Background: Uncontrolled hapatic inflammatory response is regarded as the primary pathological mechanism of acute liver failure and impairs the regeneration of hepatocytes and stem cell grafts. Interleukin-1 plays a key role for activating immune and inflammatory response. Recently, siRNA has made quite a few progresses in treating inflammatory response.
Aim. To assess the effect of IL-1β siRNA adenovirus on MSC and the therapeutic effect of MSC combined with IL-1β siRNA adenovirus in ALF.
Material and methods. We implanted MSC or/and IL-1β siRNA adenovirus via the tail vein, using CCl4-induced ALF in a mice model. Mice were sacrificed at different time points. Blood samples and liver tissues were collected. Hepatic injury, liver regeneration, cytokines (CXCL1, IL-1β, IL-10, IL-6, VEGF and HGF), animal survival and vital MSC were assessed after cell transplantation.
Results. MSC combined with IL-1β siRNA reduced the inflammatory levels and prevented liver failure. These animals administrated with MSC and IL-1β siRNA also exhibited improved liver regeneration and increased survival rates. Immunohistochemistry and fluorescence microscopy revealed the number of vital MSC in ALF + MSC + IL-1β siRNA group were significantly more than that in ALF + MSC group.
Conclusion. IL-1β siRNA adenovirus could enhance MSC ability of tissue regeneration through increasing its survival rate. Accordingly, combination of IL-1β siRNA adenovirus and MSC had a synergistic effect on acute liver failure.
Acute liver failure (ALF) is a severe clinical disease characterized by short-term loss of liver function as well as necrosis of a large number of hepatocytes. Cellular debris released by necrotic cells will lead to increased vascular permeability and strengthening the infiltration of mononuclear macrophages. More importantly, phagocytosis induced by necrotic cells will cause a cascading release of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, chemokine, leukotrienes and so on.1 At present, some studies have shown that uncontrolled immune response in the liver is the primary pathological mechanism of ALF.2
Mesenchymal stem cells (MSCs) has exhibited immunomodulatory and paracrine capabilities.3,4 Much of the research based on stem cell transplantation therapy has made remarkable achievements and provides new hopes for various diseases. MSC transplantation has also been used to treat a variety of end-stage liver diseases, including acute liver failure (ALF).5–8 However, many researchers have also found the phenomenon of poor efficacy of cell transplantation. Retrospective studies have revealed that extreme inflammation in liver inhibited the activity of MSC, limiting the efficacy of cell therapy for ALF.9 Previous studies have stated that the interaction between MSC and inflammatory microenvironment was likely to affect the efficacy of MSC. Liu, et al. indicated that inflammatory T cells could secrete TNF-α and IFN-γ which induced MSC apoptosis and restricted bone tissue regeneration.10 In addition, Shipeng Dang recently discovered that some inflammatory cytokines such as TNF-α and IFN-γ could induce MSC autophagy, in turns affecting immunomodulatory capability of MSC.11 Studies in acute lung injury also inferred inflammatory factors were toxic not only for lung tissue but also for the transplanted stem cells.12 Consequently, controlling the extreme inflammatory response in ALF was crucial for improving the efficacy of MSC transplantation.
The primary cytokines involved in inflammation include IL-1, IL-6, and TNF-α etc, in which IL-1 plays a key role for activating immune and inflammatory response. IL-1 included two subtypes, IL-1α and IL-1β, encoded by different genes. IL-1α is secreted only by macrophage while IL-1β can be produced by macrophage, monocytes, epithelial cell and tumor cells. IL-1 causes recruitment and infiltration of inflammatory cells13,14 through activating T cells and macrophages, resulting in tissue damage.15 Currently, the main methods to inhibit the inflammatory response include immunosuppressive agent and receptor antagonists of inflammation factors. However, immuno-suppressive agent has side effects of bone marrow suppression and sexual function impairment, while receptor antagonists16 must be given repeatedly in order to maintain efficacy. Accordingly, we utilized RNA interference technology to achieve the purpose of controlling the inflammation response. Small interfering RNA (siRNA) is an important intermediate role17 with the advantages of easy design, high target selectivity, and low toxicity due to metabolism to natural nucleotide components by the endogenous cell systems.18–20 Recently, siRNA has made quite a few progresses in treating acute liver failure.21–23
In this study, we constructed IL-1β siRNA adenovirus to inhibit the severe inflammation in ALF, expecting to improve the activity of infused MSC. Afterwards, we evaluated the synergetic effect of IL-1β siRNA adenovirus and MSC in ALF.
Material and MethodsAnimalsMale 7-wk-old BALB/c mice were purchased from the Laboratory Animal Center of the Affiliated Drum Tower Hospital of Nanjing University Medical School, and housed individually, placed in a ventilated cabinet under controlled air pressure and temperature conditions, and under daily cycles of alternating 12 h light/dark. They had sterile water and sterile standard pelleted rodent diet. The mice were sacrificed by cervical spine dislocation.
