presence and quantity of other reagents are key in determining the chromatic profile of the mixtures. In particular, the addition of either salt (NaCl) or tartar as fluxes appears to significantly alter the colour of the binary Naples yellows. The most striking examples are Mariani I and II. Their recipes are virtually identical, but type I, with salt, shows a bright canary yellow colour, whereas type II, with tartar, is a dark orange. The recipes by Piccolpasso and Marmi contain both alkali fluxes, but in different quantities. Their hue oscillates between dark yellow and light orange, always closer to tartar-rich Mariani II than to salt-rich Mariani I. The higher L* and b* values of the two variants of Mariani III recipes indicate that salt has a similar effect in ternary Naples yellows containing zinc or tin (Fig. 3).
The substitution of zinc for tin in otherwise identical recipes results in visible chromatic differences. Ternary pigments with zinc are invariably darker (L*<85) than their tin-modified counterparts (L*≧85). They are also redder, as reflected in the sensibly higher a* values (8.9–15.9 vs −0.69–4.7) (Table S1). On the other hand, the tin-modified recipes result in a paler yellow, with an evident decrease of the b* value (35.2–45.3). The recipe from the Darduin manuscript is the only one with calcina, the mixture of calcined lead and tin, which does not appear to bring any significant contribution to the colour of the pigment.
