In diabetes mellitus (DM) patients retinal complications were typically considered part of a vascular process. Recent research suggests that retinal degeneration in DM might also be caused by a neuropathy that could precede microvascular alterations. The present work reviews the currently available bibliography about neurodegeneration in patients with type 2 DM (DM2) without diabetic retinopathy (DR).
In patients with non-severe, early DM2 without DR and good metabolic control visual function parameters show early abnormalities that precede clinical DR (in which we diagnose with a conventional ophthalmological examination). Using optical coherence tomography (OCT) technology, a reduction in macular and peripapillary thickness has been observed in different studies. Recent researches suggest that systemic complications (especially ischaemia) and a possible microvascular alteration eventually contributes to retinal neurodegeneration, which opens the door to new studies that include new techniques for evaluating the microvascularization of the retinal layers.
La retinopatía diabética (RD) tradicionalmente se ha considerado parte de un proceso vascular. Investigaciones recientes sugieren que la degeneración de la retina en la diabetes mellitus (DM) podría ser causada también por una neuropatía y que la neurodegeneración retiniana precedería a las alteraciones microvasculares. El presente artículo revisa la bibliografía existente sobre neurodegeneración en pacientes con DM tipo 2 (DM2) sin RD.
En los pacientes con DM2 no severa, temprana, con buen control metabólico y sin RD, las pruebas de función visual muestran anormalidades precoces que anteceden a la aparición de la RD clínica (la que diagnosticamos con una exploración oftalmológica convencional) Utilizando la tomografía de coherencia óptica (OCT) se observa que en estos pacientes existe una disminución en el espesor de distintas capas de la retina tanto en el área macular como peripapilar. Recientes estudios sugieren que las complicaciones sistémicas (especialmente la isquemia) y una posible alteración microvascular contribuyen a la neurodegeneración retiniana, lo que abre la puerta a nuevos estudios que incluyan nuevas técnicas de evaluación de la microvascularización de las capas internas de la retina como la angio-OCT.
Diabetes mellitus (DM) encompasses a group of metabolic diseases characterised by chronic hyperglycemia secondary to defects in insulin secretion or resistance to insulin action, triggering a state of uncontrolled hyperglycemia that over time can produce damages in many organs and systems.1
The American Diabetes Association has classified DM into 4 main categories1:
- 1
Type 1 DM (DM1). It accounts for 5–10% of cases of DM and is characterised by an absolute insulin deficiency.
- 2
Type 2 DM (DM2). It accounts for 90% of cases of DM. The predominant common factor is resistance to the peripheral action of insulin.
- 3
Gestational DM. This subtype is diagnosed during the second or third trimester of pregnancy and is enabled by the hormonal changes of pregnancy and genetic susceptibility.
- 4
Diabetes specific to other types. This group includes subclasses of DM such as monogenic diabetes (called MODY, Maturity Onset Diabetes of the Young), diabetes caused by drugs (immunosuppressants, glucocorticoids, etc.) and diabetes caused by diseases of the exocrine pancreas (such as cystic fibrosis).
The global prevalence of adults with DM is 8.8%, approximately 425 million, and is projected to rise to about 629 million by 2045. The largest increase will occur in regions where the economy is shifting from low to middle income; it is estimated that about one third of cases are due to population growth and ageing.2 In high-income countries we can estimate the prevalence of DM2 at 87–91% and DM1 at 2–7% of the total. In Europe the overall prevalence of DM adjusted for age and sex is 6.8%.1
Complications from DM are among the leading causes of early death. In 2012, the World Health Organization estimated that approximately 1.5 million people died from DM and 2.2 million more from its complications, mainly cardiovascular.2
The main epidemiological problem related to DM2 is that in some cases the disease may remain asymptomatic for months or years, so that up to 50% of adults with DM are still undiagnosed.2 This has led to the establishment of stringent criteria for screening and diagnosis. In 2006, the United Nations General Assembly defined diabetes as "a chronic, debilitating and costly disease with serious complications, posing great risks to families, member states and the world as a whole".3
It is therefore a major public health concern due to its high and increasing prevalence and morbidity and mortality, arising from an ageing population (due to an increase in life expectancy) and lifestyle in developed countries (with less healthy diets and a decrease in physical activity). Both diabetes and its complications generate large losses in the form of direct medical costs and loss of work and income.1,2
Etiopathogenesis and risk factors of diabetes mellitusDM1, also known as insulin-dependent diabetes, is due to an autoimmune reaction in which the body itself attacks the beta cells responsible for insulin production in the pancreas, causing an absolute deficit of the hormone that requires daily exogenous administration in order to control blood glucose levels. This disease will not be the subject of this review.
DM2 (the most common and the subject of this review) is due to a progressive loss of insulin secretion by beta-pancreatic cells, often related to the development of insulin resistance which attempts to be compensated by increased production of the hormone by the pancreas and leads to a progressive failure of insulin secretion. Pancreatic insulin production may become insufficient to control blood glucose, with high levels occurring despite being asymptomatic. In these cases, there is a latent clinical manifestation that may go unnoticed for years and complications may be encountered at the time of diagnosis.
DM2 has a multifactorial origin, with a significant genetic burden, a sedentary lifestyle and obesity among the possible triggers. This category of DM was frequently diagnosed after the age of 30. although the increase in childhood and youth obesity is enabling its appearance at increasingly younger ages.
The following classical risk factors4 have been described for the development of DM2:
- -
Previous diagnosis of pre-diabetes.
- -
Obesity due to diet, lack of physical activity and other factors including individual and genetic predisposition.
- -
First-degree family history of DM2.
- -
Dyslipidemia.
- -
High blood pressure (HBP).
- -
Belonging to high-risk ethnicities (African American, Hispanic American, Native American, Asian American and Pacific Islander).
- -
Personal history of gestational diabetes or delivery of a macrosomic foetus (> 4 kg).
The sustained presence of high blood glucose levels in DM damages different organs and systems leading to the following complications:
- 1
Microvascular complications due to dysfunction of small vessels causing diabetic retinopathy (DR), diabetic nephropathy and diabetic neuropathy.
- 2
Macrovascular complications, due to dysfunction of large blood vessels, responsible for cardiovascular disease, cerebrovascular disease and peripheral arterial disease.
Diabetic neuropathy has been observed in up to 50% of patients4 and is the most frequent complication of DM. Of the different forms of presentation, the most prevalent are distal symmetrical polyneuropathy and autonomic neuropathy.
The precise mechanisms underlying this complication remain unclear. Blood glucose levels are known to play a major role.5,6 Different studies seem to stipulate 2 types of neural damage mechanisms in DM: vascular and metabolic.5
It has been suggested that changes of vascular origin would be responsible for the onset of neuropathy, since morphological changes of the vasa nervorum are present early in the course of the disease. Sustained hyperglycemia produces a metabolic cascade that causes advanced glycation products to accumulate, leading to endothelial dysfunction which results in decreased blood flow leading to hypoxia of the nerve cells. Other observed alterations include thickening of the vascular walls and neovessel formation, as well as aberrant regeneration of nerve fibres, forming microfascicles that may compress the vessel lumen.5
Both vascular-ischemic and metabolic disorders interact in a complex way and lead to long-term dysfunction of nerve fibre repair mechanisms.5
Diabetic retinopathyClassification of diabetic retinopathyThe classification proposed by the Early Treatment Diabetic Retinipathy Study (ETDRS) is considered as a reference to be followed in clinical trials. However, it is not commonly used in clinical practice due to its complexity.