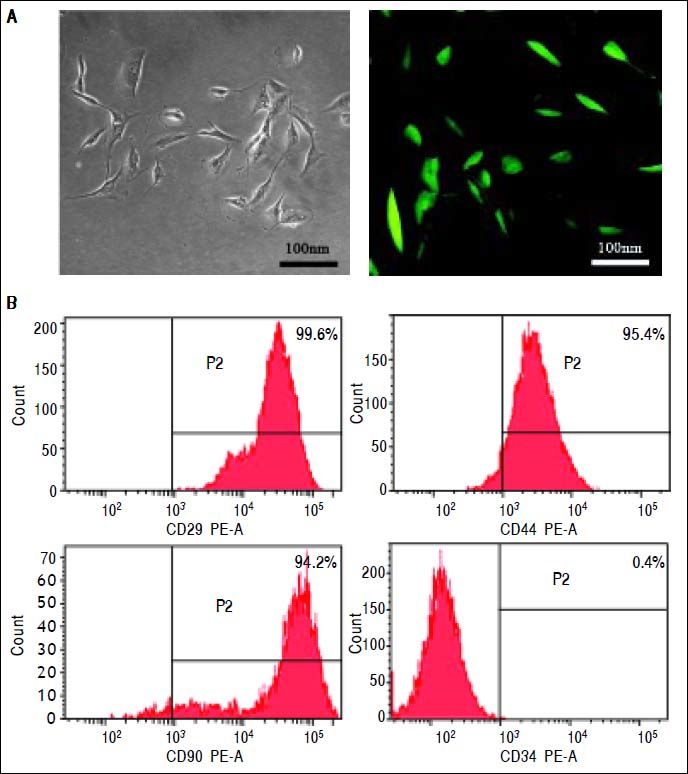
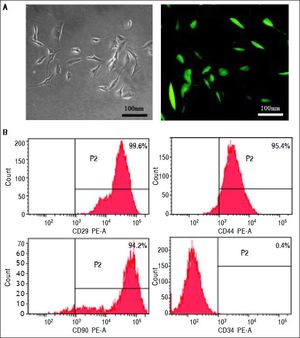
MSCs isolation and characterizationMSCs were isolated by bone marrow aspirates from the thighbone of BALB/c mice24 and transfected by lentivirus encoding green fluorescence protein (GFP). Briefly, mononuclear cells were collected by gradient centrifugation over a Ficoll histopaque layer (20 min, 400 g, density 1.077 g/mL) and seeded at a density of 1 × 106 cells/cm2 in growth medium containing low-glucose Dulbecco’s modified Eagle’s medium (DMEM-LG) supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/ mL) and streptomycin (100 mg/mL). The non-adherent cells were removed after the first 24 h and changed every 3-4 days thereafter. When cells reached 80% confluence, they were detached using 0.25% Trypsin-EDTA (w/v) and replated at a density of 1 × 104 cells/cm2 for expansion. The surface marker identification of the cultured MSCs was performed with a FACScan (Becton Dickinson, San Diego, CA) by fluorescein isothiocyanate (FITC)-labeled monoclonal antibody staining to CD29, CD44, CD90 and CD34 (Antigenix America, Huntington Station, NY). Isotypic antibodies served as the control.
Evaluation of IL-1β siRNA adenovirusRaw 264.7 cells were seeded at a density of 5 × 105 cells/ cm2 in six-well plates containing high-glucose DMEM medium with 10% FBS. 1 × 108 pfu IL-1β siRNA adenovirus was added into the six-well plates after cell adhesion and LPS (final concentration: 2 μg/mL) was added 12 h later. The culture medium was collected 24 h later and the concentration of IL-1β was detected by ELISA.
We explored the optimal dose and time point of IL-1β siRNA by qPCR. Briefly, we injected different doses of IL-1β siRNA (2 × 107 pfu, 5 × 107 pfu, 1 × 108 pfu and 2 × 108 pfu) into normal mice via tail vein and detected IL-1β level 36 h later by qPCR to identify the optimal dose. Then we injected intravenously with the optimal dose and detected the expression of IL-1β at different time points by qPCR to find out the optimal time point. Briefly, total RNA was isolated from liver tissue using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using the Superscript II Reverse Transcriptase Kit (Invitrogen). Quantitative polymerase chain reaction (qPCR) was performed using Power SYBR Green PCR Master Mix (ABI, United States). The sequences of primers were listed as follows:
- •
Mus musculus IL-1β-F: ATGCCACCTTTT-GACAGTGATG.
- •
Mus musculus IL-1β-R: TGATGTGCTGCTGCGA-GATT.
To assess whether adenoviral vectors would caused non-specific immune response, we injected intravenously null-adenoviral vector (without IL-1β siRNA) into normal mice and evaluated serum INF-γ, TNF-α and IL-6 level 4 h later by ELISA.