Once test tiles were fired at 1000°C, the chromatic differences between recipes became less evident (Fig. 2). Fig. 3 shows the colourimetric coordinates of both unpainted pigments and test tiles, indicating that the latter tend to cluster more tightly, particularly the binary and tin-modified compositions. Only the Naples yellows modified with zinc are more easily distinguishable due to their darker, orangey hue. They are characterised by a higher a* (red) value and occupy a separate area of the plot. This appears in contrast with the unpainted pigments, where it is the tin-modified compositions that are separated from the rest. On test tiles, binary and tin-modified pigments are difficult to separate based on their colour coordinates.
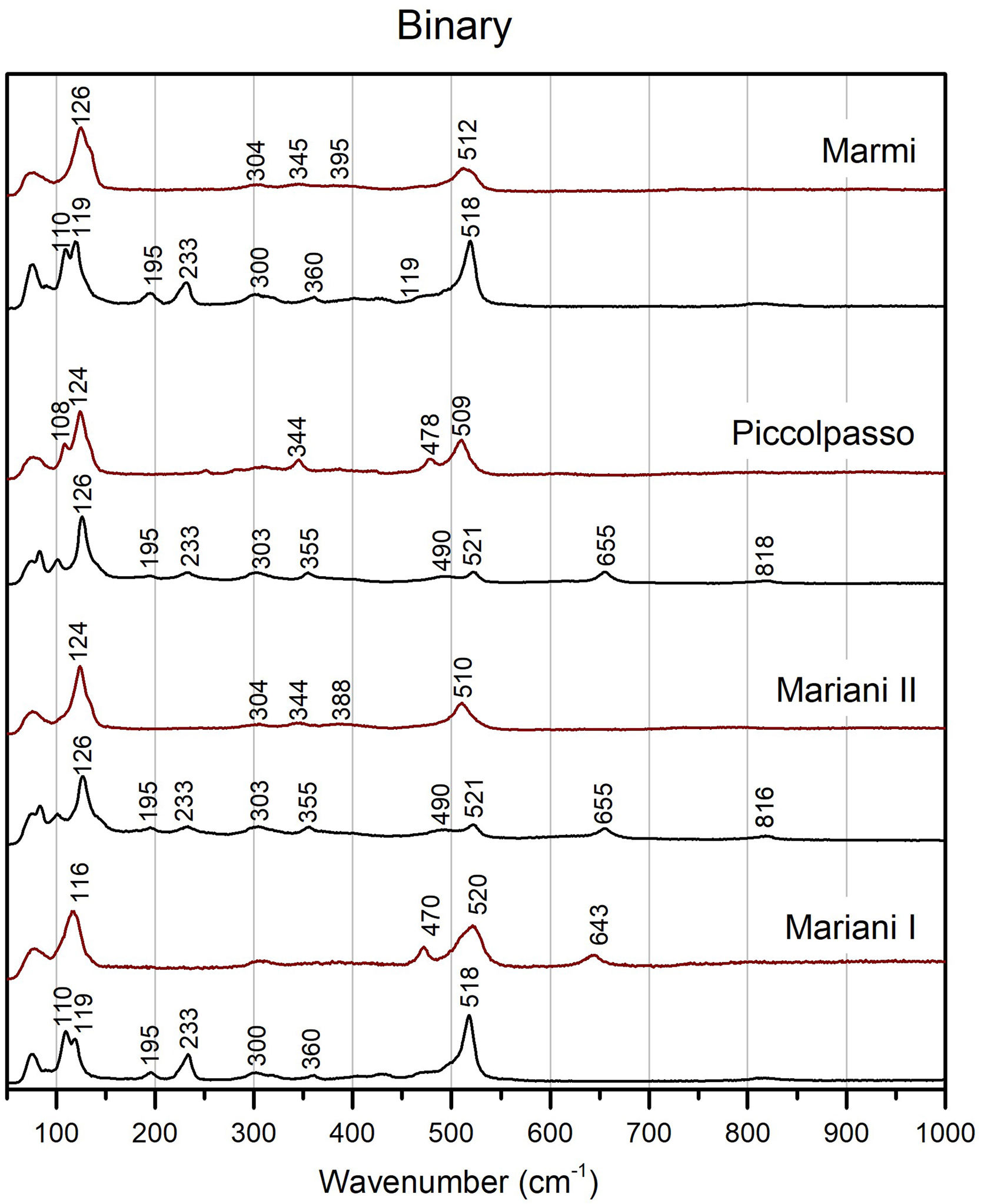
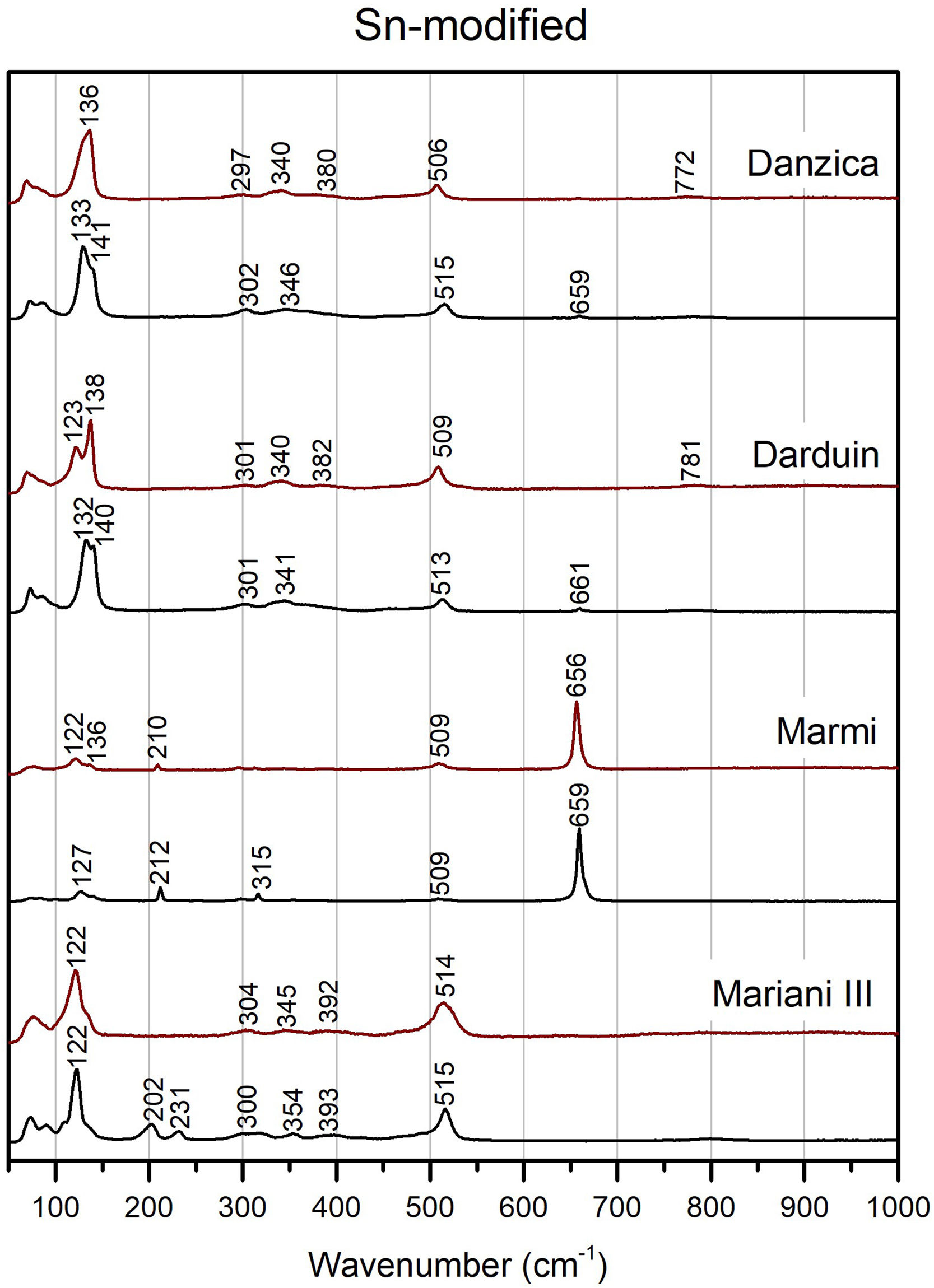
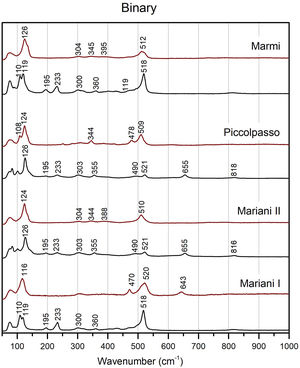
Chemical composition and molecular structureμ-Raman analysisBinary Naples yellows pigments have some common features, such as two peaks at around 195 and 230cm−1, bands of various intensities at around 301–304 and 350–360cm−1 and a weak band at around 820cm−1 (Fig. 4). All have a strong band, characteristic of the Sb-O mode, between 517 and 522cm−1. The band is better developed in Mariani I and in Marmi's recipe, while it appears weaker in Mariani II and Piccolpasso's light yellow, where a shoulder develops at 490cm−1 too. The four recipes tend to split similarly as far as the Pb–O mode bands are concerned too. While in the former pair we note a doublet between 110 and 119cm−1, the latter pigments have a single peak at around 125cm−1. They both also show a peak at around 655cm−1, indicative of the presence of the antimonate rosiaite (PbSb2O6) [43].