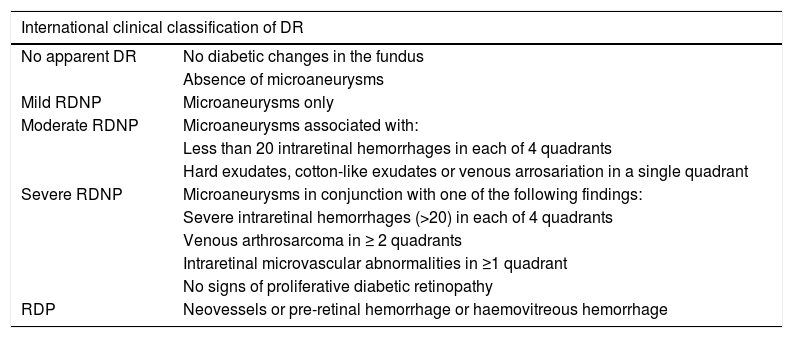
In an attempt to achieve a universal means of communication, in 2002 a group of experts (Global Diabetic Retinopathy Project Group [GDRPG]) proposed a more user-friendly classification for DR: the international retinopathy severity scale (Table 1). This classification is used at present and is based on the results of the ETDRS, therefore supported by scientific evidence. It is not intended to displace the original classification, but aims to provide a simpler and more appropriate management basis for routine clinical practice.7
International clinical classification of DR.
| International clinical classification of DR | |
|---|---|
| No apparent DR | No diabetic changes in the fundus |
| Absence of microaneurysms | |
| Mild RDNP | Microaneurysms only |
| Moderate RDNP | Microaneurysms associated with: |
| Less than 20 intraretinal hemorrhages in each of 4 quadrants | |
| Hard exudates, cotton-like exudates or venous arrosariation in a single quadrant | |
| Severe RDNP | Microaneurysms in conjunction with one of the following findings: |
| Severe intraretinal hemorrhages (>20) in each of 4 quadrants | |
| Venous arthrosarcoma in ≥ 2 quadrants | |
| Intraretinal microvascular abnormalities in ≥1 quadrant | |
| No signs of proliferative diabetic retinopathy | |
| RDP | Neovessels or pre-retinal hemorrhage or haemovitreous hemorrhage |
DR: diabetic retinopathy; DRNP: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy.
In our setting, DR is the most frequent microvascular complication of DM2 and the most frequent cause of blindness in the DR population, accounting for 1.9% of moderate and severe visual impairment worldwide, and 2.6% of cases of blindness.8
Approximately 30% of patients with DM have some degree of DR and up to 10% of these have advanced DR with severe visual impairment.9
The onset and progression of DR is conditioned by the duration of DM and its metabolic control, which in turn is the main objective of the treatment of the disease. The role of other cardiovascular risk factors such as hypertension, dyslipidemia, obesity, diabetic nephropathy and the presence of macroalbuminuria in advanced stages is also known. As in diabetic neuropathy, hyperglycemia and the metabolic alterations it generates are responsible for the appearance of microvascular lesions in the inner layer of the retina.10
The first structural alterations to appear are thickening of the vessel basement membrane, loss of pericytes and rupture of the tight junctions between endothelial cells. This decrease in the number of pericytes facilitates the formation of focal hemorrhages and microaneurysms. Thickening of the basement membrane and endothelial damage will lead to increased membrane permeability, which may lead to extravasation of fluid into the interstitial space and, secondarily, thickening of the retina and formation of hard exudates.9
The progression of said damage in the retina gives rise to a depletion of endothelial cells, favouring thrombosis and leukocyte adhesion to the damaged vascular wall. This may occlude the capillary lumen, causing hypoxia in the adjacent retina. At this stage, ophthalmoscopic examination reveals soft or cotton-like exudates, which are infarcted areas of the retina, as well as alterations in the intraretinal microcirculation (IRMA, Intraretinal Microvascular Abnormality). In the late stages of DR the degradation products of cell basement membrane destruction, together with hypoxia-stimulated antigenic agents, will facilitate the formation of neovessels. Neovascularisation produces anchorages in the posterior vitreous fibrosis, which can lead to tractional retinal detachment or can produce massive hemorrhages within the vitreous humour.9
Diabetic macular edema is relatively common in DM2 and is due to a breakdown of the blood-retinal barrier with extravasation of the contents into the interstitium, leading to protein accumulation and increased oncotic pressure.8
Traditionally, DR has been considered a disease of the retinal microcirculation. However, a growing number of studies suggest that the process of retinal neurodegeneration occurs very early, simultaneously or even before the vascular process begins, as shown by the fact that signs of retinal neurodegeneration have been detected in diabetic donor eyes with no microvascular alterations.11,12
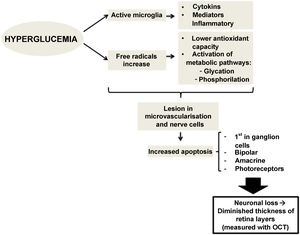
Retinal neurodegeneration in diabetes: vasculopathy or neuropathy?DR and retinal complications in patients with DM have traditionally been considered part of a vascular process. However, recent research suggests that retinal degeneration in DM may be caused not only by vasculopathy, but also (and importantly) by neuropathy.13
It is believed that retinal neurodegeneration may precede or even promote the development of microvascular alterations.14 However, the possible correlation between neuropathy and vasculopathy in this process is still unknown. The alterations that we observe in the fundus of patients with DR, microaneurysms and hemorrhages in the early stages, may be preceded by other vascular alterations not detectable in a routine fundus examination.
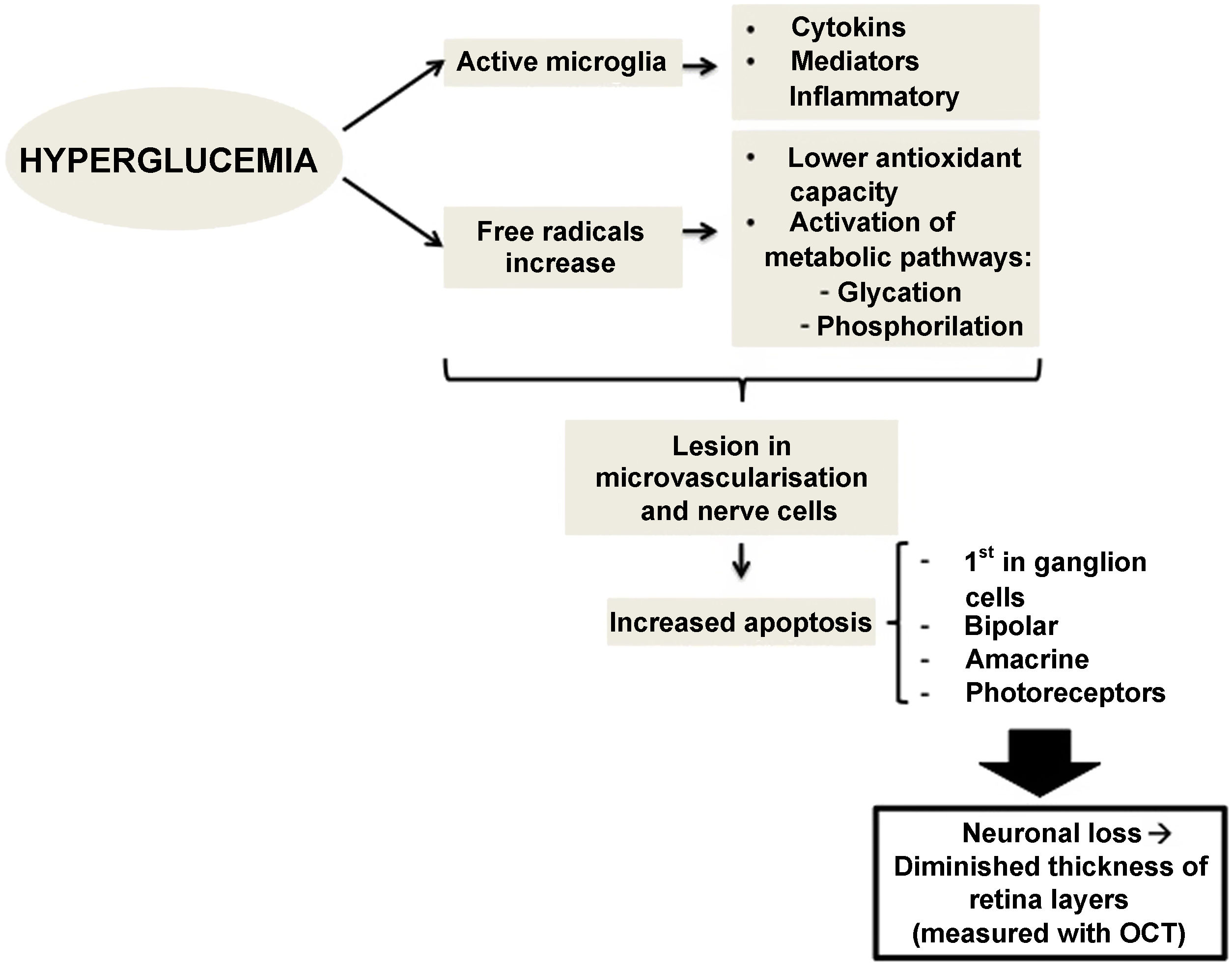
Therefore, preclinical DR (the type which we do not detect in a conventional ophthalmological examination) could be caused by changes in the calibre of retinal vessels or in the regulation of retinal blood flow.15,16 However, changes affecting retinal neurons have also been observed, such as increased apoptosis of retinal ganglion cells and activation of microglia without signs of vascular changes.11,17 Neuronal apoptosis starts in the ganglion cells of the inner retinal layer, but can also compromise other retinal nerve cells, such as bipolar cells, amacrine cells and photoreceptors.18,19 Several studies have shown this neuronal loss, which manifests as thinning of some retinal layers20.21. Alterations at the level of molecular mediators occurring from early stages of the disease have been stipulated to produce an imbalance between pro-apoptotic and cell survival signals.17 Alterations in extracellular glutamate,22 increased oxidative stress and photoxidation by-products23 have been described as capable of causing injury at the level of retinal nerve cells and microvessels.24,25
There is evidence that hyperglycemia leads to activation of microglia. Activated glial cells produce large amounts of cytokines and inflammatory mediators, which may play a role in the pathogenesis of retinal neurodegeneration by disrupting the blood-retinal barrier and decreasing neurovascular coupling, the mechanism by which vessels regulate their flow in response to a nerve signal26 (Fig. 1).