Experimental groupsTwo hundred BALB/c mice were randomly divided into five groups.
- •
Group A (n = 40): normal control group with no intervention.
- •
Group B (n = 40): ALF group, 20% v/v CCl4 (8 μL/g, dissolved in olive oil) was administered intraperitoneally to establish ALF model.
- •
Group C (n = 40): ALF + MSC group, about 1 × 106 MSCs were injected via the tail vein 24 h after CCl4 infusion.
- •
Group D (n = 40): ALF + IL-1β siRNA group, 1 × 108 pfu adenovirus containing IL-1β siRNA were treated via the tail vein 36 h prior to CCl4 infusion.
- •
Group E (n = 40): ALF + MSC + IL-1β siRNA group, 1 × 108 pfu adenovirus containing IL-1β siRNA were treated via the tail vein 36 h prior to CCl4 infusion and about 1 × 106 MSCs were injected via the tail vein 24 h after CCl4 infusion.
In each group, 10 mice were picked randomly for survival analysis. The remaining mice were sacrificed on days 1, 3, 5, 7 and 14. Serum was collected for biochemical analyses. The livers were dissected out and fixed in 4% formaldehyde.
Histological analysis and serum transaminase levelsSerum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in mouse blood were measured with an automated biochemical analyzer. For histology, livers were fixed in 4% formaldehyde for 24 h and embedded in paraffin. Five-micrometer-thick liver sections were deparaffinized and fixed. Sections were stained with hematoxylin and eosin (Sigma-Aldrich).
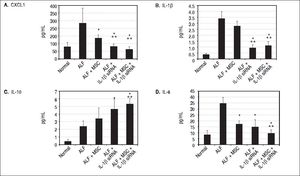
ELISA for inflammatory factors and growth factorsTo evaluate the inhibitory effect on inflammatory responses of IL-1β siRNA and MSC, we measured the concentration of the following nine cytokines at 3rd day after ALF by ELISA: G-CSF/CSF-3, IL-6, Eotaxin/CCL11, IL-1α, IL-1β, IL-10, IL-2, TNF-α and CXCL1.
Serum samples were collected at 14th day after administration of CCl4. Quantification of serum hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) was determined by ELISA as manufacturer’s instructions (R and D Systems).
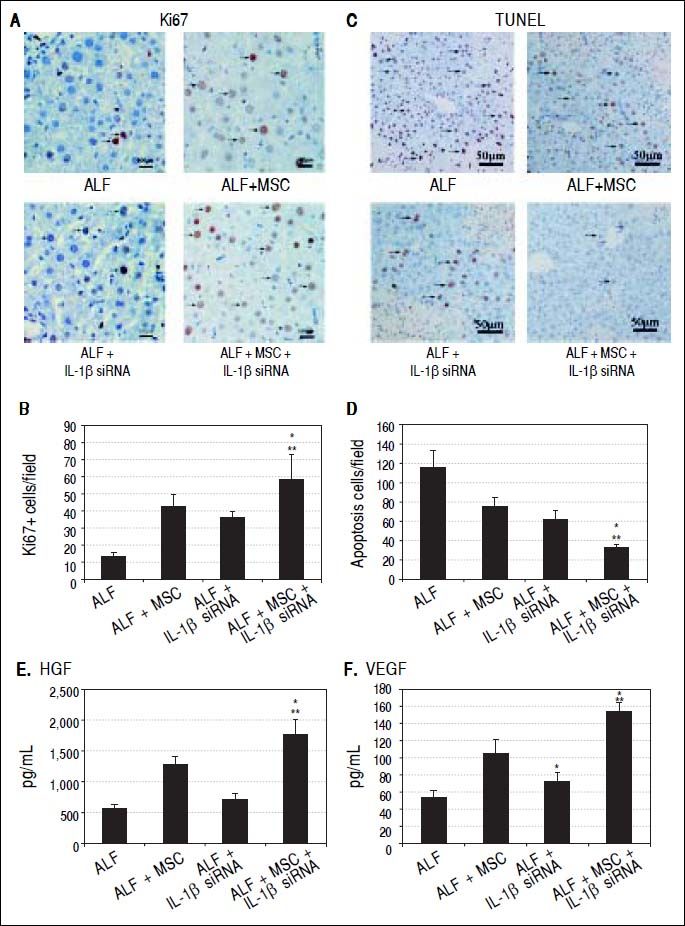
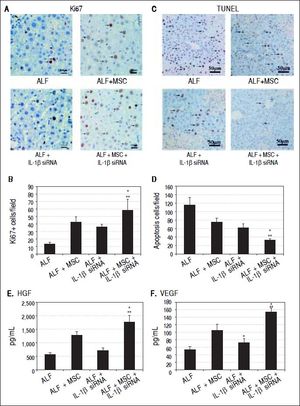
Immunohistochemistry for hepatocyte proliferation and apoptosisLiver tissues were harvested at 14th day and made into paraffin sections for Ki-67 and TUNEL immunohistochemical staining. Hematoxylin was used to indicate the nucleus of the hepatocytes. Five high-power fields were selected randomly to calculate the number of positive cells (brown nucleus).