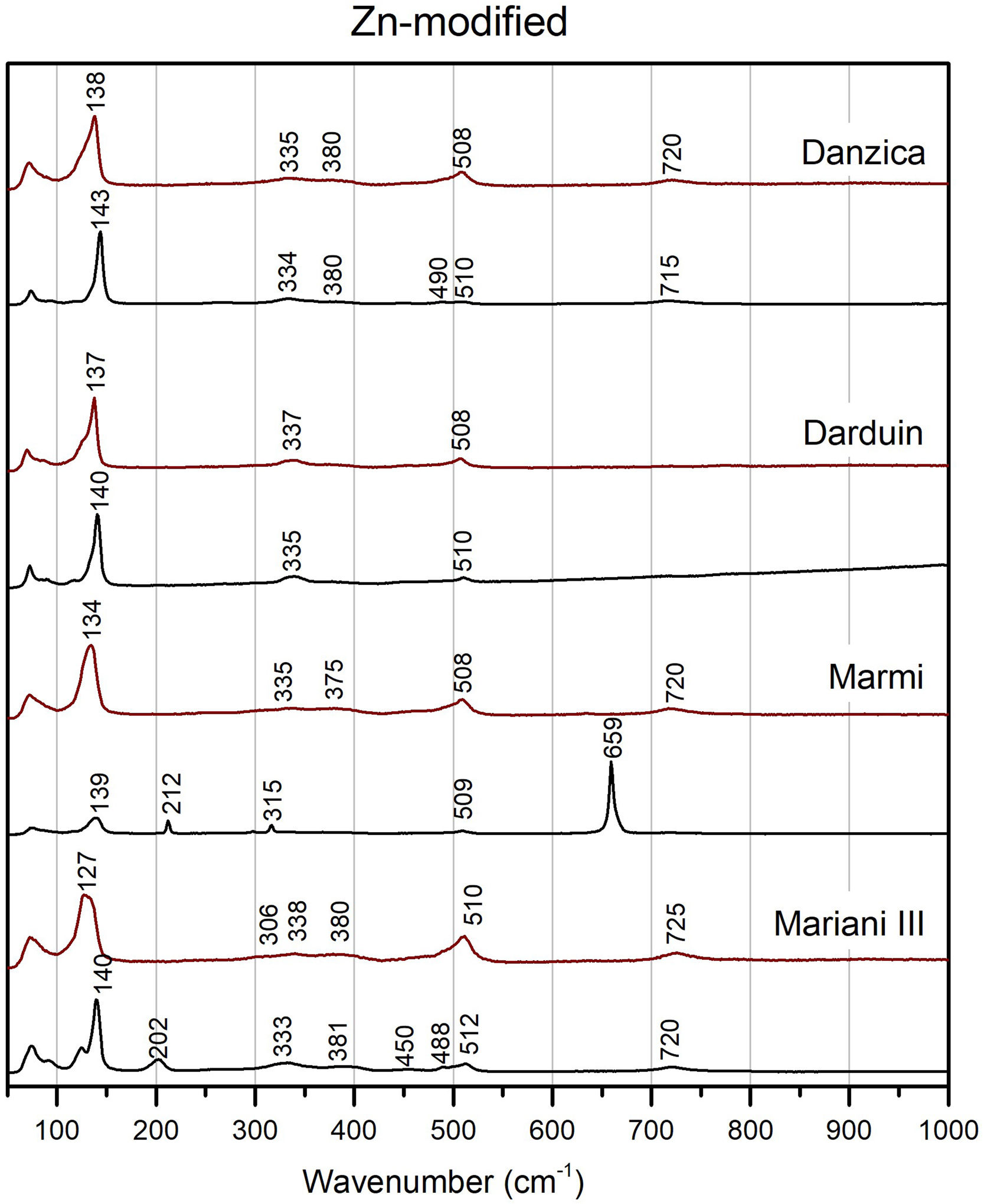
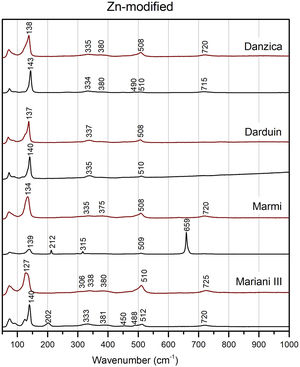
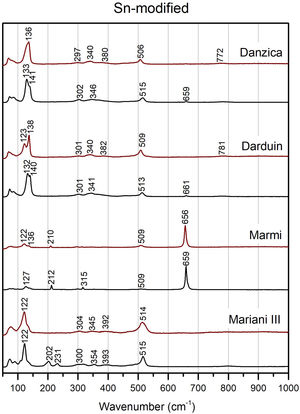
Modified variants of the unpainted pigments show significant differences concerning binary compositions (Figs. 5 and 6). Except for Mariani III, the two peaks at 195 and 230cm−1 disappear, while we note a shift of the Pb–O mode peak towards higher wavenumber in the modified variants, up to around or above 140cm−1 in Pb–Zn–Sb ones. The latter group also shows a substantial weakening of the Sb-O mode band at around 510cm−1, with some developing a shoulder at ca. 490cm−1 and a weak band at 450cm−1. Modified yellows have characteristic peaks at either 335 or 340cm−1, often accompanied by weaker bands between 380 and 390cm−1. The signal at 720cm−1 only characterises the zinc-modified variant (except for one), while it is not detected in tin-modified pigments. In both groups, the pigment made according to Marmi's recipe differs markedly from the rest. The highest level of antimony in the recipe results in the nearly exclusive presence of rosiaite peaks at 211 and 659cm−1.