Functional alteration of the retina in diabetesFunctional studies, such as electroretinogram (ERG), colour vision or contrast sensitivity tests, also show early abnormalities in DR, even before it is diagnosed.
The ERG is a fundamental tool in the evaluation of retinal function alterations and characteristic alterations in the recording (lower amplitude in voltages and greater latency in the appearance of oscillatory potentials) have been observed in both animal models and in diabetic humans, even in the absence of any microvascular alteration.27,28 In a recent study of over 400 patients with DM2, 58% of patients showed alterations in ERG recording in the absence of vascular lesions indicative of DR.13 Using multifocal ERG, it has even been observed that electrophysiological changes in the retina of an animal model for DM2 precede in time both the retinal vascular lesions and the cognitive alterations typical of this disease.29,30 All this suggests the presence of neurodegeneration prior to retinal (and even systemic) vascular alterations.
Likewise, other electrophysiological studies with visual evoked potentials have detected early changes in their results in people without DR.31,32 Lee et al. recently demonstrated an increase in P100 wave latency in newly diagnosed diabetic patients (type 1 and type 2) with apparently healthy retinas.31 This increased latency was even associated in those patients with DM2 with abnormally high levels of glycosylated hemoglobin (HbA1c) (not so in patients with DM1). In addition, this electrophysiological test has also demonstrated changes in latency in retinas of children born to mothers with gestational diabetes, especially those who are significantly overweight.33
Tests of other parameters of visual function in people with MD, such as contrast sensitivity34 and colour vision,35 have shown early abnormalities that precede the onset of clinical or established DR (which we detect on OF examination).
There are different conduction pathways for different frequencies in the processing of visual information and it is not known which pathways are most affected in MD.36 Therefore, the assessment of contrast sensitivity at different spatial frequencies is of great importance in these patients. Previous studies have barely observed loss of contrast sensitivity in type 2 diabetics without DR.36,37 However, other investigators have found changes affecting contrast sensitivity at all frequencies in patients with DM2 and without retinopathy.34,38 It seems that, in the presence of established DR, there is a decrease in contrast sensitivity,36 which may be independent of visual acuity impairment.34 However, there is not yet agreement as to whether this contrast sensitivity impairment begins before DR. It has been suggested that selective loss of contrast sensitivity for higher frequencies is a sign of parvocellular dysfunction (which is 80% of retinal ganglion cells).39 Correlation studies have also been published36,38 showing that patients with more than 10 years of disease progression or with poorer metabolic control have worse contrast sensitivity.38
Some studies have suggested the presence of changes in colour vision in patients with DM and the presence of DR, especially in the blue–yellow axis.40 This alteration in colour vision has also been observed in diabetic patients without DR35,41 and appears to be associated with the risk of developing diabetes, as well as retinal vascular alterations secondary to hyperglycemia.42 Colour vision is mainly cone-dependent, so these findings are in line with studies showing a decrease in the photoreceptor layer in the retina in the absence of visible microvascular changes.43,44 This suggests that dyschromatopsia is an early manifestation of neurological disorders in these patients.
Perimetry has also been studied in patients with DM. Visual field defects have been observed in patients with DM1 in stages of disease prior to the presence of typical DR changes.45,46 Similarly, it has been suggested that there may be diffuse visual field involvement in patients with DM2, even without DR, and that this involvement increases significantly with the amount and severity of retinal vascular changes.47 However, other authors failed to detect these changes in the perimetry of patients with DM2 without DR,48,49 or, despite detecting them, they showed no correlation with changes in retinal vasculopathy.50 A problem to highlight in these studies is that different perimetry devices were used and the patient samples were heterogeneous, mixing in most cases patients with and without DR and both types of DM. This could explain such dissimilar results.
On the other hand, other variables may influence the final results, e.g. differences in age, gender as well as cardiovascular risk factors (smoking, hyperlipidemia, obesity, etc.) or axial length may alter the optical coherence tomography (OCT) and act as confounding factors.
Visual function studies in DM have not yet succeeded in clarifying the vascular vs. neuropathic origin of DR. However, all these alterations observed in visual function tests, such as ERG,13 contrast34 sensitivity, colour35 vision and visual field scores,50,51 in patients with DM without DR or only minimal changes could support the theory of neurodegeneration in early stages of the disease. Moreover, diabetic neuropathy is associated with the same risk factors that produce other macrovascular and microvascular complications in this disease, such as dyslipidemia, poor metabolic control and oligoalbuminuria. Studies with animal models and human biopsies have shown that in diabetic neuropathy there is impaired microvascularisation of nerves, leading to endoneural hypoxia.52,53 Therefore, it could be suggested that, given that they share pathogenic mechanisms, it is possible that the presence of extraocular complications of diabetes, generated by vascular alterations, appear simultaneously with the presence of some subclinical dysfunction of retinal perfusion. This incipient vascular impairment could lead to hypoxia of retinal nerve structures, which manifests as impaired contrast sensitivity, colour vision, visual acuity and perimetry.