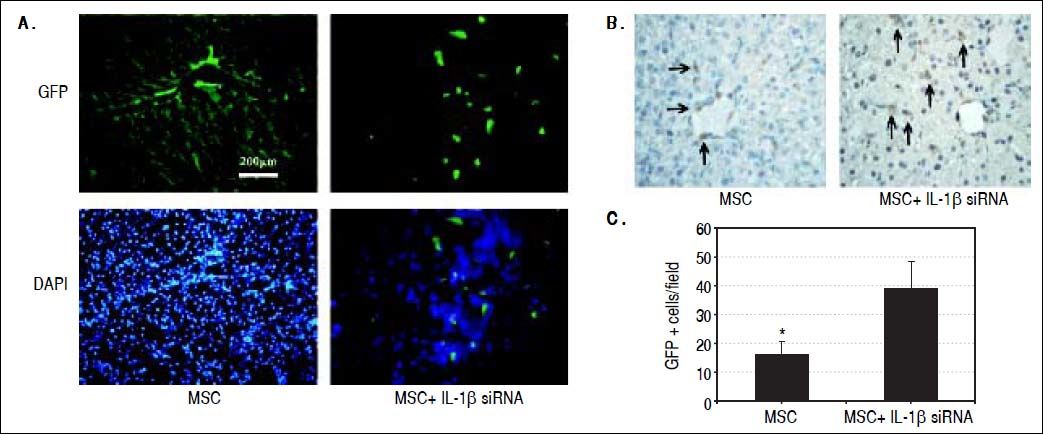
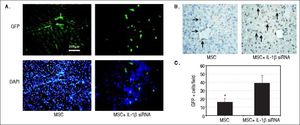
Tracking of the transplanted MSCsWe adopted two ways to assess the protection effect of IL-1β siRNA on MSC. Liver tissues were harvested at 14th day after administration of CCl4 and made into frozen and paraffin sections. Frozen sections were observed under a fluorescence microscope to search GFP-positive cells. Paraffin sections were utilized for anti-GFP immunohistochemical staining. Five high-power fields were selected randomly to calculate the number of positive cells (brown nucleus).
Statistical analysisAll experiments were replicated a minimum of three times. Statistical analysis was performed with SPSS version 19.0, and data were expressed as means ± SD. For survival analyses, a Kaplan-Meier method was used. All other data were analyzed by the independent-sample test. P < 0.05 was considered statistically significant.
ResultsIdentification of MSCsMSCs retained a fibroblastic morphology after repeated passages (Figure 1A), and their immunophenotypical characterization was confirmed by flow cytometry. Over 90% of the isolated MSCs expressed CD29, CD44 and CD90, but not CD34 (Figure 1B).
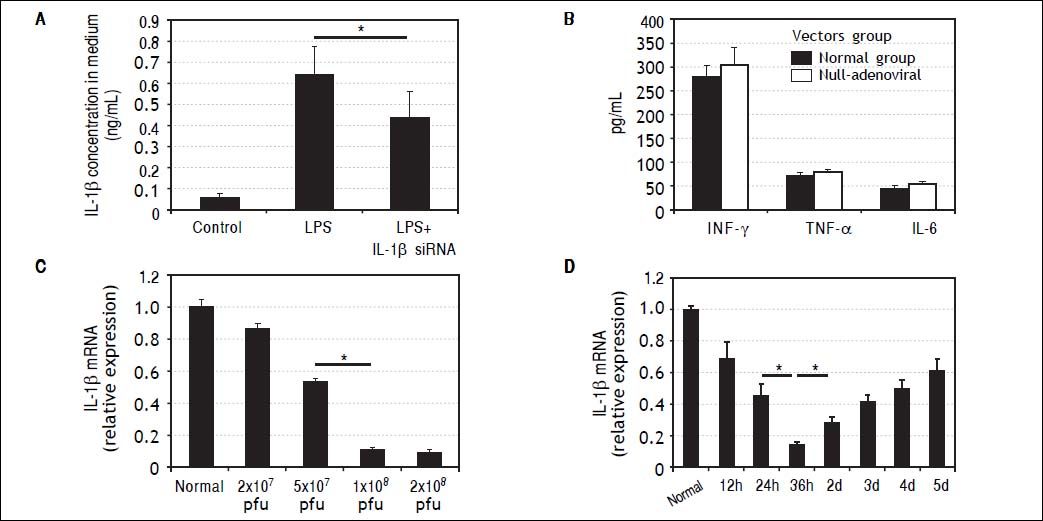
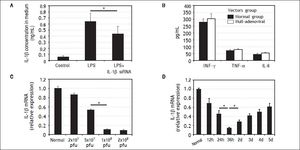
Characteristics of IL-1β siRNA adenovirusIL-1β siRNA adenovirus were designed and constructed by Life Technologies (Shanghai, China). RAW264.7 cells can secrete large amounts of IL-1β under the stimulation of LPS, while this capability was inhibited significantly when RAW264.7 cells were previously infected by Π-1β siRNA adenovirus (Figure 2A).