Figs. 4–6 also present the spectra obtained from test tiles, showing some relevant differences between unpainted and painted pigment mixtures. In the tiles, we note that in all four binary compositions the medium to strong peaks at around 200 and 230cm−1 disappear, as does the peak at around 820cm−1 (Fig. 4). The Pb–O mode bands shift to a higher wavenumber, from 108 to 120cm−1 in the unpainted pigments to between 116 and 125cm−1 in the tiles. Besides the already noted peaks at around 304cm−1 and 345cm−1, there is now another one at around 390cm−1, though this is not always well-formed. Also noteworthy is the formation of a shoulder at 470 and 478cm−1 in two of the test tiles.
In test tiles with the ternary Pb–Zn–Sb composition, we see the return of the characteristic strong band at around 510cm−1, which tends to collapse in the unpainted pigment (Fig. 5) [12]. Moreover, the band is no longer accompanied by a shoulder. Pb–O mode peaks move towards slightly lower wavenumbers, between 127 and 139cm−1, while no rosiaite is now detected in Marmi's recipe. On the other hand, tiles painted with the Pb–Sn–Sb pigments are more similar to the relative un-painted pigments. A notable difference is a peak at around 340cm−1, which is more accentuated in test tiles (Fig. 6).
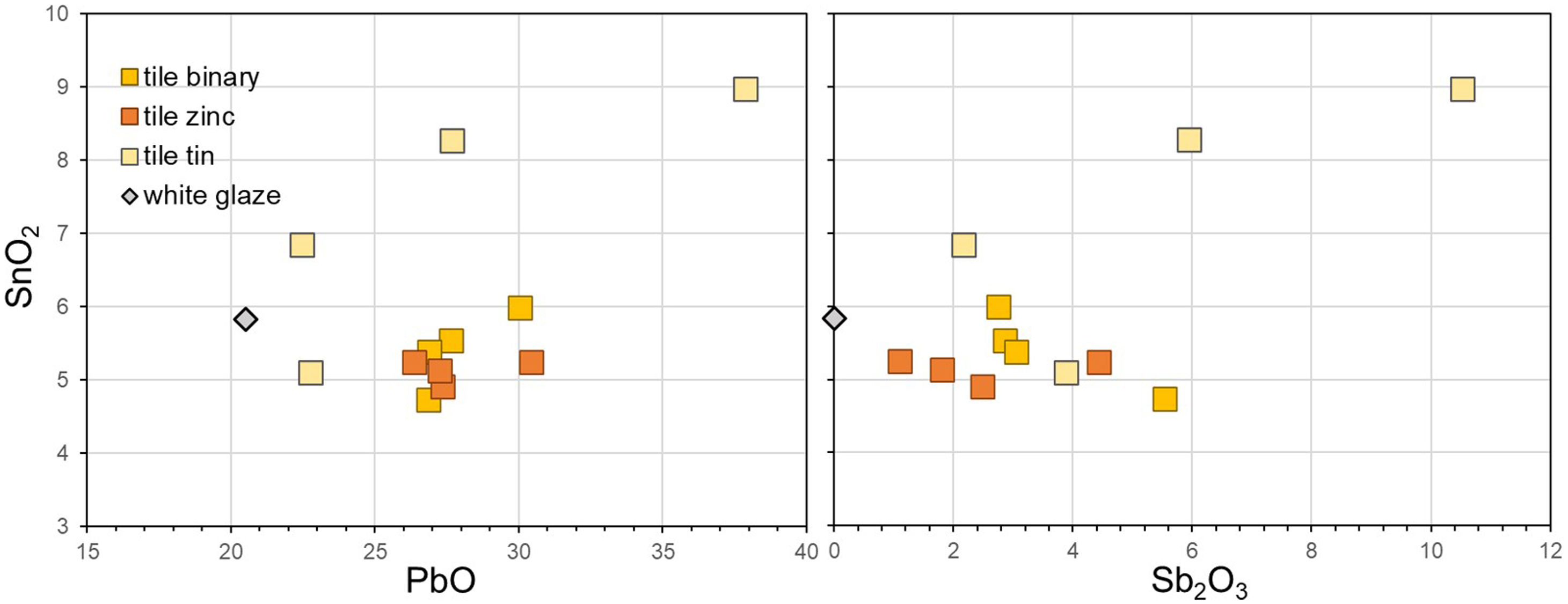
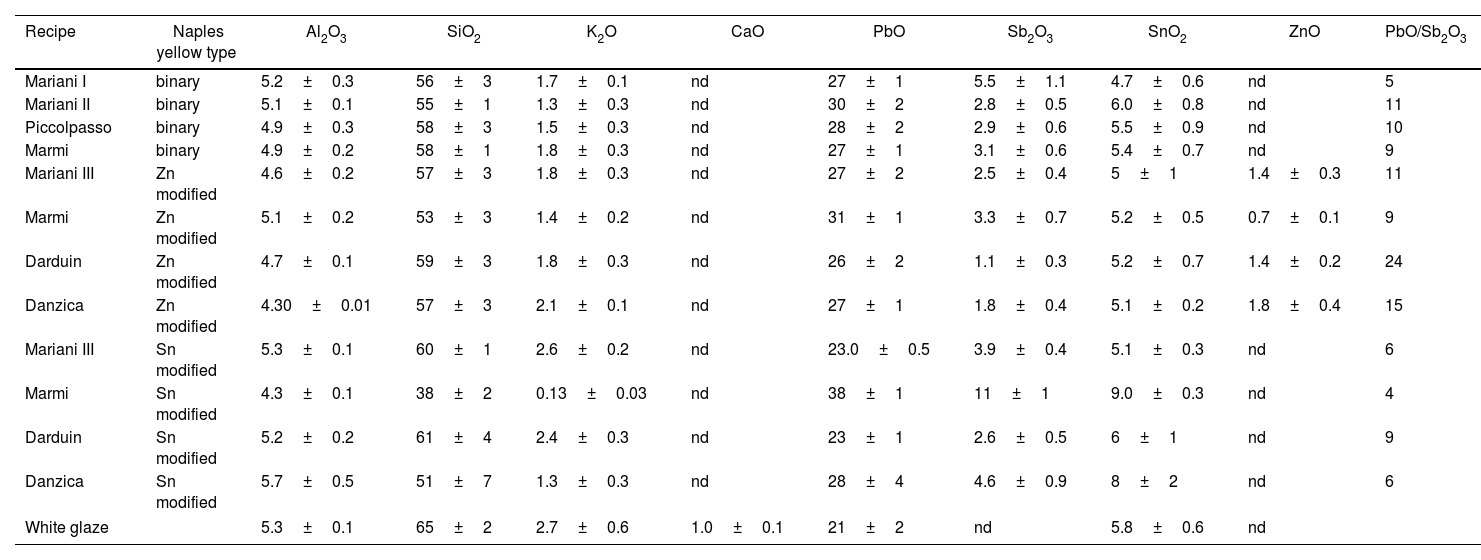
EDXRF analysisTest tiles were analysed through EDXRF to characterise the yellow areas and the white glaze (Table 2), and the results were compared to the theoretical composition of the re-worked pigments. Tin was detected in all yellow areas of the test tiles, regardless of the original pigment composition. Yellow areas painted with Pb–Sb and Pb–Zn–Sb pigments, where no tin is present in the recipe, have between 5 and 6wt% SnO2, about the same as the white glaze. All values except one rise to above 6wt% SnO2 in tiles with Pb–Sn–Sb pigments (Fig. 7). Zinc, on the other hand, is only present in tiles painted with the ternary Pb–Zn–Sb pigments, its levels ranging from 1 to 2wt% ZnO.