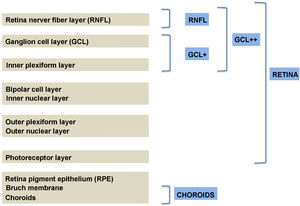
Structural alteration of the retina in diabetesCurrent structural studies aim to analyse whether DM2 produces a decrease in the thickness of the retinal nerve fibre layer (RNFL) and ganglion cell layer (GCL) that can be objectified using image analysis techniques.54 Until a few years ago, this assessment was performed by ophthalmoscopy and monochromatic retinal photography, which suggested the presence of early structural alterations in the RNFL of diabetic patients.55 However, these imaging techniques are not quantitative and have an important explorer-dependent variability, and a loss of more than 50% of the ganglion cells is necessary to detect defects in the RNFL, which makes them ineffective in early diagnosis.56
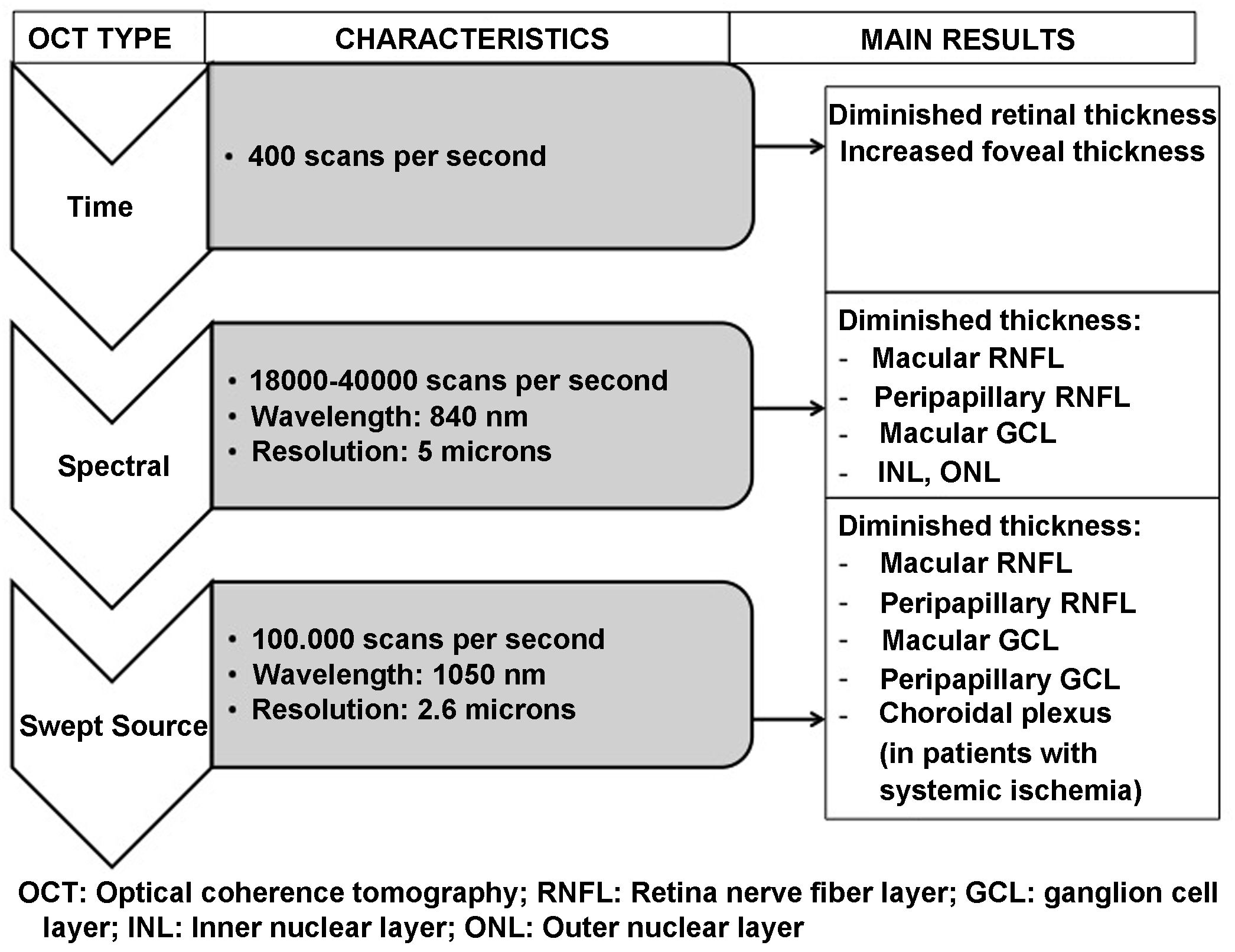
OCT is capable of obtaining images of the retina and optic nerve similar to a histological slice of the retina, performed in vivo and in real time. It allows the thickness of the different layers of the retina, in the macula and in the peripapillary area, to be quantified accurately, quickly and without harm to the patient. OCT systems have been evolving since their introduction in 1991. The most recent Swept Source OCT (SS-OCT) devices use longer wavelength light sources (1050 nm) reaching a scanning speed of 100.000 A-scans per second and producing better axial and transverse resolution. Accordingly, they perform analysis at a higher scanning frequency than their time-domain or spectral predecessors. This avoids the high reflectivity of the pigment epithelium and choroidal vasculature, allowing greater penetration of light at the tissue level and an even greater increase in resolution and scanning speed. This technology provides better quality examinations and analysis of deeper layers (reaching the choroid) compared to previous spectral domain technology.57,58
Many different studies have been published analysing retinal changes in patients with DM using OCT; however, none seem to clarify which comes first, neurodegeneration or vasculopathy. Those studies assessing whether alterations affecting the RNFL and other retinal layers precede any vascular changes in patients with DM have obtained opposing results,21,34,59 but a recent meta-analysis suggests that this may be the case.60