Characteristics of IL-1βsiRNA adenovirus. A. IL-1βsiRNA can inhibit LPS-stimulated RAW264.7 cells to secrete IL-1β. B.Levels of nonspecific immunological factors (INF-γ, TNF-αand IL-6) after injection of null-adenoviral vectors. C. Interference efficiency of different doses of IL-1βsiRNA in vitro. D.Interference efficiency of IL-1βsiRNA at different time points i n vitro. * P < 0.05, data are shown as mean±SD.
To assess whether IL-1β siRNA adenoviral vectors would cause undesirable immune response, we injected null-adenoviral vectors (avoiding interference effect of IL-1β siRNA) and detected serum INF-γ, TNF-α and IL-6 by ELISA. The results showed that there were no statistical differences of these inflammatory cytokines compared with normal mice (Figure 2B).
We injected different volume of adenovirus solution to confirm the optimal interference dose. When the dose was gradually increased from 2 × 107 pfu (20 μL) to 1 × 108 pfu (100 μL), the interference efficiency was improved correspondingly from 13 to about 89%. Inconsistently, the interference efficiency did not continue to increase when we injected 2 × 108 pfu (200 μL) (Figure 2C). Based on the above results, we choose 1 × 108 pfu (100 μL) as a follow-on dose.
We injected 1 × 108 pfu IL-1β siRNA adenovirus to normal mice and detected IL-1β gene expression in liver tissues at different time points by qPCR. It showed the maximal interference efficiency (about 76%) of Π.-1β siR-NA adenovirus occurred at 36h, and then decreased gradually (Figure 2D). Based on these findings, we choose to inject IL-1β siRNA adenovirus 36 h before administration of CCl4.
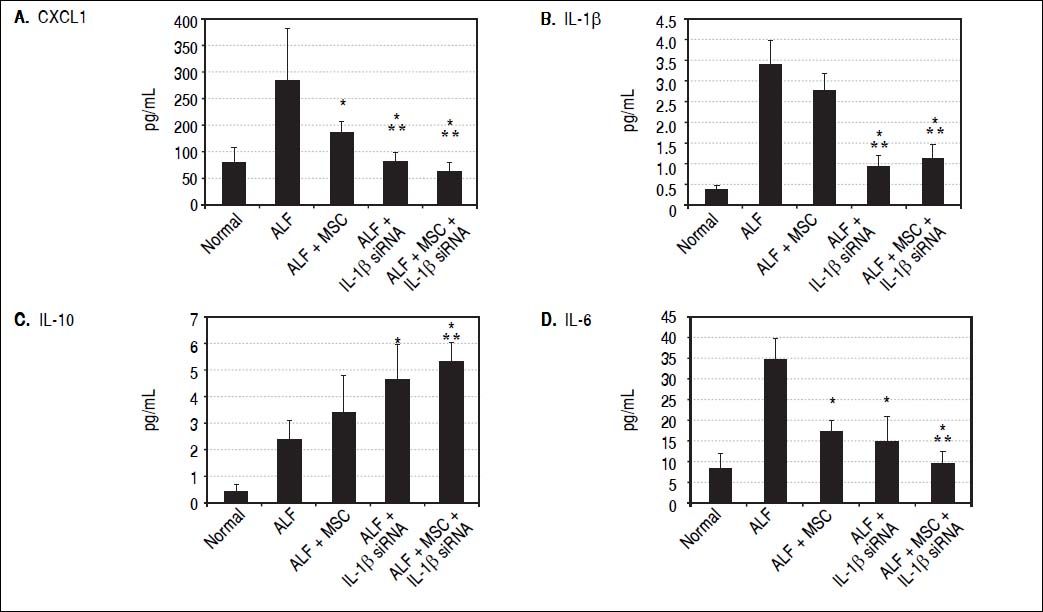
IL-1β siRNA attenuated inflammatory responsesWe detected the levels of serum inflammatory factors (G-CSF/CSF-3, IL-6, Eotaxin/CCL11, IL-1 α, Η.-1β, IL-10, IL-2, TNF-α and CXCL1) by ELISA at 3rd day after ALF. Eventually we listed CXCL1, Κ.-1β, IL-10 and IL-6 levels (changes of the other factors were not significant). We demonstrated that compared with ALF group, the levels of CXCL1 and IL-6 in ALF + MSC group were significantly lower, while CXCL1, IL-1β and IL-6 in ALF+IL-1β siRNA group reduced significantly. However, the level of IL-10 in ALF + IL-1β siRNA group was higher than that in ALF group. In addition, CXCL1, IL-1β and IL-6 levels in ALF + IL-1β siRNA+MSC group were lower than that in either ALF + IL-1β siRNA group or ALF + MSC group, and IL-10 level in ALF + IL-1β siRNA + MSC group was higher than that in ALF group or ALF + MSC group. Interestingly, there were no significantly differences between ALF + IL-1β siRNA + MSC group and ALF + IL-1β siRNA group regarding to the levels of CXCL1, IL-1β, IL-10 and IL-6 levels (Figures 3A-3D).