EDXRF results of yellow areas on test tiles.
| Recipe | Naples yellow type | Al2O3 | SiO2 | K2O | CaO | PbO | Sb2O3 | SnO2 | ZnO | PbO/Sb2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mariani I | binary | 5.2±0.3 | 56±3 | 1.7±0.1 | nd | 27±1 | 5.5±1.1 | 4.7±0.6 | nd | 5 |
| Mariani II | binary | 5.1±0.1 | 55±1 | 1.3±0.3 | nd | 30±2 | 2.8±0.5 | 6.0±0.8 | nd | 11 |
| Piccolpasso | binary | 4.9±0.3 | 58±3 | 1.5±0.3 | nd | 28±2 | 2.9±0.6 | 5.5±0.9 | nd | 10 |
| Marmi | binary | 4.9±0.2 | 58±1 | 1.8±0.3 | nd | 27±1 | 3.1±0.6 | 5.4±0.7 | nd | 9 |
| Mariani III | Zn modified | 4.6±0.2 | 57±3 | 1.8±0.3 | nd | 27±2 | 2.5±0.4 | 5±1 | 1.4±0.3 | 11 |
| Marmi | Zn modified | 5.1±0.2 | 53±3 | 1.4±0.2 | nd | 31±1 | 3.3±0.7 | 5.2±0.5 | 0.7±0.1 | 9 |
| Darduin | Zn modified | 4.7±0.1 | 59±3 | 1.8±0.3 | nd | 26±2 | 1.1±0.3 | 5.2±0.7 | 1.4±0.2 | 24 |
| Danzica | Zn modified | 4.30±0.01 | 57±3 | 2.1±0.1 | nd | 27±1 | 1.8±0.4 | 5.1±0.2 | 1.8±0.4 | 15 |
| Mariani III | Sn modified | 5.3±0.1 | 60±1 | 2.6±0.2 | nd | 23.0±0.5 | 3.9±0.4 | 5.1±0.3 | nd | 6 |
| Marmi | Sn modified | 4.3±0.1 | 38±2 | 0.13±0.03 | nd | 38±1 | 11±1 | 9.0±0.3 | nd | 4 |
| Darduin | Sn modified | 5.2±0.2 | 61±4 | 2.4±0.3 | nd | 23±1 | 2.6±0.5 | 6±1 | nd | 9 |
| Danzica | Sn modified | 5.7±0.5 | 51±7 | 1.3±0.3 | nd | 28±4 | 4.6±0.9 | 8±2 | nd | 6 |
| White glaze | 5.3±0.1 | 65±2 | 2.7±0.6 | 1.0±0.1 | 21±2 | nd | 5.8±0.6 | nd | ||
Data is shown as wt% of the oxides, nd=not detected.
The Pb/Sb ratio, mostly falling at around 1.5:1 in un-painted pigments, is always increased in the test tiles (see Table 2), where XRF detects both Pb from the white glaze and the yellow pigment. Tiles with tin-modified pigments display a tendency towards a lower ratio than the rest, while the opposite is true for those with zinc-modified pigments, even though the two groups share the same quantities of the two ingredients in the original pigment recipes. In each of the two modified groups, the highest Pb/Sb ratio is displayed by the two variants from Darduin's manuscript, where calcina is present in the recipe.