Early clinical studies using time-domain OCT pointed to increased macular thickness in subjects with DM (with and without DR) compared to healthy individuals.61 More recent research suggested a significant reduction in macular area in patients with DM without DR.44,62,63
Our own group performed a structural analysis of the retina (using Spectralis OCT) in MD patients without fundoscopic or OCT signs of DR, demonstrating a significant reduction in macular thickness in all ETDRS sectors.64
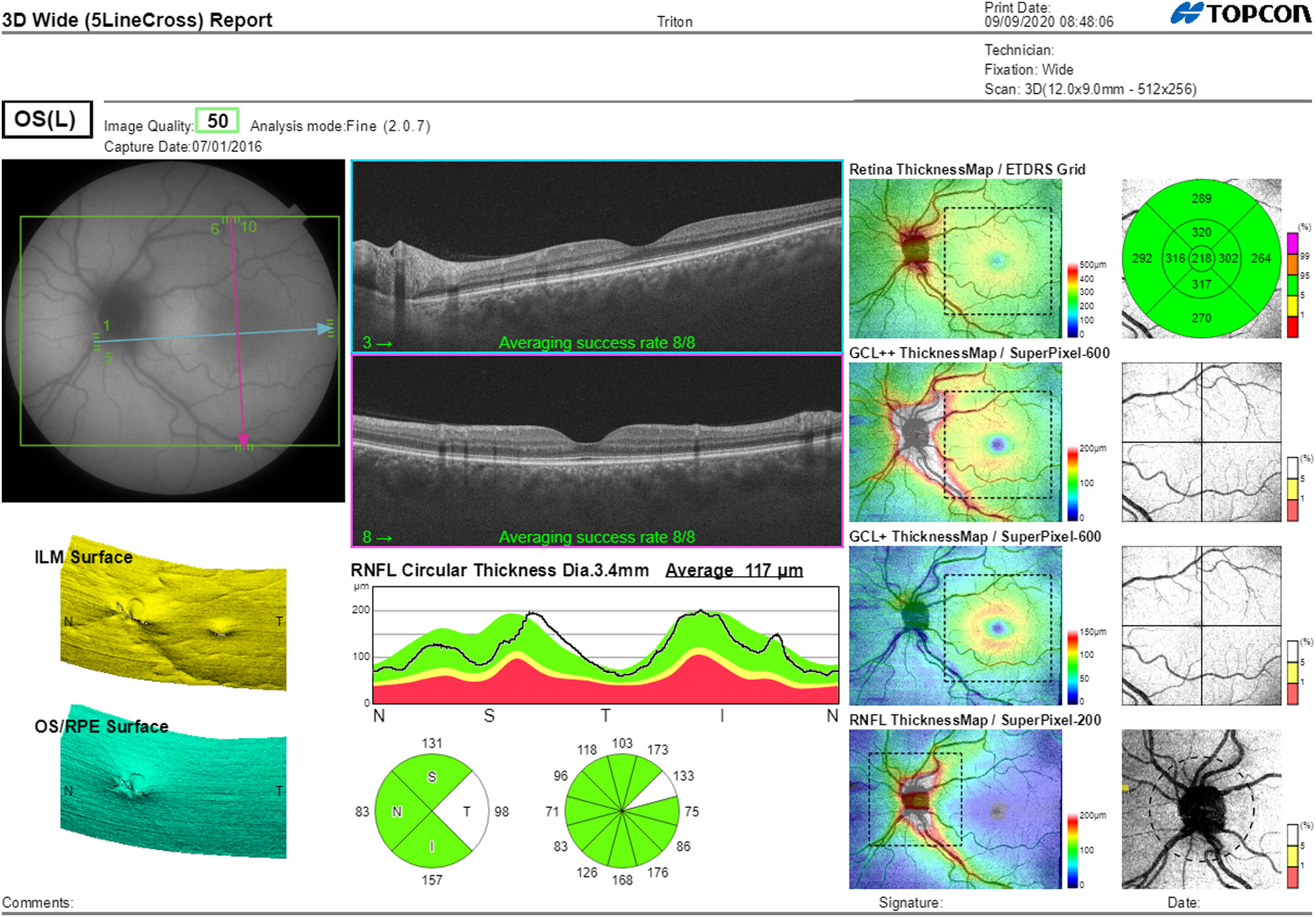
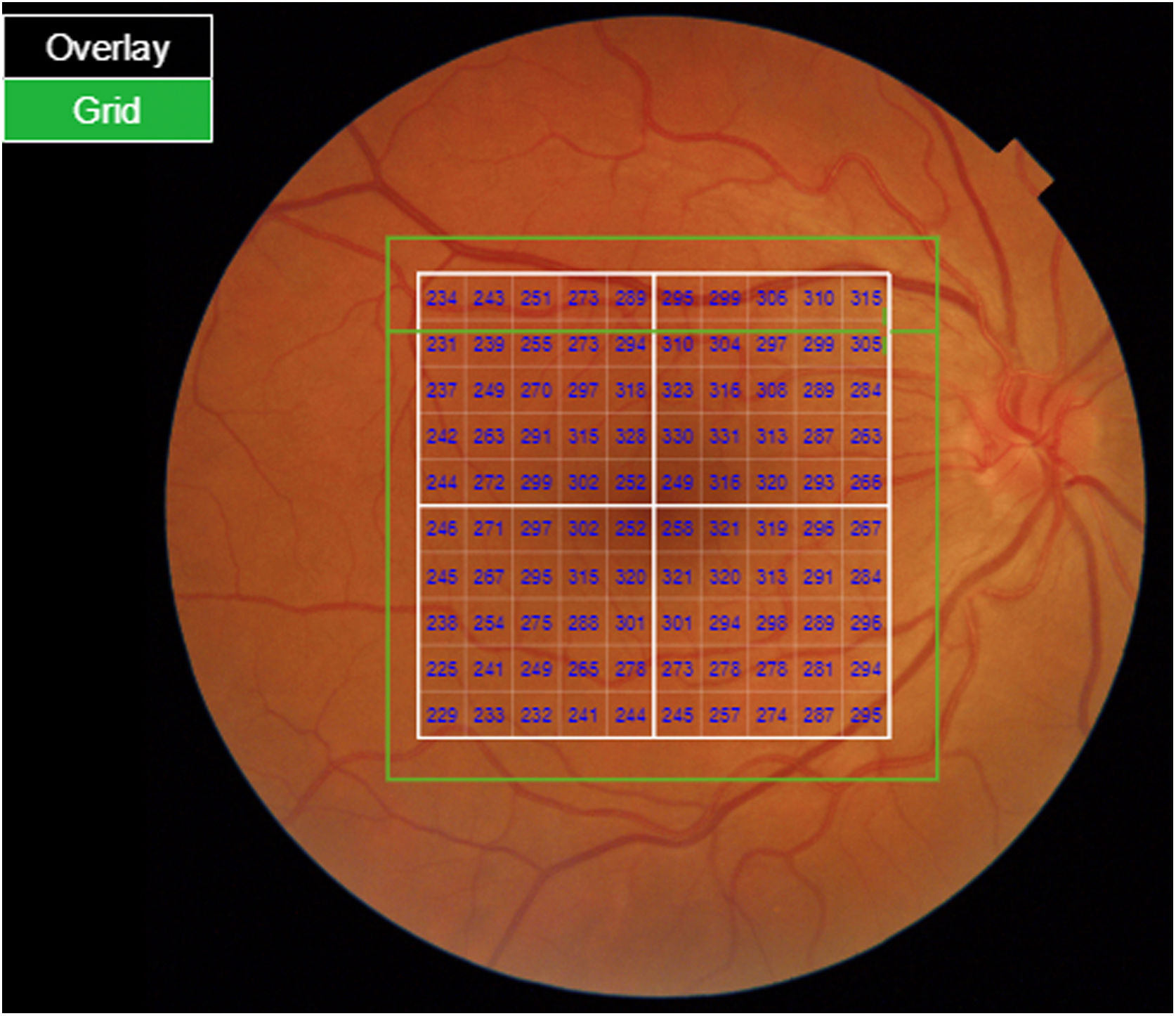
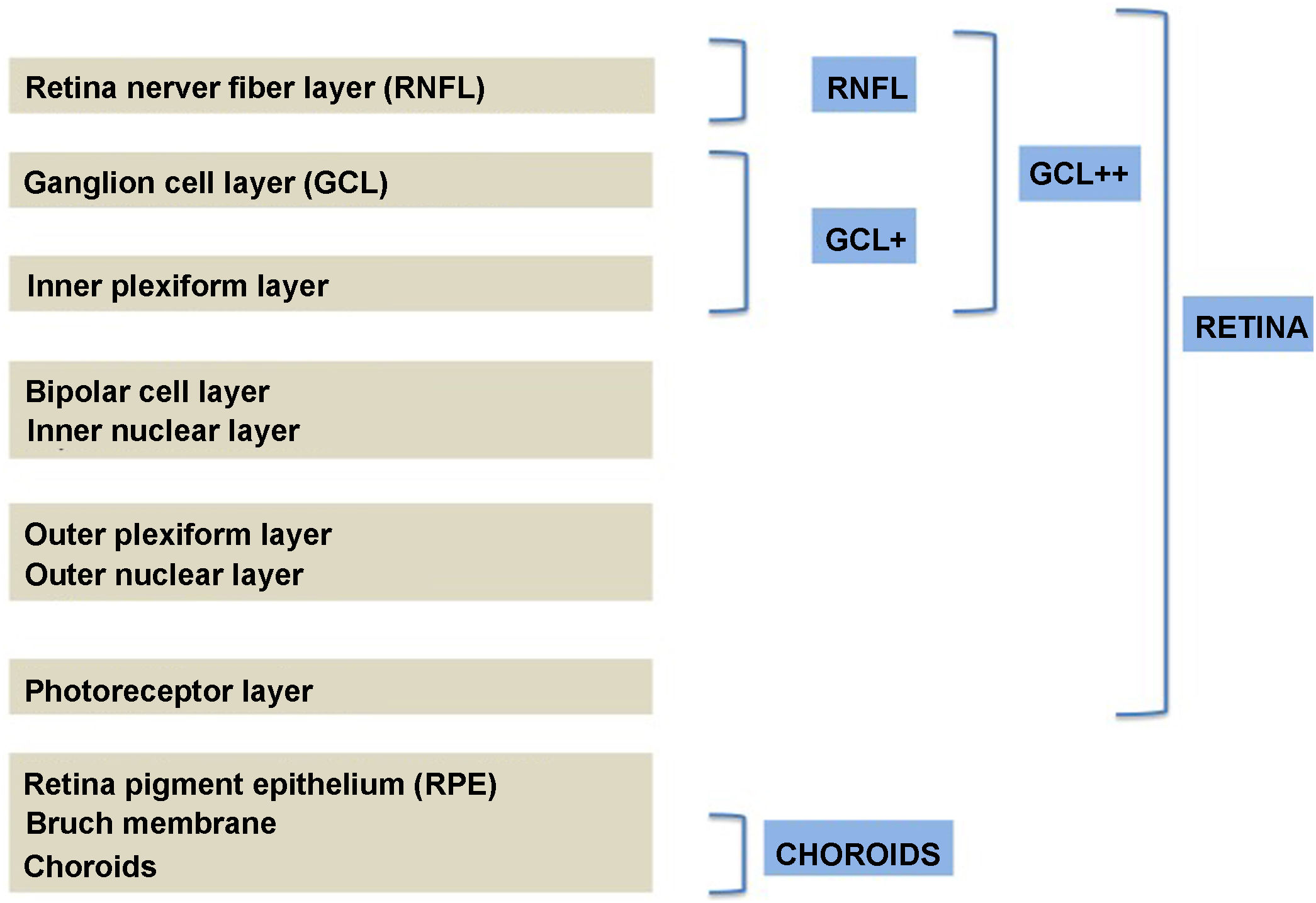
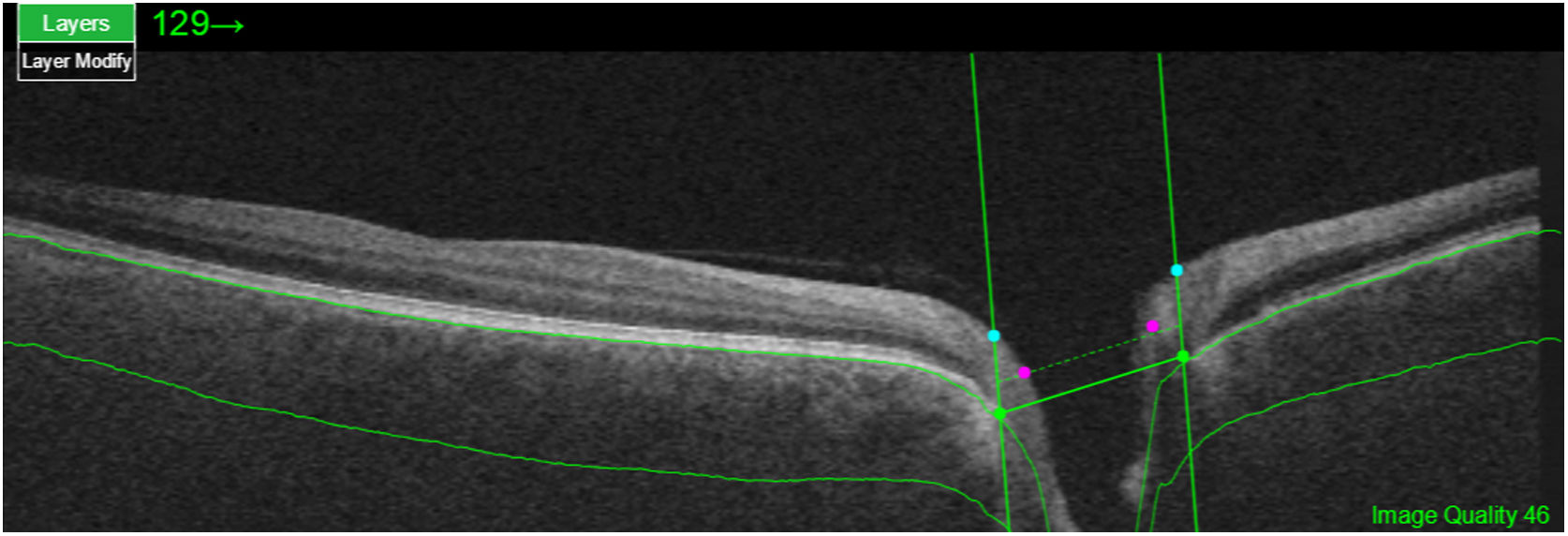
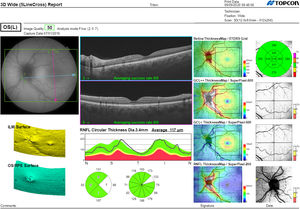
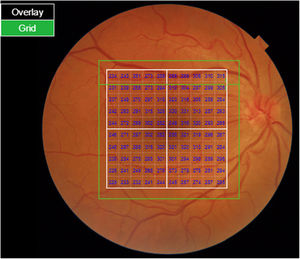
Segmentation of the different macular layers in patients with DM has also shown different results between studies: recent studies31,59,65,66 demonstrated a reduction of the macular area GCL in patients with DM (type 1 in the studies by Scarinci et al. and Gundogan et al.) without DR, but some of them could not find differences in macular RNFL thickness between the groups.65,66 Another recent study found a reduction of macular RNFL and ganglion cells in type 2 DM patients without DR, but changes in RNFL thickness were not significant after age correction for the diabetic group.67 These results suggest that neurodegeneration (i.e. ganglion cell degeneration) is present from the early stages of DM, but axonal loss (the RNFL) in the macular area may not be simultaneously detectable. In our study with Spectralis OCT we observed a reduction in GCL thickness affecting all sectors (except the fovea) but hardly any difference in macular RNFL thickness, which supports the studies mentioned above.64 More recently, our group has studied the retina of diabetic patients using the latest SS-OCT technology with the Triton device (Topcon Corporation, Tokyo, Japan) using Deep Range Imaging (DRI) technology.68 the study included 60 patients with DM2 without DR and 60 healthy controls. Recorded patient data comprised age at diagnosis, duration of disease, prescribed treatments and the presence of vascular complications (macrovascular: cardiovascular and cerebrovascular diseases, and microvascular: neuropathy and nephropathy). To perform the analysis we used the 3D Wide protocol of the device that includes a wide scanning range and focuses on both macular (ETDRS) and