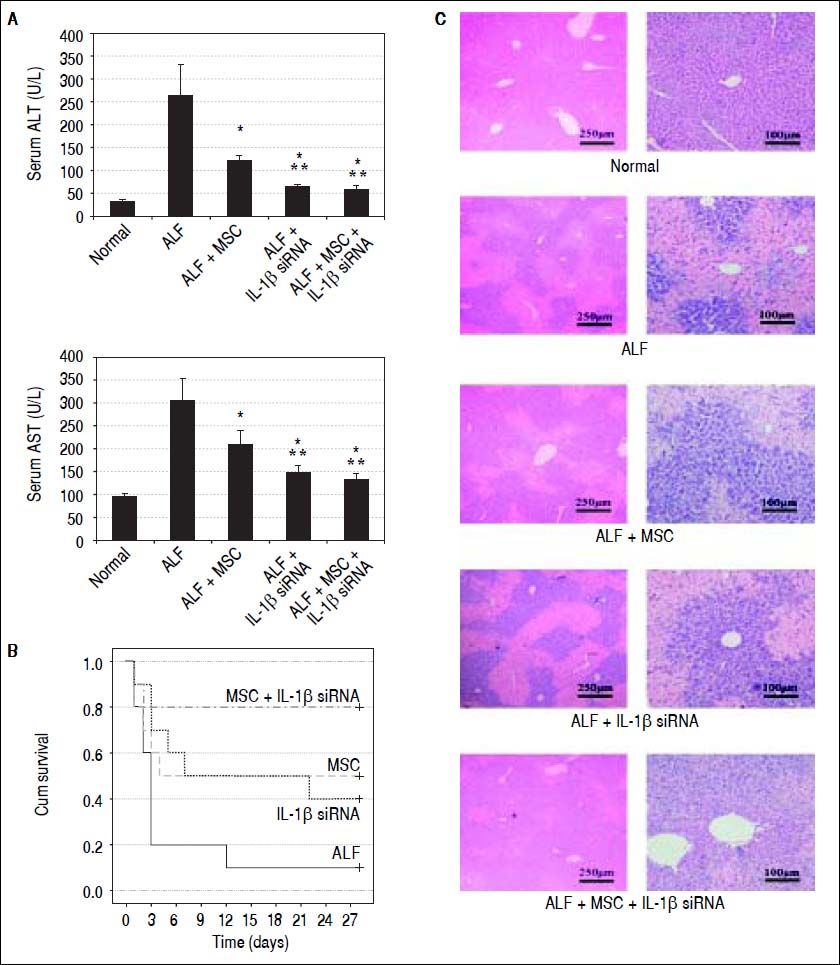
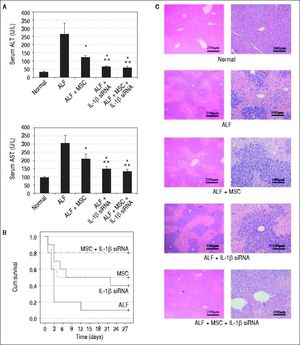
IL-1β siRNA enhances the therapeutic efficacy of MSCsSerum ALT and AST was detected at 7th day after CCl4 infusion. The levels of ALT in each group were displayed as follows:
- •
ALF group, 263.11 U/L ± 71.05 U/L.
- •
ALF + MSC group, 121.16 U/L ± 11.93 U/L.
- •
ALF + IL-1β siRNA group, 62.92 U/L ± 7.82 U/L.
- •
ALF + MSC + Π.-1β siRNA group, 57.34 U/L ± 8.51 U/L.
AST levels in each group were (Figure 4A):
- •
ALF group, 304.89 U/L ± 28.47 U/L.
- •
ALF+MSC group, 210.02 U/L ± 22.43 U/L.
- •
ALF + IL-1β siRNA group, 146.34 ± 11.89 U/L.
- •
ALF+MSC+IL-1β siRNA group, 130.56 ± 16.87 U/L.
Therapeutic efficacy was evaluated by histological analyses, liver enzyme and survival rate. A. ALT and AST enzyme levels in peripheral blood samples collected at 7th day after transplantation. B. Survival analysis of CCl4-treated mice. C. Liver HE staining of each group at 2nd week after cell transplantation. * P < 0.05 vs. ALF group, ** P < 0.05 vs. ALF + MSC group, data are shown as mean ± SD. ALT: aspartate aminotransferase. AST: alanine aminotransferase.