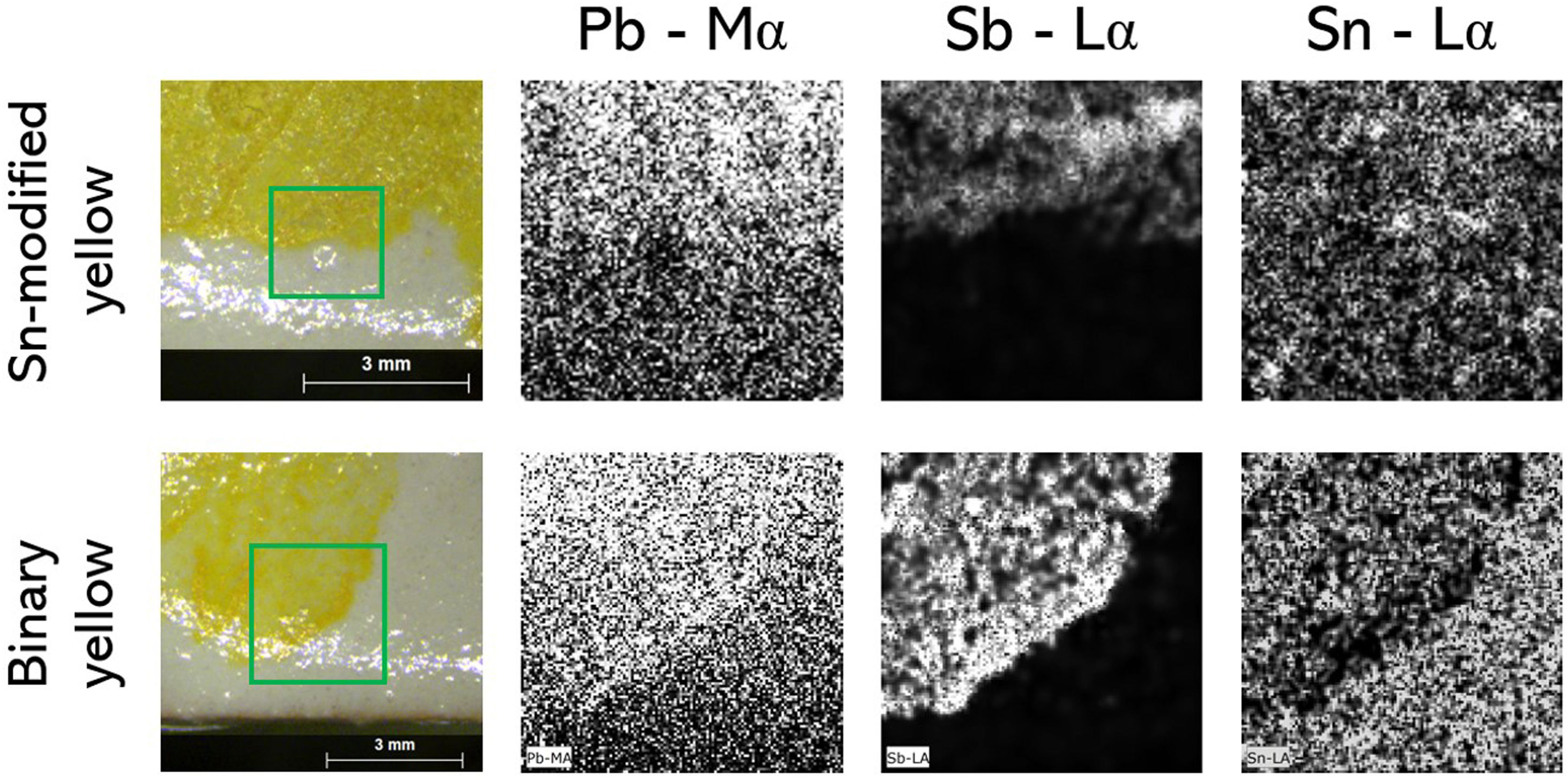
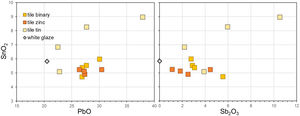
In Fig. 7, SnO2 and Sb2O3 exhibit a tendency towards an inverse correlation for the binary and Zn-modified recipes, suggesting that less SnO2 from the glaze is detected in thicker layers of yellow pigment. This is also visible in the elemental maps obtained by EDXRF for Pb–Ma, Sb–La and Sn–La on the surface of test tiles (Fig. 8): while there is no significant change in the Sn map for Sn-modified yellow, we can see a difference in the binary yellow's map, which shows Sn more concentrated in the glaze than in the pigment. Also, in the area where the yellow is more intense, the Sn is less concentrated.
No sodium was detected, although the data shows variable amounts of potassium (0.13–2.6wt% K2O), which may come from either the pigment or the glaze. Finally, several elements were detected in the composition of the test tiles which come uniquely from the white glaze, i.e., Si and Al.
DiscussionThe influence of fluxesFrom the results outlined in the previous sections, we can argue that the choice of alkali flux was key to developing a palette of yellows that artists could choose from, thus accommodating their chromatic needs. This is why the Italian miniaturist Valerio Mariani provides two recipes for simple potters’ yellows: type I, with the addition of salt and type II with tartar, which result in remarkably different hues. Mariani tells the reader that the first is “more subtle and works well for colouring drawings”, while the second “shows more body” [2]. It is worth keeping in mind that what is being described here is the pigment in its powder form, before any application. Our results show that the purest and most intense yellow is achieved by adding salt, while tartar produces a reddish-orange colour (Fig. 1), so we can tentatively link these differences with Mariani's reference to “body”. However, there is no unanimity in the existing literature. While some studies confirm that the addition of salt appears to improve the intensity of the colour [29,32], others observe less marked differences [44]. Dik and collaborators, the only ones testing both recipes by Mariani as we do here, observe that type II comes out paler than type I [2]. Nevertheless, what is probably more important to highlight here is that the high amount of salt in Mariani I makes this the only binary pigment displaying a bright, light yellow hue. Much in the same way, Mariani's tin-modified variant, again with salt as a flux, stands out for its brightness among the other pigments in its group (Fig. 1).