peripapillary (TSNIT: Temporal-Superior-Nasal-Inferior-Nasal-Temporal) scans (Fig. 2). To perform the macular segmentation we used the Super Pixel Grid-600 application of the Triton OCT. This application performs measurements of the macular area underlying a grid composed of 100 cells each measuring 600 μm × 6002 and provides an automated analysis of the different retinal layers in the cell area (Fig. 3). For the analysis, we calculated the mean value for each layer in 3 different composite zones: total (T), which is the sum of all mean values of the cells within the macular grid; superior (S), which is the sum of all mean values of the cells corresponding to the upper half of the macular grid; and inferior (I), which is the sum of all mean values corresponding to the lower half. All subjects in our study were treated by assessment for the ETDRS and TSNIT protocols (the latter includes the following layers: RNFL, the GCL+ [which includes measurements between the RNFL to the boundaries of the inner nuclear layer] and GCL++ [between the ILM and the boundaries of the inner nuclear layer] thickness measurements in the 4 basic quadrants and the 6 peripapillary sectors), and macular analysis with the Super Pixel Grid-600 application (which equally provides data for the layers: whole retina, RNFL, GCL+, GCL++ in the T, S and I zones) (Fig. 4). We also obtained choroidal thickness measurements for both the macular area (ETDRS and grid analysis) and the peripapillary area (Fig. 5).
Structural measurements of macular area with the Wide protocol (whole retina and choroidal thickness) showed significant retinal thinning in patients with DM2 compared to healthy subjects in all areas of ETDRS measured, except in the outer (T-sector). Total macular volume showed no difference between groups and the choroid plexus was not reduced in patients compared to controls. Macular thickness segmentation using the Super Pixel Grid-600 application showed a significant reduction in patients with DM2 in all layers, which would be in agreement with previous studies.68
Interestingly, we performed a post hoc ANOVA analysis to assess the influence of systemic ischemia on these measurements and observed that only differences in external areas of ETDRS and those related to GCL+ (total and higher), GCL++ (total and higher) and RNFL (higher) were due to DM2 alone.