We randomly selected 10 mice from each group for survival rate analysis. During the 4 weeks after cell transplantation, only 10% mice (1/10) in ALF group survived at fourth week, and 50% (5/10) mice in ALF + MSC group survived up to 4 wk, while 40% (4/10) mice in ALF + IL-1β siRNA group survived up to 4 wk. Excitingly, 80% (8/ 10) mice in ALF + IL-1β siRNA + MSC group survived until the fourth week (Figure 4B).
To investigate the liver histology of the mice, hematoxylin and eosin staining was performed. The liver histology of the normal mice showed uniform cellular morphology. In contrast, failing liver showed extensive hepatocyte necrosis with hemorrhaging involving entire lobules, and the hepatocytes presented swollen cytoplasm (light-staining areas). In the images of mice transplanted with MSC or IL-1β siRNA, light-staining areas decreased. However, this tendency was improved in the images of mice transplanted with MSC + IL-1β siRNA (Figure 4C).
To evaluate whether stem cell transplantation enhanced the proliferation and suppressed the apoptosis of hepatocytes in failing liver, Ki-67 and TUNEL expression were assessed by immunohistochemistry. The results showed that Ki67+ cells in ALF + MSC group were slightly more than that in ALF + IL-1β siRNA group, while apoptotic cells were slightly less than that in ALF + IL-1β siRNA group, but there was no statistically difference between the two groups (Figures 5A and 5B). However, Ki-67+ cells in ALF + IL-1β siRNA + MSC group were significantly more than that in ALF + MSC group or ALF + IL-1β siRNA group, and apoptotic cells were significantly less than that in ALF + MSC group or ALF + IL-1β siRNA group (Figures 5C and 5D).
Cell transplantation promoted proliferation and suppressed apoptosis of hepatocytes via growth factors. A, C. Immunohistochemical staining of Ki67 and TUNEL assay on 14th day after cell perfusion. B, D. Graph of the average percentage of Ki67-positive cells and TUNEL-positive cells. E, F. Levels of HGFand VEGF on days 14 after cell perfusion. * P < 0.05 vs. ALF + MSC group. ** P < 0.05 vs. ALF + IL-1βsiRNA group, data are shown as mean±SD. TUNEL: 2’-deoxyuridine 5-triphosphate nick-end labeling. HGF: hepatocyte-generating factors. VEGF: vascular endothelial growth factor.
Two weeks after cell transplantation, serum showed a significant increase in HGF and VEGF levels in ALF + MSC group when compared with ALF group or ALF + IL-1β siRNA group. The levels of HGF and VEGF in ALF + IL-1β siRNA + MSC group were higher significantly than that in ALF + MSC group (Figures 5E and 5F).
IL-1β siRNA improved the therapeutic efficacy of MSCs by promoting the survival of MSCsWe observed the colonization of MSCs in liver tissues by fluorescence microscopy and anti-GFP immunohistochemical staining. Fluorescence microscopy exhibited that in ALF + IL-1β siRNA+MSC group, MSC distributed in lobular parenchyma dispersedly, while along the vascular in ALF + MSC group (Figure 6A). Immunohistochemistry displayed that the number of GFP+ cells in ALF + IL-1β siRNA + MSC group was significantly higher than that in ALF + MSC group (Figures 6B and 6C), consistently with the result of fluorescence microscope.
Protective effects of IL-1βsiRNA on MSC. A. Expression of GFP-labeled MSCs engrafted in liver tissues, detected by fuorescence microscope. B. Immunohistochemical staining of anti-GFP assay. GFP+ cells exhibited brown. C. Quantitative image analyses of GFP+ cells. *P < 0.05vs. MSC + IL-1βsiRNA group. Data are shown as mean±SD. DAPI: 4’, 6-diamidino-2-phenylindole.
Uncontrolled hepatic immunoactivation is regarded as the primary pathological mechanism of ALF. And the blockade of inflammatory cytokines has been considered beneficial in the treatment of acute liver injury. In our previous studies, we employed IL-1Ra to inhibit inflammation of ALF.16,25 Unfortunately, exogenous IL-1Ra is rapidly cleared by the liver and thus has a short biological half-life. It is therefore almost impossible to sustain a constant high level of exogenous IL-1Ra in the circulation, even by using repeated injections of IL-1Ra protein at short intervals. So injection of exogenous IL-1Ra is not a feasible intervention way. Zheng, et al.26 implanted IL-1Ra-expressing AF-MSCs into injured liver and their results showed that AF-MSCs over-expressing IL-1Ra prevented liver failure and reduced mortality in rats with FHF. Genetic modification solved the problem of rapid clearing of exogenous IL-1Ra, but they didn’t detect viable MSCs, nor explore the protective effect of controlling inflammation on MSCs.