Besides colour, salts seem beneficial to the structural homogeneity of the pigment too. Raman data shows that the two binary recipes where salt is the predominant flux (i.e. Mariani I and Marmi's light yellow), have strong and well-developed bands at around 518cm−1, which indicates that cubic Pb2Sb2O7 is the dominant antimonate (Fig. 4) [12]. In contrast, the other two pigments from the binary group (i.e. Mariani II and Piccolpasso), where little or no salt was added, have a considerably weaker band that develops a shoulder at 490cm−1. Their structure is less homogenous, and they contain different crystalline phases, as indicated by the presence of rosiaite peaks. In this respect, our data is consistent with previous experimental studies that characterised Naples yellows made with tartar [29,30,32].
The role of zinc and tinOur experiments with modified Naples yellow variants are also in general agreement with data outlined in the literature [2,29,32,45,46]. We note that the addition of tin to the lead-antimony base affords a lighter and purer yellow, while zinc tends to produce a darker, orange-like hue. The trend can be better appreciated by comparing Mariani I (Pb–Sb) against the two versions of Mariani III (Pb–Zn–Sb and Pb–Sn–Sb) (Fig. 1). The three recipes are virtually identical, the only major difference being the presence of either zinc or tin in the latter two. Besides changing the pigment's colour, the addition of a third oxide also modifies the pyrochlore's structure with visible differences in the Raman signature.
The shifts in the fundamental bands of the Pb–O mode, changing from 110 to 119cm−1 in the binary yellow, to 122cm−1 and 140cm−1 in the tin and zinc-modified variants respectively, have been used before to distinguish between binary and ternary forms of the pigment [16,25,27]. However, more detailed experiments pointed out how the Pb–O vibration pattern is highly sensitive to firing temperature and lead-to-antimony ratio, thereby raising a note of caution [12]. Instead, more reliable indicators of a modified pyrochlore structure are (1) the presence of new, medium-intensity bands between 330 and 350cm−1 (2) the collapse of the strong band at around 510cm−1 and (3) the appearance of a shoulder at 450cm−1, the latter two especially visible in the Naples yellows with zinc. All of these features appear in our experiments.
Zinc and tin play a major role in defining the chromatic properties of Naples yellow, allowing tile and maiolica painters to expand their palette. We mentioned earlier that there has been confusion around the term tutty, whether it may have meant zinc or tin [2], although zinc is generally accepted as the most likely translation [13,14,30,45,47]. Most material and textural evidence seem to point to the fact that at least in Europe, the term was employed to describe the “artificial” compound of zinc (i.e. the oxide), as opposed to the natural carbonate, referred to as cadmia[28,48–50]. Moreover, tin does appear as an ingredient in the very same texts taken into account here. Cipriano Piccolpasso, for instance, mentions stagno (tin) when writing recipes for the white glaze [34], which he differentiates from tuzia employed in the preparation of yellows. No confusion around the meaning of tutty seems to exist among early modern practitioners. Nonetheless, what is important here is that yellow pigments with a ternary Pb–Sn–Sb composition do exist and that they were discovered on numerous early modern works of art, including tiles [11,19–23,27]. If we adhere to the most widespread interpretation of tutty as zinc, we also need to address the possible reasons that lead to the formation of tin-modified variants of Naples yellow.
Test tiles: observations and contextOur experiments show that, as pigments are applied and fired on tiles, they undergo relevant changes, with major consequences on the study of historical glazes. The changes involve critical aspects of historical artwork, such as colour, chemical composition and molecular structure, and affect our understanding of technological processes. At least some of these modifications could have been triggered by the extra firing to which the pigments were subjected after painting. As this study places the accent on recipe variations, we decided not to make further tests in this sense. However, the little data available in the literature indicates that firing time concentrates the hue, without fundamentally changing the colour, nor does it modify the molecular structure of the pigment [46,51]. More likely, significant modifications arose from the interaction between the white glaze and the pigment, with the formation of a glass phase.
The most immediately visible consequence is on colour, as the range of hues becomes sensibly narrower once the pigments are applied and fired on the tiles (Fig. 2). All pigments become less bright in the glaze, but binary Pb–Sb yellows are especially affected. Their red coordinates decrease quite dramatically, and they lose the orange tone that before made them very similar to zinc-modified variants (Fig. 3). The same value appears slightly increased in the Pb–Sn–Sb yellows, so that the two groups of tiles are chromatically very similar. As a consequence, the glazing procedure seems to make it easier to distinguish recipes containing zinc, due to their characteristic orange hue.
Glazing is also responsible for chemical and structural modifications of the pigment. Most notably, test tiles show less structural diversity than un-painted pigments do, so distinctions based on Raman spectra alone become difficult. Spectra from the three tiles painted with Mariani's recipes show that potters’ yellows III with zinc and tin are nearly identical (Figs. 4–6). The only possible exception is a peak of unclear nature at around 720–725cm−1, which only appears in the zinc-modified variants, both before and after painting [45].
What is especially interesting, however, is that the binary yellows now show features of a partially modified pyrochlore, effectively making their spectra similar to those of zinc and tin variants. The distortion occurs when tin ions migrate from the glaze to the pigment, as first observed by Rosi and collaborators [12]. They argue that this phenomenon is very likely the reason why similar, partially modified spectra are often found on Renaissance glazes. In other words, modifications in the Raman signature do not necessarily reflect that the artist chose a modified pigment. The observation is of major relevance, in that it provides us with a framework to address the discrepancy between the widespread presence of tin-rich yellows in historical artwork and the absence of tin in Naples yellow recipes of the same period.