In general, macular assessment in patients with DM without DR should be analysed with caution as macular thickness (often affected by macular edema occurring in DR) in patients with DM varies greatly with disease progression and does not have a uniform behaviour. Retinal damage caused by disease progression may trigger an immune response leading to intra- and extracellular inflammation and macular changes secondary to the presence of edema. In addition, vascular alterations may mask other concomitant neurodegenerative processes affecting the same area of the retina.69,70
The peripapillary area contains a higher density of axons (RNFL) compared to the macular area and, therefore, analysing ganglion cells and RNFL thickness in this area could be the best option to detect neurodegeneration early in these patients, being the optimal location to assess axonal loss caused by the disease. Many studies have evaluated by OCT the thickness of the RNFL in the peripapillary area in patients with DM2 without DR, finding a significant thinning.44,71 Several authors have described a decrease in the overall thickness of the RNFL in patients with DM2 without DR.59,62,72,73 Carpineto et al.73 demonstrated additional damage to the RNFL, as they observed a reduction in RNFL involving all peripapillary quadrants (superior, inferior, nasal and temporal). However, this reduction in the lower quadrant had only been published by Carpineto et al.,73 Salvi et al.59 and recently by Mehboob et al.71 who included the largest sample sizes in their study.
Our group analysed peripapillary RNFL using 2 different Spectralis OCT protocols: the glaucoma application (which is the one most widely used in routine clinical practice): RNFL-G and the axonal application (RNFL-N); the latter protocol was specifically designed for the assessment of neurodegenerative damage in the peripapillary area.74,75 In our patients, we found a significant reduction in RNFL thickness compared to controls not only in the average value, but especially in the infero-temporal sectors. We also found a reduction in the papillary-macular bundle suggesting a degeneration of the axonal pathway between the optic disc and the macular ganglion cells.64 These results also present anatomical correspondence with the distribution of the macular ganglion cells whose axons correspond to the RNFL of the temporal and inferior sectors of the optic disc.
This involvement of the temporal sectors had hardly been observed in previous studies73 and the most important studies on this subject found no differences involving the temporal quadrant. Since the inferior sector is the most frequently affected by hypoxia, it is reasonable to find a significant reduction in this quadrant. The reduction of temporal areas could also indicate the presence of neurodegeneration due to different causes other than hypoxia, similar to those observed in neurodegenerative diseases.76,77
In contrast to the macular area, the GCL of the peripapillary area has hardly been studied in subjects with DM2 to date. Analysis of the GCL at the papillary level provides more information on neurodegeneration in these patients, since the RNFL is mostly the axonal extension of ganglion cells located at the macular level, but does not provide sufficient data on the ganglion somas in the peripapillary area. In 2018, Şahin et al. found significant alterations in the nasal quadrant of this layer in a group of patients with prediabetes.78 Our group performed peripapillary analysis with the Triton OCT device and observed a significant reduction in the group of patients affecting the average, superior, inferior and infero-temporal RNFL thickness. However, only the inferior quadrant was independent of systemic ischemia. We also observed a significant reduction in the GCL++ layer (hardly assessed by previous investigations) affecting different sectors but these changes were also influenced by systemic ischemia in these patients.68
As previously discussed, our group assessed whether systemic ischemia plays a role in retinal degeneration in these patients. Those with chronic systemic vascular complications had an additional reduction in RNFL and GCL + layer in the temporal quadrant, suggesting that systemic ischemia contributes to further damage in retinal neurodegeneration. Also, GCL + thickness was reduced in the group with longer disease duration, indicating the presence of different factors contributing to neuronal damage. In our study, systemic ischemia was considered a confounding factor affecting retinal measurements, which might even explain the differences between previously published studies. We observed that systemic ischemic complications in these patients may explain several areas showing retinal thinning and could therefore affect the overall results.
These results should, however, be analysed with caution as in our study we did not assess retinal ischemia with either fluorescein angiography or OCT angiography (OCTA), i.e. we did not include purely vascular tests to determine the presence or absence of vascular alterations and retinal ischemia not observable by fundoscopy or OCT/retinography. Nevertheless, it is interesting to note that when taking into account the systemic ischemia factor, which may reflect underlying retinal ischemia, different results are obtained68; this suggests a possible microvascular alteration in these patients that eventually contributes to retinal neurodegeneration and opens the door to further studies including new techniques to assess the microvascularisation of the inner retinal layers.
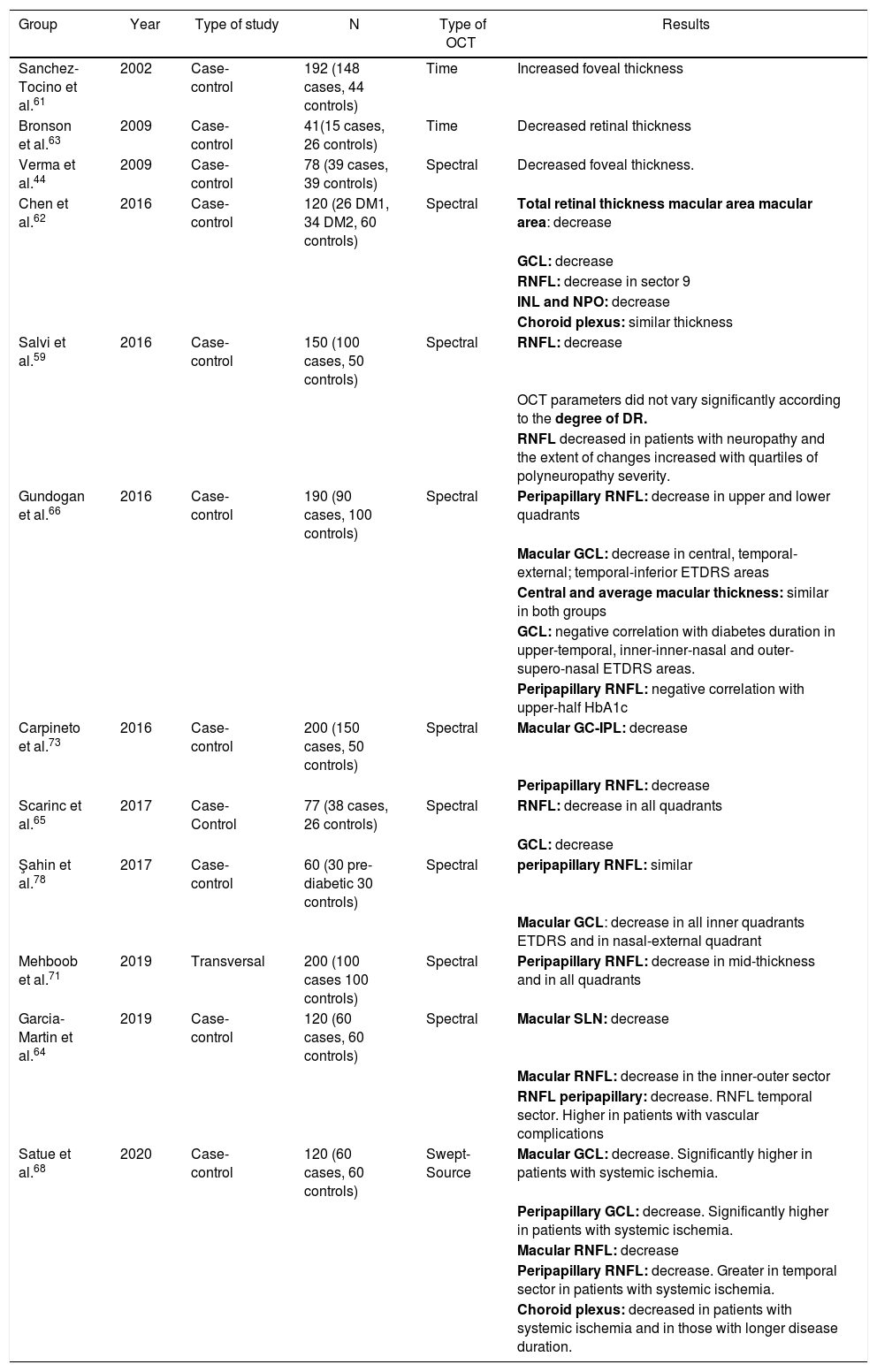
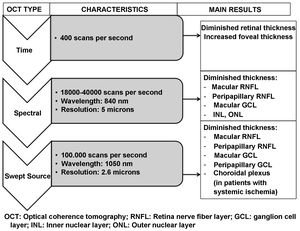
Fig. 6 and Table 2 summarise the main studies that have been published to date on retinal structure (studied by OCT) and neurodegeneration in patients with DM2.