In this study, we utilized IL-1β siRNA to control the inflammatory response. However, naked siRNA is very unstable in vivo and27,28 readily digested by nucleases. In order to facilitate the effect of siRNA delivery, it is important to employ suitable carriers. In vivo delivery techniques have historically included plasmid vectors, viral vectors and cationic liposomes.29–31 Considering that retrovirus can only infect cells in M phase and lentivirus are potentially harmful to staff,32 we chose adenovirus as vectors in this study. Although gene expression of adenoviral vector is short, considering the most dramatic inflammatory response in ALF is approximately one week, adenoviral vector is sufficient to control the inflammation.
Our study demonstrated that when compared with MSC group, CXCL1, IL,-1β and IL-6 levels in IL,-1β siRNA + MSC group were significantly lower, while IL-10 levels were significantly higher. But no differences of CXCL1, IL-1β, IL-6 and IL-10 levels were observed between IL-1β siRNA+MSC group and IL-1β siRNA group. Above results showed that in the aspect of controlling inflammation, IL-1β siRNA+MSC and IL-1β siRNA have similar effects. The possible reason we inferred was that compared with IL-1β siRNA, the immunosuppression effect of MSC could be ignored. We found an interesting phenomenon in the detection of transaminases: levels of ALT and AST in ALF + IL-1β siRNA + MSC group changed significantly when compared with ALF + MSC group, but no statistical differences were observed when compared with ALF + IL-1β siRNA group. The possible reason we supposed was that transaminase reflects damage of hepatocytes, which was triggered mainly by acute inflammatory response. However, inflammation was suppressed in either ALF + IL-1β siRNA + MSC group or ALF + IL-1β siRNA group due to the administration of IL-1β siRNA. Accordingly, levels of transaminases were similar in ALF + IL-1β siRNA+MSC group and ALF + IL-1β siRNA group. Based on the above results, it seemed that the combination of MSC was hardly inevitable, but we frowned on it. Although IL-1β siRNA had a similar effect on the control of inflammation compared with IL-1β siRNA + MSC, it had no effect on promoting liver regeneration.
The Ki-67 and TUNEL examination showed that Ki-67+ cells in ALF + IL-1β siRNA + MSC group were significantly more than that in ALF + MSC group or ALF + IL-1β siRNA group, and apoptotic cells were significantly less than that in ALF + MSC group or ALF+IL-1β siRNA group. We concluded that ALF + IL-1β siRNA + MSC group exhibited a better effect on promoting liver regeneration than ALF + MSC group or ALF + IL-1β siRNA group. Consistently, the survival rate of mice in ALF+IL-1β siRNA+MSC group was 80%, higher than ALF + MSC group (50%) or ALF + IL-1β siRNA group (70%). In fact, controlling inflammation and promoting liver regeneration are two equally crucial aspects in the treatment of ALF, only two-pronged approach could achieve the best results.
In order to explain the mechanism of combination therapy, we examined serum HGF and VEGF at 14th day. There was a significant increase in ALF + IL-1β siRNA + MSC group when compared with ALF + MSC group or ALF + IL-1β siRNA group. We hypothesized that IL-1β siRNA inhibited liver inflammation response and in turns increased the activity of MSCs. We detected the colonization of MSCs in liver tissues by fluorescence microscopy and anti-GFP immunohistochemical staining to confirm our hypothesis. The results were consistent with our expectation: the number of GFP+ cells in ALF+IL-1β siRNA+MSC group was significantly higher than that in ALF + MSC group, indicating IL-1β siRNA could improve the survival rate of MSC in failing liver.
Although our study showed that MSC transplantation represent a promising therapy related to liver failure, present therapeutic efficacy was limited to some extent. Efficient delivery of cells to target organs is critical to improving their effectiveness. Some researchers have revealed that low colonization of transplanted MSCs in the liver restricted the efficacy of cytotherapy.33 Our previous study demonstrated that genetically modified MSCs expressing CXCR4 showed greater colonization and conferred better functional recovery in damaged liver.34 That observation may be beneficial for improving efficacy of MSCs, although further studies are required to confirm this.
In clinical trials, the administration of MSCs has also been reported to be well tolerated and confers beneficial effects in patients with liver failure, by enhancing liver function and reducing Child-Pugh and MELD scores, ascites, and overall mortality.35–37 However, some concerns and critical issues remain unanswered regarding the longterm safety and efficacy of clinical stem cell therapies. More randomised clinical trials with low-risk of bias may help in defining the role of stem cell transplantation in the treatment of liver disease.
In summary, IL-1β siRNA could effectively inhibit the inflammatory response in failing liver and enhance the MSC ability of tissue repair through increasing the survival rate of MSC. Based on this, IL-1β siRNA combined with MSC has a synergistic therapy effect on ALF.
SupportNational Natural Science Foundation of China, No. 81170418; Natural Science Foundation of Jiangsu Province, No. BK20131084; Strategic Priority Research Program of the Chinese Academy of Science, No.XDA01030602.
Compliance With Ethical RequirementsHucheng Ma, Xiaolei Shi, Xianwen Yuan and YiTao Ding declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.