As mentioned earlier, EDXRF data show that tin dioxide is indeed present in all our test tiles. Where the original pigment contained none, the concentrations are stable between 5 and 6wt% SnO2, comparable with the tin dioxide content in the white glaze (table). The levels increase to between 6 and 9wt% SnO2 in the four test tiles painted with the ternary Pb–Sn–Sb pigments. On the other hand, zinc oxide is only detected on the four test tiles where the Pb–Zn–Sb pigment was employed, thus EDXRF makes its identification much more immediate than μ-Raman. Moreover, and unlike tin, the amount of zinc oxide in the test tiles (0.7–1.8wt% ZnO) roughly follows the proportions in the original recipe, so that pigments with more zinc result in tiles with more zinc. What is more important to stress here is that even in the absence of a modified variant of Naples yellow, the interaction between pigment and glaze is enough to cause the enrichment in tin. To the investigation of historical tiles and maiolica, this means that analyses through EDXRF will very likely result in the detection of tin, regardless of the original pigment employed. Like our test tiles, the Renaissance maiolica plates analysed by Rosi and collaborators [12] contain tin in both the light-yellow decorations and the orange, Zn-rich areas. Thus, without more analyses that would enable to target discrete pigment crystals, the presence of tin cannot be automatically used as evidence of the artist employing a tin-modified Naples yellow.
The ratio of lead to antimony is also severely affected by the underlying glaze, so yellow decorations on test tiles are always heavily enriched in lead over antimony. Similarly to what was noticed above with zinc, the Pb/Sn ratios of test tiles generally mirror the quantities of lead and tin in the original pigment recipe. Most notably, the two preparations with calcina display the highest ratio, since the latter introduces even more lead in the mix. The ratio is sufficiently higher when compared to that of the other tiles, that this parameter can be used to introduce the possibility that the original pigment contained calcina. But our chemical data indicates that, aside from zinc, most assumptions regarding reagents added to the basic lead-antimony mix are extremely difficult to make. For instance, we could not detect any sodium in the test tiles, even when salt is part of the original pigment. Sodium characteristic X-rays are of very low energy, and they are especially difficult to detect in lead-rich environments like the ones discussed here, where they become strongly attenuated and are often not picked up. Similarly, nothing can be said about tartar, whether it had been present as an ingredient in the pigment recipe. In fact, potassium was found in all test tiles, regardless of what mixture was applied to them. Moreover, the pigments with the highest proportion of tartar do not result in increased quantities of K2O in the relative tiles. We conclude that most tartar is either lost during the glazing procedure or that it remains below the detection limit of the equipment so that what we do see comes from the white glaze only.
ConclusionsAnalyses with techniques commonly employed in the in situ characterisation of yellow pigments suggest that some important modifications occur that affect the colour, chemical composition and crystallographic structure of the pigments. We noted that once pigments are fired onto tiles, the colour of yellow decorations seems to become more similar, with obvious consequences on our ability to distinguish between binary and ternary variants of Naples yellow. Only modified Pb–Zn–Sb pigments are sufficiently different, exhibiting a marked orange hue. Besides colour, we argue that distinguishing is also an issue when using μ-Raman alone, since some characteristic features of modified pyrochlore structures appear in binary pigments as a result of glaze-pigment interaction.
In this sense, we raise a note of caution when identifications of modified Naples yellow on glazed ceramics or other vitreous materials are achieved through comparisons with spectra from un-painted (in powder form) reference pigments. Even when XRF data is available, our experiments show that only zinc-modified variants can be securely identified, as the pigment is usually the sole possible source of zinc. On the other hand, the migration of tin from the white glaze underneath or its detection through thin and micro-fractured layers of pigment makes it virtually impossible to establish whether tin was part of the original pigment recipe.
To achieve a secure identification of each Naples yellow variant, the analysis of discrete pigment crystals within the glass phase is required. Mimoso et al. [19] were able to make the distinction between unmodified and tin-modified Naples yellows through SEM-EDS, in polished cross-section samples from 16th-century azulejo panels from Lisbon, though data is still very limited. In collaboration with the Museu Nacional do Azulejo, we aim to reproduce this methodology in historical tiles. For now, the combination of experimental re-working of historical recipes and an in situ analytical approach is capable of securely identifying the Zn-modified Naples yellow, which, contrary to what has been identified in other parts of Europe, seems to have been the main yellow pigment used in Portuguese and Spanish tiles between the late 16th and the early 18th centuries.
FundingThis work is the result of the projects “ChromAz: the Chromatic Journey of the Portuguese Azulejo” (PTDC/HAR-HIS/1899/2020), White Glazes (CEECIND/00882/2017), and LumiGLAD (2020.00252.CEECIND). Funding has been provided by the Fundação para a Ciência e a Tecnologia (FCT); The work was carried out at the VICARTE Research Unit (UIDB/00729/2020 and UIDP/00729/2020), the Associated Laboratory for Green Chemistry – Clean Technologies and Processes (LA/P/0008/2020) and LIBPhys-UNL Research Unit (UIDB/04559/2020 and UIDP/04559/2020).
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are indebted to Dr. Mathilda L. Coutinho for providing her expertise in re-working the white glaze recipe, to Dr. Paula Nabais for helping with the initial colorimetric measurements, and to Dr. Teresa Palomar for reviewing the Spanish text.