Schematic summary of the evolution of the different optical coherence tomography (OCT) devices, their characteristics and the main results obtained in type 2 diabetic patients without diabetic retinopathy.
GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer; RNFL: retinal nerve fibre layer.
Results of major published studies on retinal structure and retinal neurodegeneration in patients with DM2.
| Group | Year | Type of study | N | Type of OCT | Results |
|---|---|---|---|---|---|
| Sanchez-Tocino et al.61 | 2002 | Case-control | 192 (148 cases, 44 controls) | Time | Increased foveal thickness |
| Bronson et al.63 | 2009 | Case-control | 41(15 cases, 26 controls) | Time | Decreased retinal thickness |
| Verma et al.44 | 2009 | Case-control | 78 (39 cases, 39 controls) | Spectral | Decreased foveal thickness. |
| Chen et al.62 | 2016 | Case-control | 120 (26 DM1, 34 DM2, 60 controls) | Spectral | Total retinal thickness macular area macular area: decrease |
| GCL: decrease | |||||
| RNFL: decrease in sector 9 | |||||
| INL and NPO: decrease | |||||
| Choroid plexus: similar thickness | |||||
| Salvi et al.59 | 2016 | Case-control | 150 (100 cases, 50 controls) | Spectral | RNFL: decrease |
| OCT parameters did not vary significantly according to the degree of DR. | |||||
| RNFL decreased in patients with neuropathy and the extent of changes increased with quartiles of polyneuropathy severity. | |||||
| Gundogan et al.66 | 2016 | Case-control | 190 (90 cases, 100 controls) | Spectral | Peripapillary RNFL: decrease in upper and lower quadrants |
| Macular GCL: decrease in central, temporal-external; temporal-inferior ETDRS areas | |||||
| Central and average macular thickness: similar in both groups | |||||
| GCL: negative correlation with diabetes duration in upper-temporal, inner-inner-nasal and outer-supero-nasal ETDRS areas. | |||||
| Peripapillary RNFL: negative correlation with upper-half HbA1c | |||||
| Carpineto et al.73 | 2016 | Case-control | 200 (150 cases, 50 controls) | Spectral | Macular GC-IPL: decrease |
| Peripapillary RNFL: decrease | |||||
| Scarinc et al.65 | 2017 | Case-Control | 77 (38 cases, 26 controls) | Spectral | RNFL: decrease in all quadrants |
| GCL: decrease | |||||
| Şahin et al.78 | 2017 | Case-control | 60 (30 pre-diabetic 30 controls) | Spectral | peripapillary RNFL: similar |
| Macular GCL: decrease in all inner quadrants ETDRS and in nasal-external quadrant | |||||
| Mehboob et al.71 | 2019 | Transversal | 200 (100 cases 100 controls) | Spectral | Peripapillary RNFL: decrease in mid-thickness and in all quadrants |
| Garcia-Martin et al.64 | 2019 | Case-control | 120 (60 cases, 60 controls) | Spectral | Macular SLN: decrease |
| Macular RNFL: decrease in the inner-outer sector | |||||
| RNFL peripapillary: decrease. RNFL temporal sector. Higher in patients with vascular complications | |||||
| Satue et al.68 | 2020 | Case-control | 120 (60 cases, 60 controls) | Swept-Source | Macular GCL: decrease. Significantly higher in patients with systemic ischemia. |
| Peripapillary GCL: decrease. Significantly higher in patients with systemic ischemia. | |||||
| Macular RNFL: decrease | |||||
| Peripapillary RNFL: decrease. Greater in temporal sector in patients with systemic ischemia. | |||||
| Choroid plexus: decreased in patients with systemic ischemia and in those with longer disease duration. |
DM1: diabetes mellitus type 1; DM2: diabetes mellitus type 2; ETDRS: Early Treatment Diabetic Retinopathy Study; GCL: ganglion cell layer; HbA1: glycated hemoglobin; INL: inner nuclear layer; IPL: inner plexiform layer; OCT: optical coherence tomography; ONL: outer nuclear layer; RNFL: retinal nerve fibre layer; OCT: optical coherence tomography.
In bold, layers and sectors of the retina in which changes have been detected.
Although the aforementioned studies suggest the presence of clear neuronal degeneration, there is some discordance in some aspects of retinal thickness in patients with DM who do not have DR. Several studies have found no differences between diabetics without DR lesions and the healthy population in the thickness of the RNFL13,20 or find differences only in the subgroup with DM2,63 but not in the subgroup with DM1. The striking disparity in the results of these studies may be explained by the heterogeneity of the study designs, which often include type 1 and type 2 diabetics, with and without DR, and the different OCT devices used for structural analysis. Many of these studies were performed with older OCT devices with lower resolving power than current Swept Source or spectral domain OCT.
One of the largest studies that has been performed on the changes observed by OCT in patients with DM concluded that retinal thinning in these patients is caused by both vascular changes and neurodegeneration and suggests 2 different phenotypes, one vascular (in which neurodegeneration is absent or plays a minimal role) and the other (up to 60% of patients) more prone to neurodegeneration without vascular changes.13 However, it did not establish whether microvascular (subclinical) alterations played an important role in the pathogenesis of retinal changes in the neurodegenerative phenotype and whether patients with neurodegenerative changes are more likely to develop microvascular disease.
The results of recent studies carried out by our group suggest that, in patients with non-severe, early, type 2 DM without DR and good metabolic control, neurodegeneration caused by DM is present, especially affecting neurons in the macular area and the RNFL in the inferior quadrant of the optic disc. Furthermore, they suggest that systemic ischemia may be an important factor contributing to neurodegeneration in DM2. Our results add to previous evidence of neurodegeneration observed by other working groups and provide new data on the peripapillary GCL that were scarcely evaluated by previous investigators. OCT Triton provides high-quality images and performs automated segmentation of the choroid, facilitating the study of this vascular layer in patients with DM2. The future of retinal neurodegeneration studies in these patients is expected to focus on the use of new imaging techniques such as OCT-A to establish the possible role of subclinical vascular alterations in the pathogenesis of retinal neurodegeneration.
The detection of this neurodegeneration in early stages of DM2 could have important therapeutic implications as it seems reasonable to think that these patients would benefit from therapeutic strategies based on neuroprotection. This opens the possibility of developing topical or systemic therapy with neuroprotective drugs in the early stages of DR, where currently available therapies (such as laser photocoagulation or intravitreal injections) have no role.
FundingThis research has not received specific support from public sector agencies, the commercial sector or non-profit organisations.
Conflict of interestNo conflicts of interest were declared by the authors.
Please cite this article as: Ciprés M, Satue M, Melchor I, Gil-Arribas L, Vilades E, Garcia-Martin E. Neurodegeneración retiniana en pacientes diabéticos tipo 2 sin retinopatía diabética. Arch Soc Esp Oftalmol. 2022;97:205–218.